Abstract
Objective
Despite advancements in surgical techniques, operations for infective endocarditis (IE) remain associated with relatively high mortality. The aim of this study was to develop a nomogram model to predict the early postoperative mortality in patients undergoing cardiac surgery for infective endocarditis based on the preoperative clinical features.
Methods
We retrospectively analyzed the clinical data of 357 patients with IE who underwent surgeries at our center between January 2007 and June 2023. Independent risk factors for early postoperative mortality were identified using univariate and multivariate logistic regression models. Based on these factors, a predictive model was developed and presented in a nomogram. The performance of the nomogram was evaluated through the receiver operating characteristic (ROC) curve, calibration plot, and decision curve analysis (DCA). Internal validation was performed utilizing the bootstrapping method.
Results
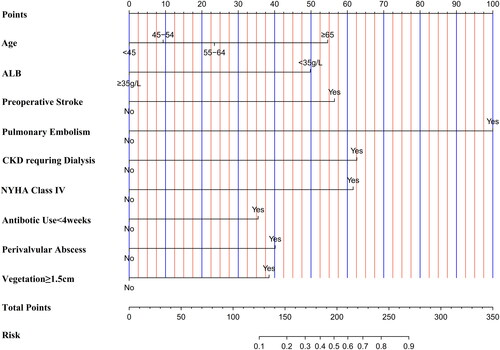
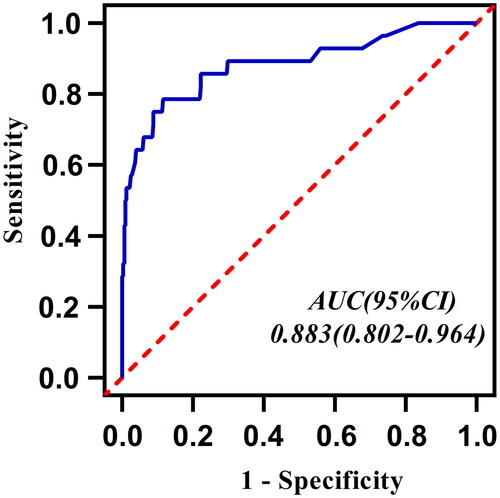
The nomogram included nine predictors: age, stroke, pulmonary embolism, albumin level, cardiac function class IV, antibotic use <4weeks, vegetation size ≥1.5 cm, perivalvular abscess and preoperative dialysis. The area under the ROC curve (AUC) of the model was 0.88 (95%CI:0.80–0.96). The calibration plot indicated strong prediction consistency of the nomogram with satisfactory Hosmer-Lemeshow test results (χ2 = 13.490, p = 0.142). Decision curve analysis indicated that the nomogram model provided greater clinical net benefits compared to “operate-all” or “operate-none” strategies.
Conclusions
The innovative nomogram model offers cardiovascular surgeons a tool to predict the risk of early postoperative mortality in patients undergoing IE operations. This model can serve as a valuable reference for preoperative decision-making and can enhance the clinical outcomes of IE patients.
Introduction
Infective endocarditis (IE) is marked by significant inflammation affecting the heart valves or other parts of the endocardium, mainly caused by bacteria but occasionally by other microorganisms [Citation1]. Often leading to fatal complications such as embolism, acute heart failure, and sepsis, IE is recognized as a highly life-threatening cardiovascular disorder [Citation2]. Despite its relatively low incidence of 1.8–9.7 cases per 100,000 people/year [Citation3], IE has a high in-hospital mortality rate of 15–20% and a 1-year mortality rate of nearly 40% [Citation4]. Since the first reported operation for IE in 1965 [Citation5], surgical treatment has evolved as the most effective treatment for IE patients, aimed at managing heart failure and preventing further embolization [Citation1, Citation6]. Approximately 50% of IE patients undergo surgical procedures during hospitalization [Citation6, Citation7]. Despite significant advancements in surgical techniques, procedures for IE still have the highest mortality rates among all valve surgeries [Citation8, Citation9]. Recent reports showed early postoperative mortality rates for IE patients ranged from 6% to 36% [Citation6, Citation10–12], considerably higher than most types of routine cardiac surgeries [Citation13]. Therefore, early identification of patients at high risk of postoperative mortality is crucial to enhance the prognosis of IE.
The nomogram serves as a prognostic prediction tool that calculates the probabilities of specific clinical outcomes by developing statistical prediction models and presents the results in simple graphs [Citation14]. Nomogram has been increasingly utilized in prognostic studies due to its accuracy and simplicity [Citation15]. Moreover, it is widely recognized that nomogram can offer personalized risk estimates and contribute to clinical decision-making [Citation16].
