Abstract
Objective
This paper was performed to decipher the serum microRNA (miR)-125b-5p expression in patients with dilated cardiomyopathy (DCM) combined with heart failure (HF) and its effect on myocardial fibrosis.
Methods
Serum miR-125b-5p expression, LVEDD, LVESD, LVEF, LVFS, and NT-proBNP levels were evaluated in clinical samples. A rat DCM model was established by continuous intraperitoneal injection of adriamycin and treated with miR-125b-5p agomir and its negative control. Cardiac function, serum TNF-α, hs-CRP, and NT-proBNP levels, pathological changes in myocardial tissues, cardiomyocyte apoptosis, and the expression levels of miR-125b-5p and fibrosis-related factors were detected in rats.
Results
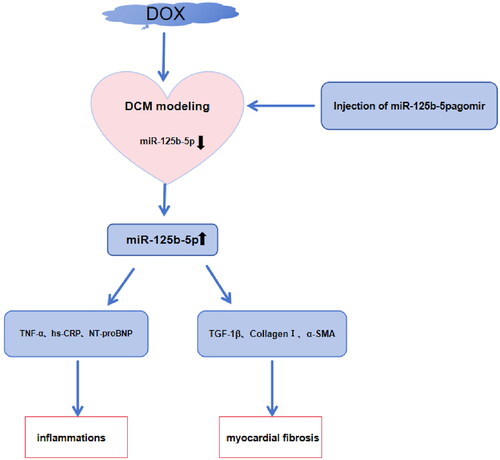
In comparison to the control group, the case group had higher levels of LVEDD, LVESD, and NT-pro-BNP, and lower levels of LVEF, LVFS, and miR-125b-5p expression levels. Overexpression of miR-125b-5p effectively led to the improvement of cardiomyocyte hypertrophy and collagen arrangement disorder in DCM rats, the reduction of blue-stained collagen fibers in the interstitial myocardium, the reduction of the levels of TNF-α, hs-CRP, and NT-proBNP and the expression levels of TGF-1β, Collagen I, and α-SMA, and the reduction of the number of apoptosis in cardiomyocytes.
Conclusion
Overexpression of miR-125b-5p is effective in ameliorating myocardial fibrosis.
Introduction
Dilated cardiomyopathy (DCM) is a chief reason for serious heart failure (HF) and is featured with fluid retention (edema) in the lungs and other areas, resulting in breathing difficulties, decreased physical fitness, limitations in normal daily activities, and death [Citation1,Citation2]. DCM has genetic combined with other reasons, while it typically develops to symptomatic HF via four stages (A-D), which are summarized in the mouse model of DCM combined with HF with decreased ejection fraction [Citation3]. Myocardial fibrosis is defined as a frequent pathological feature in DCM, which contributes to ventricular dysfunction and HF [Citation4]. Further, DCM is featured with clinical, electrocardiographic, imaging abnormalities, as well as circulating biomarker. The diagnosis of clinical DCM combined with CHF is simple, while the recognition of the preclinical stage may be challenging [Citation5].
The structural proteins are mostly utilized DCM biomarkers in routine genetic and clinical testing, but the role of microRNA (miRNA) has also become a biomarker as they can be obtained through non-invasive methods in the past few years [Citation6]. miRNAs are able to induce gene silencing together with translational repression via binding to specific sequences in target mRNAs, thus functioning in biological processes of diverse diseases [Citation7]. Accumulating publications have disclosed that miRNAs are capable of modulating immunity and inflammation, and these miRNAs might be potential treatment methods for clinical problems [Citation8,Citation9]. miR-125 is considered a crucial modulator in hematological malignancies and belongs to highly-conserved miRNAs in different species [Citation10]. Especially, the potency of miR-125b in cardiovascular diseases has recently received increasing attention. For instance, it has been revealed that elevation of miR-125b mitigates hypoxia/reoxygenation and ischemia/reperfusion-evoked cell injury and death in H9C2 cardiomyoblasts and adult cardiacmyocytes [Citation11]. Another article has demonstrated that the cardiac-specific miR-125b-1 knockout reduces the expression of fibrosis-associated genes at the neonatal stage, indicating that miR-125b in cardiomyocytes may modulate cardiac fibrosis [Citation12]. Furthermore, several studies have unveiled that miR-125b-5p is downregulated in end-stage DCM or ischemic cardiomyopathy patients [Citation13,Citation14]. Therefore, this paper was performed to decipher the serum miR-125b-5p expression in patients with DCM combined with HF and its effect on myocardial fibrosis.
Materials and methods
Participants
Forty-nine patients with DCM combined with HF who were diagnosed and treated in Hunan Provincial People’s Hospital were selected as the case group. Inclusion criteria: (1) patients met the diagnostic criteria for DCM in the Recommendations for the Diagnosis and Treatment of Cardiomyopathy in China [cardiac enlargement: left ventricular end-diastolic internal diameter (LVEDD) > 5.5 cm (men) or > 5.0 cm (women); left ventricular ejection fraction (LVEF) < 45%]; (2) those met the diagnostic criteria for HF of the European Society of Cardiology; (3) those belonging to the NYHA cardiac function class II to IV. Patients with primary and secondary hypertension, heart valve disease, congenital heart disease, ischemic heart disease, hypertrophic cardiomyopathy, diabetes cardiomyopathy, serious immune disease, liver and kidney dysfunction, serious infection, malignant tumor and other diseases were excluded. Forty-nine healthy medical examiners were also selected as the control group.
Determination of cardiac function indicators and serum NT-proBNP levels
LVEDD, left ventricular end-systolic internal diameter (LVESD), LVEF, and left ventricular short-axis fraction shortening (LVFS) were measured using a diagnostic ultrasound device (LOGIQ-700). Fasting peripheral venous blood of 5 mL was collected on the second day of enrollment, centrifuged for 10 min (3000 r/min), and the supernatant was stored in the refrigerator at −80 °C after centrifugation. The serum NT-proBNP levels were assessed by using enzyme-linked immunosorbent assay (ELISA, Shanghai Jinsui Bio-Technology Co., Ltd., Shanghai, China).
Detection of miR-125b-5p expression levels
Total RNA was extracted from patient serum and rat myocardial tissue as per the requirements of TRIzol kit (Shanghai Yuduo Biotechnology Co., Ltd., Shanghai, China), and the concentration and purity of total RNA were evaluated by NanoDropND12000 spectrophotometer (Thermo, USA). RNA was reverse transcribed into cDNA using the PrimeScript cDNA RT Kit (Beijing Kang Ruina Biotechnology Co., Ltd., Beijing, China). miR-125b-5p, TGF-1-β, Collagen I, and α-SMA expression was evaluated by RT-qPCR (Bio-Rad, USA) with the internal reference of U6 or GAPDH. The primer sequences are shown in . To minimize the experimental error, each sample was repeated three times, and the relative expression of miR-125b-5p was calculated using the 2-ΔΔCt method.
Table 1. Primer sequences for genes in RT-qPCR (rat).
Grouping for the embellishment of animal models of DCM and methods of intervention
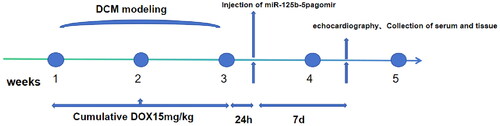
SD rats were randomized into four groups: control (CON) group, doxorubicin (DOX) group, DOX + negative control (NC) agomir group, and DOX + miR-125b-5p agomir group (n = 5 rats), and these rats were housed for about 7 days in the SPF-grade Animal Center for acclimatization to the new environment. The rats in the DOX, DOX + NC agomir, and DOX + miR-125b-5p agomir groups were given DOX, which was administered intraperitoneally to the rats over a 3-week period in six equal injections (each containing 2.5 mg/kg) for a total cumulative dose of 15 mg/kg [Citation15]. During ADR injection, take care not to inject liquid into the intestinal lumen and be aseptic. All rats were successfully modeled. Rats in the CON group were injected with equal volumes of saline according to body weight by the same route during the same period. When the EF values of rats in the DOX, DOX + NC agomir, and DOX + miR-125b-5p agomir groups were < 60%, it was suggested that the DCM combined with HF model in rats was successfully prepared. Twenty-four hours after successful modeling, miR-125b-5p agomir (15 mg/kg) was injected into the tail vein of rats in the DOX + miR-125b-5p agomir group, and a NC (miR-125b-5p NC, 15 mg/kg) was injected into rats in the DOX + NC agomir group, via tail vein injections on days 1, 3 and 8 [Citation16].