However, compared with other common cardiovascular disorders, nomogram-based studies predicting the prognosis of infective endocarditis (IE) are relatively scarce [Citation17]. Besides, these existing studies commonly included both surgical and non-surgical IE patients [Citation1, Citation17, Citation18]. Thus, it is essential to develop a novel prediction model especially for surgical patients. This study aims to construct a novel nomogram model based on specific preoperative features to predict postoperative mortality for IE patients.
Method
Study population
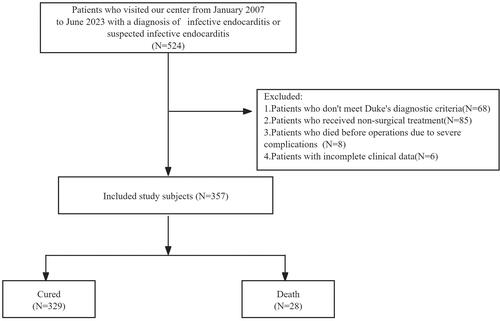
We retrospectively collected the clinical data of the patients with infective endocarditis who were treated at our center from January 2007 to June 2023 by using the electronic medical record system of our hospital. The inclusive criteria were as follows: (1) met the modified Duke diagnostic criteria for infective endocarditis [Citation19] (2) met the surgical indications of the guidelines [Citation7, Citation20] (3) received surgical operation for IE in our center. The flow chart describing the inclusion process, with the reasons for exclusion, is presented in . Based on the early postoperative outcomes, the 357 enrolled patients were divided into surgical-cured group (N = 329) and a surgical-death group (N = 28) ().
Data collection and definition of study endpoint
We collected 64 preoperative variables associated with IE covering the aspects of baseline information, commobidities, embolic events, predisposing factors, echocardiographic data, laboratory tests data and blood culture results ( and ). Blood cultures were taken from all patients at the time of admission. All the operations were performed by 8 experienced surgical groups of our center. The primary study endpoint was early postoperative death, which was defined as the mortality occurring within 30 days postoperatively or without discharge [Citation6].
Table 1. Comparison of baseline clinical characteristics.
Table 2. Comparison of preoperative echocardiographic and laboratory data.
Development of the nomogram prediction model
The 357 patients were divided into two groups (death versus survival) based on the early postoperative outcomes. We firstly performed inter-group comparisons for each preoperative variable. The variables with the p-value < 0.10 in inter-group comparison were included in the univariate logistic regression analysis. And then, only the variables significant at the 0.05 level in the univariate analysis can be included in the subsequent multivariate logistic regression analysis. Based on the results of multivariate regression, we obtained the independent risk factors of early postoperative death. The nomogram prediction model was then developed using these screened independent risk factors.
Display and validation of the nomogram prediction model
The developed prediction model was displayed in a nomogram. We adopted AUC(area under the ROC curve) to evaluate the discrimination of the model by drawing the Receiver Operator Characteristic curve(ROC). Internal validation of the model was realized by the Boot-strapped method with 1000 repetitions. We used the calibration curve to evaluate the consistency between the observed probabilities and the model-predicted probabilities. And the Hosmer–Lemeshow test was employed to examine the fitness of the model. Finally, the clinical utility of the nomogram model was assessed by decision curve analysis(DCA).
Statistical analysis
Normally-distributed continuous variables were presented as mean ± standard deviation, while non-normally distributed continuous variables were expressed by median (interquartile range). Categorical variables were shown as counts (percentages). Inter-group comparisons were conducted by using the Student’s t-test for normally distributed continuous variables and the Mann–Whitney U test for non-normally distributed continuous variables. And categorical variables were compared by the chi-square test or Fisher’s exact test. We adopted a backward stepwise method to perform multivariate logistic regression analysis. A two-tailed α level of 0.05 was chosen and all statistical analysis were performed by using R(4.2.2)software or SPSS(25.0)statistical software.
Results
A total of 28 early postoperative deaths were observed with a mortality rate of 8%(28/357). The remaining 329 cases were surgically cured and discharged. Inter-group comparison revealed 18 preoperative variables that displayed significance at the 0.10 level, including gender, age, NYHA class IV, emergent operation, durtation of antibiotic use, Chronic kidney disease(CKD) requiring dialysis, stroke, pulmonary embolism, congenital heart disease, perivalvular abscess, mitral valve(MV) involved, aortic valve(AV) involved, multiple valves involved, vegetation size ≥ 1.5 cm, WBC, Hb, serum creatinine and ALB ( and ). However, there was no significant difference in the type of causative microorganisms between groups (p > 0.10).
The result of univariate logistic regression analysis showed that 13 variables of them were significant at the 0.05 level, including age, Hb, serum creatinine, ALB, NYHA class IV, emergent operation, duration of antibotic use, stroke, pulmonary embolism, CKD requiring dialysis, perivalvular abscess, vegetation size ≥ 1.5 cm, and multiple valves involved (). The multivariate logistic regression screened 9 variables as the independent risk factors of early postoperative death, including age, stroke, pulmonary embolism, ALB, NYHA class IV, durtation of antibotic use <4weeks, vegetation size ≥1.5 cm, perivalvular abscess, CKD requiring dialysis (). Although the P-value of perivalvular abscess and CKD requiring dialysis were slightly higher than 0.05, R softare still included these two variables into the final model based on the minimum AIC(Akaike information criterion)[Citation21] principles. Based on the above 9 variables, a novel nomogram prediction model for early postoperative death of IE operation was constructed ().
Table 3. Univariate and multivariate logistic regression analysis of in-hospital mortality.
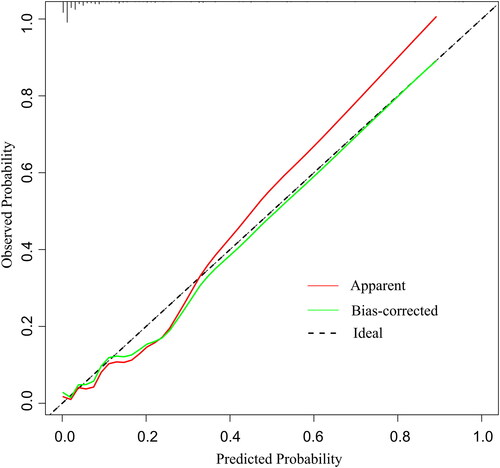
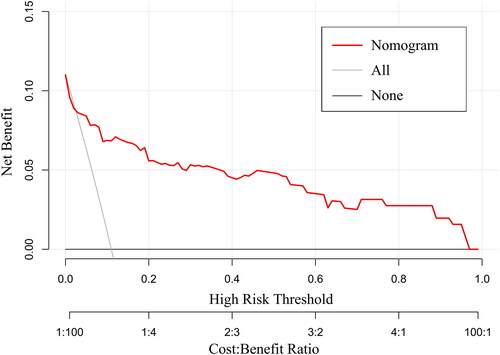
This study validated the performance of the nomogram prediction model from the following aspects. The AUC of nomogram model was calculated as 0.88 with a 95% confidence interval (CI) of 0.80–0.96 (). The optimal cut-off value of the ROC curve was 0.117. At this threshold, the specificity and sensitivity of the nomogram model were 88.4% and 78.6%, respectively. The ROC curve of the nomogram prediction model was then verified internally by Boot-strapping method (1000 repetitions) and the results showed that the boot-strapped AUC was 0.89(0.81–0.96). The calibration curve of the model was also verified internally by bootstrap-1000 times, and both the original curve and the bias-corrected curve fit well with the standard ideal curve (). The results of Hosmer–Lemeshow test (χ2 = 13.490, p = 0.142) indicated a good fitness of the model. Taking together, the above results indicated the good calibration of the nomogram model. To further evaluate the clinical utility of the model, we found that at an approximate threshold probability of 3%-97%, utilizing nomogram to classify patients at high risk of IE operations can offer more net benefit than either the “operate-all-patients” scheme or the “operate-none-patients” scheme. If we use the optimal cut-off value of 0.117 of the ROC curve as the threshold probability, the benefit-cost ratio is at least more than 4:1 ().
Discussion
The purpose of this study was to develop a novel nomogram model to predict early postoperative mortality especially for surgical IE patients based on preoperative variables. And the major finding was the identification of nine preoperative variables (age, stroke, pulmonary embolism, albumin level, cardiac function class IV, antibotic use < 4 weeks, vegetation size ≥1.5 cm, perivalvular abscess and preoperative dialysis) as the independent risk factors for early postoperative mortality. And these factors were subsequently integrated into the final prediction model. Furthermore, this prediction model was proven to have satisfied predictive performance by comprehensive evaluation of discrimination, calibration and clinical utility.