Cardiac function in rats by echocardiography
The miR-125b-5p agomir and NC agomir drug injection was completed with continuous observation for 1 week. All rats were housed in a certified SPF-grade facility maintained at approximately 24 °C with a 12-h light/dark cycle and free access to food and water. The rats to be tested were subjected to thoracic and abdominal skin preparation about 20 min in advance, and the rats were anesthetized by inhalation of 2.0% isoflurane [Citation17] until they were unresponsive to nose pinching. LVEDD and LVESD were measured using a Phillips SONOS 5500 color Doppler ultrasound device, and the LVEF and LVFS were also calculated. The model establishment and detection process diagram is shown in .
HE and Masson stainings
After the cardiac ultrasound examination, the rats were immediately executed, the hearts were dissected and quickly removed, the ventricular muscle was repeatedly cleaned and separated, and paraffin sections were routinely fixed, stained with HE staining [Citation18] and Masson stainings [Citation19], and observed under an ordinary light microscope.
Detection of serum TNF-α, hs-CRP, and NT-proBNP levels
Three milliliters of blood was taken from rats before execution, centrifuged at high speed and low temperature (3000 rpm, 4 °C) for 10 min. Subsequently, the serum was labeled and then the specimen was stored at −20 °C for examination. The levels of TNF-α, hs-CRP and NT-proBNP were determined by double antibody sandwich ELISA (Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China). The flowchart of detection indicators is shown in .
TUNEL staining
The paraffin sections of each group were operated under the TUNEL kit procedure and blocked with drops of anti-fluorescence quencher, and the apoptotic cardiomyocytes were counted under the fluorescence microscope, and green fluorescent nuclei were considered as positive results.
Statistical analysis
SPSS25.0 statistical professional software was applied to analyze and calculate the data. The categorical data was expressed by n, which were analyzed by χ2 test or Fisher’s exact test; the measurement data was expressed by mean ± standard deviation, which were analyzed by t test or one-way ANOVA. The receiver operating characteristic (ROC) curves of the subjects were plotted, and the area under the curve (AUC) was calculated to evaluate the clinical diagnostic value of miR-125b-5p level for DCM with HF. Pearson correlation analysis was employed to analyze the relationship between miR-125b-5p and LVEDD, LVESD, NT-proBNP, LVEF, and LVFS, and Spearman correlation analysis was employed to analyze the relationship between miR-125b-5p and NYHA cardiac function classification. p < 0.05 represented statistical significance.
Results
General data comparison in clinical samples
The differences in age, gender, body mass index, course of disease, smoking history, drinking history, and NYHA classification between the two groups were not statistically significant (p > 0.05) and were comparable ().
Table 2. Comparison of the general data between the two groups.
Cardiac function, miR-125b-5p and NT-pro-BNP levels
Lower miR-125b-5p expression levels and higher NT-pro-BNP levels were observed in the case group compared with the control group (p < 0.05). LVEDD and LVESD were markedly higher and LVEF and LVFS were significantly lower in the case group in contrast to the control group (p < 0.05; ).
Table 3. Comparison of cardiac function between the two groups.