Consistent with previous findings [Citation2], cerebral stroke and splenic infarction were the two most prevalent embolic events in this cohort, with incidences of 17.1% and 4.2% among the patients, respectively. Embolic events of IE primarily result from dislodged debris from intracardiac vegetations [Citation22]. It is recognized that systemic embolisms are closely linked to left-sided IE, whereas pulmonary embolisms are more commonly associated with right-sided IE [Citation2, Citation23]. In our study, the incidence of pulmonary embolism was 1.12%, lower than that of cerebral stroke, splenic infarction, and renal infarction. Despite the low incidence, pulmonary embolism remains a significant independent predictor of postoperative mortality (OR = 24.71). This finding strongly suggested that the presence of pulmonary embolism highly indicated poorer postoperative prognosis of IE.Therefore, patients exhibiting this risk factor warrant special attention, and efforts should be made to improve their prognosis through meticulously designed surgical strategies and rigorous postoperative management. A multi-center study involving 1345 consecutive IE patients revealed that stroke was independently associated with early mortality (HR = 1.63), and vegetation size emerged as an independent risk factor for neurological complications in IE [Citation24]. Moreover, Fosbol et al. summarized the findings of a multi-national prospective registry containing 1006 IE patients, indicating that vegetation size was correlated with higher 6-month mortality following non-surgical treatment [Citation25]. In our study, cerebral stroke (OR = 5.63) and vegetation size (OR = 3.17) were identified as independent risk factors for postoperative mortality and collectively included in the final prediction model. These findings indicated that vegetation size can serve not only as a parameter for assessing embolic risk [Citation2, Citation26], but also as a predictor for early postoperative mortality.
In a large European multi-center retrospective study by Di Mauro and colleagues [Citation12], the perivalvular abscess was identified as an independent risk factor for early mortality following infective endocarditis (IE) operations. A perivalvular abscess signifies a more aggressive infection status, potentially complicating surgical repair and increasing the chances of operative failures. Similarly, Sameh M. Said et al. retrospectively analyzed the data of 801 IE patients and concluded that both root abscess and preoperative dialysis were predictors for early death after IE operations [Citation11]. Our study employed a backward stepwise method for multi-regression analysis. The results indicated that the P-values for perivalvular abscess and chronic kidney disease requiring dialysis were slightly above 0.05. However, based on the principle of minimum AIC (Akaike information criterion) [Citation21] and clinical significance, we retained these two variables in the final nomogram prediction model.
Age is the most common clinical characteristic that impacts the survival outcomes of IE patients [Citation1]. Studies have shown that advanced age correlates with a poorer prognosis than younger individuals [Citation12, Citation17]. Our findings also demonstrated that the advanced age was an independent risk factor contributing to postoperative mortality (OR = 1.7). Albumin, an acute-phase protein, is crucial in anti-inflammatory processes [Citation27]. Heightened inflammation can hinder albumin synthesis and increase catabolism [Citation28]. Low albumin levels were associated with mortality in various critical surgical conditions, particularly in IE patients [Citation28]. Furthermore, our study identified decreased albumin levels as a predictor of early postoperative mortality (OR = 4.03).
Only a limited number of studies have employed nomogram models to predict the in-hospital mortality of IE patients [Citation1, Citation17, Citation18]. However, these previous studies included both surgical and non-surgical patients. By contrast, our study included a relatively large cohort of 357 IE patients undergoing cardiac surgeries, and developed a nomogram model for the first time to predict postoperative mortality especially for surgical patients. Early identification of high-risk patients by comprehensive evaluation of preoperative features is crucial for devising effective strategies in subsequent clinical treatment [Citation29]. To achieve this goal, cardiac surgeons urgently require a reliable prediction model to quantify postoperative death risk for surgical IE patients. It is generally accepted that discrimination is the first concern of a nomogram prediction model. In our study, AUC was adopted to identify the nomogram model’s ability to discriminate between surgical survivals and surgical deaths. An AUC greater than 0.75 is generally considered to indicate good discrimination. Our model exhibited an AUC of 0.88 (95%CI:0.80–0.96), indicating excellent discriminatory abilities. Internal validation was performed by the bootstrapping method with 1000 repetitions, yielding a bootstrapping-corrected AUC of 0.89 (95%CI:0.81–0.96), showing the model’s strong discriminatory aptitude in the validation queue as well. Moreover, calibration curve was employed to assess the consistency between the nomogram-predicted probabilities and the actual outcomes. The calibration curve indicated that the nomogram-predicted postoperative mortality showed good consistency with the actual postoperative mortality. The 1000-bootstrapping lines also performed well in the calibration curve plot, and again confirmed that the model was stable with good prediction consistency. In addition, the results of the Hosmer-Lemeshow test(χ2 = 13.490, p = 0.142 > 0.05) also demonstrated the good fitness of the model. Decision curve analysis(DCA) can assess the clinical applicability of a prediction model based on the threshold probability from which the benefit can be derived. Our findings revealed that utilizing this nomogram model for postoperative mortality prediction would yield greater net benefits than either the “operate-all-patients” scheme or the “operate-none-patients” scheme if the threshold probability was 3%–97%. This broad effective range suggests the model’s suitability for extensive application in clinical practice.