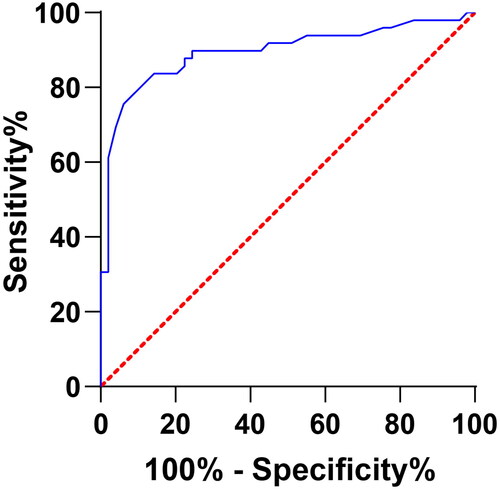
Clinical diagnostic value of miR-125b-5p levels in DCM with HF
The miR-125b-5p level was effective in predicting DCM with HF with a sensitivity of 0.776, a specificity of 0.918, and a Youden index of approximately 0.694 ().
Analysis of the correlation between miR-125b-5p and each indicator in patients
Pearson correlation analysis revealed that serum miR-125b-5p levels of patients in the case group were negatively correlated with LVEDD, LVESD, NT-proBNP, and positively correlated with LVEF and LVFS. Spearman correlation analysis suggested that serum miR-125b-5p levels were negatively correlated with NYHA cardiac function classification (p < 0.05; ).
Table 4. Analysis of the correlation between miR-125b-5p and each indicator in patients.
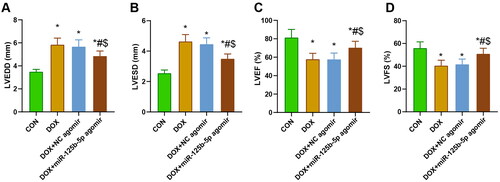
Cardiac function of rats in each group
After modeling, echocardiography was performed to assess the cardiac function of rats in each group, and the findings demonstrated that LVEDD and LVESD were expanded and LVEF and LVFS were decreased in rats in the DOX, DOX + NC agomir, and DOX + miR-125b-5p agomir groups compared with those in the CON group. Compared with rats in the DOX and DOX + NC agomir groups, rats in the DOX + miR-125b-5p agomir group had reduced LVEDD and LVESD and elevated LVEF and LVFS ().
Overexpression of miR-125b-5p effectively ameliorates myocardial fibrosis in DCM rats
miR-125b-5p expression in rats in each group was measured by RT-qPCR, which suggested that miR-125b-5p levels in the DOX and DOX + NC agomir groups were significantly lower than those in the CON group, and miR-125b-5p levels in rats in the DOX + miR-125b-5p agomir group were significantly higher than those in the CON, DOX and DOX + NC agomir groups (p < 0.05; ).
Figure 5. Overexpression of miR-125b-5p effectively improves myocardial fibrosis in DCM rats. A. RT-qPCR was utilized to detect miR-125b-5p expression in rats of each group. B. HE and masson stainings were employed to observe the histologic changes of myocardial pathology in rats. C-E. ELISA was adopted to measure the levels of TNF-α, hs-CRP, and NT-proBNP in rats of each group. F-H. RT-qPCR was implemented to test the expression of TGF-1β, collagenI, and α-SMA factors in rats of each group. I. TUNEL staining was performed to examine apoptotic cardiomyocytes in rats of each group. * p < 0.05 vs. CON group; # p < 0.05 vs. DOX group; $ p < 0.05 vs. DOX + NC agomir group.
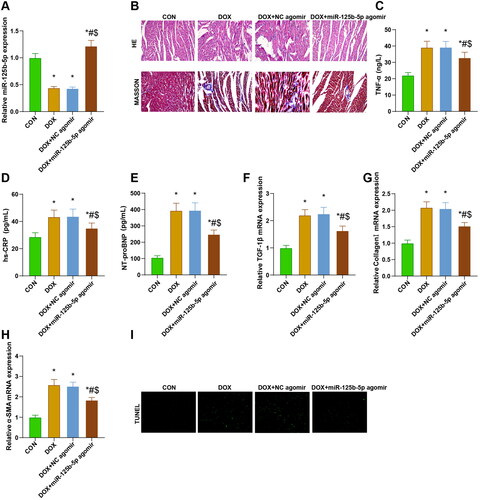
Myocardial fibers in the CON group were neatly aligned, with clear cellular structure and homogeneous nuclei size; myocardial fibers in the DOX and DOX + NC agomir groups were disorganized and sparsely aligned, with hypertrophic degeneration of cardiomyocytes, heterogeneous nuclei sizes, and an increase in bluish-stained collagen fibers in the interstitial space; hypertrophy of cardiomyocytes and disorganization of collagen alignment in the rats of the DOX + miR-125b-5p agomir group was ameliorated, and there were fewer bluish-stained collagen fibers in the interstitial space ()
The levels of TNF-α, hs-CRP, and NT-proBNP were notably higher in the DOX, DOX + NC agomir, and DOX + miR-125b-5p agomir groups than those in the CON group (p < 0.05), and the levels of these factors were remarkably lower in the DOX + miR-125b-5p agomir group than in the DOX and DOX + NC agomir groups (p < 0.05) ().
The mRNA expression levels of TGF-1β, CollagenI, and α-SMA in the DOX, DOX + NC agomir, and DOX + miR-125b-5p agomir groups were significantly higher versus the CON group (p < 0.05), and the levels of these genes in the DOX + miR-125b-5p agomir group were notably lower than those in the DOX and DOX + NC agomir groups (p < 0.05) ().
Nuclei of normal cardiomyocytes were blue-black and nuclei of apoptotic cells were green-fluorescent. The number of apoptotic cardiomyocytes was increased in the DOX and DOX + NC agomir groups compared with the CON group (p < 0.05); apoptotic cells were reduced in the DOX + miR-125b-5p agomir group in contrast to both the DOX and DOX + NC agomir group (p < 0.05) ().
In summary, overexpression of miR-125b-5p can effectively improve myocardial fibrosis in DCM rats.
Discussion
DCM is usually linked with an elevated risk of severe arrhythmia, indicating pathological implications of the cardiac conduction system. Diastolic dysfunction and injured right ventricular function will develop as the disease progresses, ultimately resulting in HF and premature death [Citation20]. The current nursing standards do not address the potential molecular mechanisms related to hereditary forms of HF, therefore, it is imperative to develop new DCM treatment methods [Citation21]. Evidence has now concentrated on using genetic testing to recognize risk markers for DCM patients [Citation22]. Therefore, this paper was performed to decipher the serum miR-125b-5p expression in patients with DCM combined with HF and its effect on myocardial fibrosis.
Combined analysis of miRNA and mRNA may supply diagnostic and prognostic information for end-stage cardiomyopathy [Citation23], which reveals the essential role of miRNAs in the overall modulation of cardiac function. It has been reported that cardiac-specific deletion of dicer results in remarkable reduction in mature miRNA levels, contributing to DCM and HF, which implies that miRNAs may function in globally modulating cardiac signaling and function [Citation24,Citation25]. In our study, we found that miR-125b-5p was underexpressed in patients with DCM combined with HF, and overexpression of miR-125b-5p was effective in ameliorating myocardial fibrosis. As previously reported, delivery of miR-125b-expressing lentivirus into the myocardium attenuates sepsis-evoked cardiac dysfunction through restraining inflammatory responses and myocardial apoptosis via blocking the p53-mediated apoptotic signaling [Citation26]. Another article has indicated that miR-125b-mediated suppression of the TNF-α/NF-κB pathway is critical for protecting endothelial progenitor cells and could be a new therapeutic hallmark for advancing the cell therapy effectiveness for ischemic heart disease [Citation27]. Moreover, miR-125b-5p upregulation has been revealed to exert a suppressive effect on hypoxia/reoxygenation-induced apoptosis via negatively modulating NLRC5 [Citation28].