Limitations
There were still some limitations to this study. Firstly, this study was a retrospective study. Risk of selective bias was inevitable due to the retrospective nature. Thus, prospective and randomized clinical studies were still needed. Secondly, this was a single-center study. Considering the sample size, we haven’t externally validated the nomogram. Therefore, multi-center studies with larger sample size were still required. Thirdly, the nomogram model built on the clinical data of our center may have lower predictive ability in other areas due to factors such as race and different etiology constituting distribution in different regions. As a result, continuous optimization of the model should be considered during the process of clinical popularization.
Conclusion
Our study identified several independent predictors of postoperative death for IE operations, such as age, stroke, pulmonary embolism, albumin, NYHA class IV, antibiotic duration, vegetation size, perivalvular abscess, and preoperative dialysis. Based on these predictors, a nomogram was developed and validated to show good predictive performance with favorable discrimination abililty, calibration values and clinical applicability. This nomogram model can offer individualized prognosis prediction, aiding cardiovascular surgeons in the preoperative identification of high-risk patients and implementing targeted interventions to mitigate the potential mortality associated with IE operations.
Ethical approval and consent to participate
This study was approved by the Institutional Ethical Review Board of the First Affiliated Hospital of Anhui Medical University(No.PJ2023-10-48). Written informed consent about the surgical procedure was obtained from all patients enrolled in this study.
Authors’contributions
Concept-Yanyi Liu, Shenglin Ge; Design-Shenglin Ge, Yanyi Liu;Writing-Yanyi Liu;Supervision-Shenglin Ge;Data Collection-Yanyi Liu, Xin Li, Zhuang Liu, Chenghao Lu;Analysis-Yanyi Liu;Literature Review-Yanyi Liu;Critical review-Shenglin Ge.
Acknowledgements
None.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The clinical data used to support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Yu ZJ, Dou Z, Li J, et al. Nomogram for predicting in-hospital mortality in infective endocarditis based on early clinical features and treatment options. Front Cardiovasc Med. 2022;9:882869. doi: 10.3389/fcvm.2022.
- Mohananey D, Mohadjer A, Pettersson G, et al. Association of vegetation size with embolic risk in patients with infective endocarditis: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(4):502–510. doi: 10.1001/jamainternmed.2017.8653.
- Bin AA, Baddour LM, Erwin PJ, et al. Global and regional burden of infective endocarditis, 1990–2010: a systematic review of the literature. Glob Heart. 2014;9(1):131–143. doi: 10.1016/j.gheart.2014.01.002.
- Pettersson GB, Coselli JS, Pettersson GB, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg. 2017;153(6):1241–1258.e29. doi: 10.1016/j.jtcvs.2016.09.093.
- Wallace AG, Young WJ, Osterhout S. Treatment of acute bacterial endocarditis by valve excision and replacement. Circulation. 1965;31(3):450–453. doi: 10.1161/01.cir.31.3.450.
- Üstünışık ÇT, Duman ZM, Timur B, et al. Early mortality predictors in infective endocarditis patients: a single-center surgical experience. Braz J Cardiovasc Surg. 2022;37(6):829–835. doi: 10.21470/1678-9741-20.
- Delgado V, Ajmone MN, de Waha S, et al. 2023 ESC guidelines for the management of endocarditis. Eur Heart J. 2023;44(39):3948–4042. doi: 10.1093/eurheartj/ehad193.
- Huang JB, Lu CC, Wen ZK, et al. Surgical treatment of left-sided infective endocarditis with symptomatic neurological complications before surgery in China. Front Cardiovasc Med. 2023;10:1217148. doi: 10.3389/fcvm.2023.1217148.
- Sousa C, Pinto FJ. Infective endocarditis: still more challenges than convictions. Arq Bras Cardiol. 2022;118(5):976–988. doi: 10.36660/abc.20200798.
- Habib G, Erba PA, Iung B, et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J. 2019;40(39):3222–3232. doi: 10.1093/eurheartj/ehz620.
- Said SM, Abdelsattar ZM, Schaff HV, et al. Outcomes of surgery for infective endocarditis: a single-centre experience of 801 patients. Eur J Cardiothorac Surg. 2018;53(2):435–439. doi: 10.1093/ejcts/ezx341.
- Di Mauro M, Dato G, Barili F, et al. A predictive model for early mortality after surgical treatment of heart valve or prosthesis infective endocarditis. The EndoSCORE. Int J Cardiol. 2017;241:97–102. doi: 10.1016/j.ijcard.2017.03.148.
- San Román JA, Vilacosta I, López J, et al. Critical questions about left-sided infective endocarditis. J Am Coll Cardiol. 2015;66(9):1068–1076. doi: 10.1016/j.jacc.2015.07.016.
- Jalali A, Alvarez-Iglesias A, Roshan D, et al. Visualising statistical models using dynamic nomograms. PLOS One. 2019;14(11):e225253. doi: 10.1371/journal.pone.0225253.
- Deng X, Hou H, Wang X, et al. Development and validation of a nomogram to better predict hypertension based on a 10-year retrospective cohort study in China. Elife. 2021;10:e66419. doi: 10.7554/eLife.66419.
- Wang H, Ou Y, Fan T, et al. Development and internal validation of a nomogram to predict mortality during the ICU stay of thoracic fracture patients without neurological compromise: an analysis of the MIMIC-III clinical database. Front Public Health. 2021;9:818439. doi: 10.3389/fpubh.2021.818439.
- Yu ZJ, Ni ZJ, Li J, et al. Construction and internal validation of a novel nomogram for predicting prognosis of infective endocarditis. Sci Rep. 2022;12(1):17249. doi: 10.1038/s41598-022-22173-5.
- Li Z, Gao Q, Ren Z, et al. Nomogram based on neutrophil-to-platelet ratio to predict in-hospital mortality in infective endocarditis. Biomark Med. 2021;15(14):1233–1243. doi: 10.2217/bmm-2021-0085.
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–638. doi: 10.1086/313753.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):2440–2492. doi: 10.1161/CIR.0000000000000029.
- Bozdogan H. Akaike’s information criterion and recent developments in information complexity. J Math Psychol. 2000;44(1):62–91. doi: 10.1006/jmps.1999.1277.
- Jacobwitz M, Favilla E, Patel A, et al. Neurologic complications of infective endocarditis in children. Cardiol Young. 2023;33(3):463–472. doi: 10.1017/S1047951122001159.
- Chahoud J, Sharif YA, Saad H, et al. Right-sided infective endocarditis and pulmonary infiltrates: an update. Cardiol Rev. 2016;24(5):230–237. doi: 10.1097/CRD.0000000000000095.
- García-Cabrera E, Fernández-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation. 2013;127(23):2272–2284. doi: 10.1161/CIRCULATIONAHA.112.000813.
- Fosbøl EL, Park LP, Chu VH, et al. The association between vegetation size and surgical treatment on 6-month mortality in left-sided infective endocarditis. Eur Heart J. 2019;40(27):2243–2251. doi: 10.1093/eurheartj/ehz204.
- Song SJ, Kim JH, Ku NS, et al. Vegetation size, multiplicity, and position in patients with infective endocarditis. Ann Thorac Surg. 2022;114(6):2253–2260. doi: 10.1016/j.athoracsur.2021.10.071.
- Kelesoglu S, Yilmaz Y, Elcık D. Relationship between c-reactive protein to albumin ratio and coronary collateral circulation in patients with stable coronary artery disease. Angiology. 2021;72(9):829–835. doi: 10.1177/00033197211004392.
- Karaca B, Esin F, Tiryaki MM, et al. Combining C-reactive protein, procalcitonin, and serum albumin to predict long-term mortality in patients with infective endocarditis. J Int Med Res. 2023;51(10):3000605231208910. doi: 10.1177/03000605231208910.
- Fernandez-Felix BM, Barca LV, Garcia-Esquinas E, et al. Prognostic models for mortality after cardiac surgery in patients with infective endocarditis: a systematic review and aggregation of prediction models. Clin Microbiol Infect. 2021;27(10):1422–1430. doi: 10.1016/j.cmi.2021.05.051.