Currently, BNP and NT-proBNP are broadly utilized as diagnostic biomarkers for HF and cardiac dysfunction in clinical medicine, serving as postmortem biomarkers in forensic medicine to reflect the cardiac function of the deceased before death [Citation29]. Higher NT-proBNP levels are tightly linked with HF and mortality post-acute coronary syndrome, and therapies with reduced NT-proBNP levels are bound up with improved prognosis [Citation30]. It is accepted that the increase in LVEDD and LVESD reflect the ventricular remodeling in myocardial infarction [Citation31], while the decrease in LVEF and LVFS indicate the decline in the heart systolic function [Citation32]. Meanwhile, the common hallmark of fibrosis is featured with the accumulation of diverse fibrillar collagens, which chiefly serve as a hallmark of fibrosis, especially Collagen 1 [Citation33]. Also, TGF-β1 can fundamentally strengthen cardiac fibrosis, thereby notably precipitating the HF progression [Citation34]. In this paper, cardiac function, including LVEF, LVFS, LVESD and LVEDD, serum TNF-α, hs-CRP, and NT-proBNP levels, pathological changes in myocardial tissues, cardiomyocyte apoptosis, and the expression levels of fibrosis-related factors TGF-1β, Collagen I and α-SMA were detected in rats. The corresponding findings demonstrated that overexpression of miR-125b-5p effectively led to the improvement of cardiomyocyte hypertrophy and collagen arrangement disorder in DCM rats, the reduction of blue-stained collagen fibers in the interstitial myocardium, the reduction of the levels of TNF-α, hs-CRP, and NT-proBNP and the expression levels of TGF-1β, Collagen I, and α-SMA, and the reduction of the number of apoptosis in cardiomyocytes. Interestingly, reduction of miR-125b-5p correlates with an enhanced initiation of acute myocardial infarction in humans [Citation35]. In the meantime, miR-125b-5p overexpression protects against ischemia/reperfusion injury via modulating cardiomyocyte apoptosis in mouse studies [Citation11], but downregulation of miR-125b-5p diminishes angiotensin II-evoked cardiac fibrosis by modulating fibroblast proliferation [Citation16]. Another recent publication has disclosed that miR-125b-5p advances cell viability and diminishes cell apoptosis and inflammatory factors, thereby mitigating myocardial infarction injury in oxygen glucose deprivation-induced human coronary artery endothelial cells along with human cardiac microvascular endothelial cells [Citation36]. All these findings validate the importance of miR-125b-5p in heart-related diseases.
To conclude, this paper reveals that miR-125b-5p is underexpressed in patients with DCM combined with HF, and overexpression of miR-125b-5p is effective in ameliorating myocardial fibrosis. This paper underlines that regulation of miR-125b-5p acts as an alternative intervention for DCM combined with HF. Moreover, a miRNA-network diagram should be established in the development of DCM combined with HF to lay a basis for the exploration of new therapeutics.
Ethical approval
The study got approval from the Medical Ethics Committee of Hunan Provincial People’s Hospital (approval number: 20201009) and all subjects supplied written informed consent.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet. 2017; 390(10092):400–414. doi: 10.1016/S0140-6736(16)31713-5.
- Metra M, Teerlink JR. Heart failure. Lancet. 2017; 390(10106):1981–1995. doi: 10.1016/S0140-6736(17)31071-1.
- Tripathi R, Sullivan RD, Fan TM, et al. In experimental dilated cardiomyopathy heart failure and survival are adversely affected by a lack of sexual interactions. Int J Mol Sci. 2020; 21(15):5450. doi: 10.3390/ijms21155450.
- Malgija B, Kumar NS, Piramanayagam S. Collective transcriptomic deregulation of hypertrophic and dilated cardiomyopathy - Importance of fibrotic mechanism in heart failure. Comput Biol Chem. 2018;73:85–94. doi: 10.1016/j.compbiolchem.2018.01.011.
- Bonagura JD, Visser LC. Echocardiographic assessment of dilated cardiomyopathy in dogs. J Vet Cardiol. 2022; 40:15–50. doi: 10.1016/j.jvc.2021.08.004.
- Giri P, Mukhopadhyay A, Gupta M, et al. Dilated cardiomyopathy: a new insight into the rare but common cause of heart failure. Heart Fail Rev. 2022;27(2):431–454. doi: 10.1007/s10741-021-10125-6.
- Vishnoi A, Rani S. MiRNA biogenesis and regulation of diseases: an overview. Methods Mol Biol. 2017;1509:1–10. doi: 10.1007/978-1-4939-6524-3_1.
- Nejad C, Stunden HJ, Gantier MP. A guide to miRNAs in inflammation and innate immune responses. Febs J. 2018; 285(20):3695–3716. doi: 10.1111/febs.14482.
- Mikami Y, Philips RL, Sciumè G, et al. MicroRNA-221 and -222 modulate intestinal inflammatory Th17 cell response as negative feedback regulators downstream of interleukin-23. Immunity. 2021;54(3):514–525 e6. doi: 10.1016/j.immuni.2021.02.015.
- Rodriguez A, Griffiths-Jones S, Ashurst JL, et al. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14(10A):1902–1910. doi: 10.1101/gr.2722704.
- Wang X, Ha T, Zou J, et al. MicroRNA-125b protects against myocardial ischaemia/reperfusion injury via targeting p53-mediated apoptotic signalling and TRAF6. Cardiovasc Res. 2014;102(3):385–395. doi: 10.1093/cvr/cvu044.
- Chen CY, Lee DS, Choong OK, et al. Cardiac-specific microRNA-125b deficiency induces perinatal death and cardiac hypertrophy. Sci Rep. 2021; 11(1):2377. doi: 10.1038/s41598-021-81700-y.
- Voellenkle C, van Rooij J, Cappuzzello C, et al. MicroRNA signatures in peripheral blood mononuclear cells of chronic heart failure patients. Physiol Genomics. 2010;42(3):420–426. doi: 10.1152/physiolgenomics.00211.2009.
- Marques FZ, Vizi D, Khammy O, et al. The transcardiac gradient of cardio-microRNAs in the failing heart. Eur J Heart Fail. 2016; 18(8):1000–1008. doi: 10.1002/ejhf.517.
- Zhang Y, Zhang M, Xu W, et al. The long non-coding RNA H19 promotes cardiomyocyte apoptosis in dilated cardiomyopathy. Oncotarget. 2017;8(17):28588–28594. doi: 10.18632/oncotarget.15544.
- Nagpal V, Rai R, Place AT, et al. MiR-125b Is Critical for Fibroblast-to-Myofibroblast Transition and Cardiac Fibrosis. Circulation. 2016;133(3):291–301. doi: 10.1161/CIRCULATIONAHA.115.018174.
- Sheu JJ, Chai HT, Sung PH, et al. Double overexpression of miR-19a and miR-20a in induced pluripotent stem cell-derived mesenchymal stem cells effectively preserves the left ventricular function in dilated cardiomyopathic rat. Stem Cell Res Ther. 2021;12(1):371. doi: 10.1186/s13287-021-02440-4.
- Li Y, Li Z, Liu J, et al. miR-190-5p alleviates myocardial ischemia-reperfusion injury by targeting PHLPP1. Dis Markers. 2021;2021:8709298–8709211. doi: 10.1155/2021/8709298.
- Han J, Zhang Z, Zhang Z, et al. Artemisinin relieves myocardial ischemia-reperfusion injury via modulating miR-29b-3p and hemicentin 1. Front Pharmacol. 2022;13:918966. doi: 10.3389/fphar.2022.918966.
- Zhang L, Zhang G, Lu Y, et al. Differential expression profiles of plasma exosomal microRNAs in dilated cardiomyopathy with chronic heart failure. J Cell Mol Med. 2023; 27(14):1988–2003. doi: 10.1111/jcmm.17789.
- Yang J, Grafton F, Ranjbarvaziri S, et al. Phenotypic screening with deep learning identifies HDAC6 inhibitors as cardioprotective in a BAG3 mouse model of dilated cardiomyopathy. Sci Transl Med. 2022; 14(652):eabl5654. doi: 10.1126/scitranslmed.abl5654.
- Rosenbaum AN, Agre KE, Pereira NL. Genetics of dilated cardiomyopathy: practical implications for heart failure management. Nat Rev Cardiol. 2020;17(5):286–297. doi: 10.1038/s41569-019-0284-0.
- Matkovich SJ, Van Booven DJ, Youker KA, et al. Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation. 2009; 119(9):1263–1271. doi: 10.1161/CIRCULATIONAHA.108.813576.
- Ono K, Kuwabara Y, Han J. MicroRNAs and cardiovascular diseases. Febs J. 2011;278(10):1619–1633. doi: 10.1111/j.1742-4658.2011.08090.x.
- Ikeda S, Kong SW, Lu J, et al. Altered microRNA expression in human heart disease. Physiol Genomics. 2007;31(3):367–373. doi: 10.1152/physiolgenomics.00144.2007.
- Ma H, Wang X, Ha T, et al. MicroRNA-125b prevents cardiac dysfunction in polymicrobial sepsis by targeting TRAF6-mediated nuclear factor kappaB activation and p53-mediated apoptotic signaling. J Infect Dis. 2016;214(11):1773–1783. doi: 10.1093/infdis/jiw449.
- Yang K, Liu X, Lin W, et al. Upregulation of MicroRNA-125b leads to the resistance to inflammatory injury in endothelial progenitor cells. Cardiol Res Pract. 2020;2020:6210847–6210847. doi: 10.1155/2020/6210847.
- Liu Z, Liu J, Wei Y, et al. LncRNA MALAT1 prevents the protective effects of miR-125b-5p against acute myocardial infarction through positive regulation of NLRC5. Exp Ther Med. 2020; 19(2):990–998. doi: 10.3892/etm.2019.8309.
- Cao Z, Jia Y, Zhu B. BNP and NT-proBNP as diagnostic biomarkers for cardiac dysfunction in both clinical and forensic medicine. Int J Mol Sci. 2019;20(8):1820. doi: 10.3390/ijms20081820.
- Kwee LC, Neely ML, Grass E, et al. Associations of osteopontin and NT-proBNP with circulating miRNA levels in acute coronary syndrome. Physiol Genomics. 2019;51(10):506–515. doi: 10.1152/physiolgenomics.00033.2019.
- Wu L, Maimaitirexiati X, Jiang Y, et al. Parkin regulates mitochondrial autophagy after myocardial infarction in rats. Med Sci Monit. 2016;22:1553–1559. doi: 10.12659/msm.898722.
- Oz F, Cizgici AY, Ucar A, et al. Doppler-derived strain imaging detects left ventricular systolic dysfunction in children with Turner syndrome. Echocardiography. 2014;31(8):1017–1022. doi: 10.1111/echo.12500.
- Hosper NA, van den Berg PP, de Rond S, et al. Epithelial-to-mesenchymal transition in fibrosis: collagen type I expression is highly upregulated after EMT, but does not contribute to collagen deposition. Exp Cell Res. 2013;319(19):3000–3009. doi: 10.1016/j.yexcr.2013.07.014.
- Kapur NK, Wilson S, Yunis AA, et al. Reduced endoglin activity limits cardiac fibrosis and improves survival in heart failure. Circulation. 2012;125(22):2728–2738. doi: 10.1161/CIRCULATIONAHA.111.080002.
- Huang S, Chen M, Li L, et al. Circulating MicroRNAs and the occurrence of acute myocardial infarction in Chinese populations. Circ Cardiovasc Genet. 2014;7(2):189–198. doi: 10.1161/CIRCGENETICS.113.000294.
- Wu Z, Geng J, Bai Y, et al. miR-125b-5p alleviates the damage of myocardial infarction by inhibiting the NFAT2 to reduce F2RL2 expression. Regen Med. 2023;18(7):543–559. doi: 10.2217/rme-2022-0150.