?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Acute Type A Aortic Dissection (AAAD) is one of the most life-threatening diseases, often associated with transient hyperglycemia induced by acute physiological stress. The impact of stress-induced hyperglycemia on the prognosis of ST-segment elevation myocardial infarction has been reported. However, the relationship between stress-induced hyperglycemia and the prognosis of AAAD patients remains uncertain.
Methods
The clinical data of 456 patients with acute type A aortic dissection were retrospectively reviewed. Patients were divided into two groups based on their admission blood glucose. Cox model regression analysis was performed to assess the relationship between stress-induced hyperglycemia and the 30-day and 1-year mortality rates of these patients.
Results
Among the 456 patients, 149 cases (32.7%) had AAAD combined with stress-induced hyperglycemia (SIH). The results of the multifactor regression analysis of the Cox model indicated that hyperglycemia (RR = 1.505, 95% CI: 1.046–2.165, p = 0.028), aortic coarctation involving renal arteries (RR = 3.330, 95% CI: 2.237–4.957, p < 0.001), aortic coarctation involving superior mesenteric arteries (RR = 1.611, 95% CI: 1.056–2.455, p = 0.027), and aortic coarctation involving iliac arteries (RR = 2.034, 95% CI: 1.364–3.035, p = 0.001) were independent influences on 1-year postoperative mortality in AAAD patients.
Conclusion
The current findings indicate that stress-induced hyperglycemia measured on admission is strongly associated with 1-year mortality in patients with AAAD. Furthermore, stress-induced hyperglycemia may be related to the severity of the condition in patients with AAAD.
1. Introduction
Acute Type A Aortic Dissection (AAAD) is a severe and potentially fatal disease with a high mortality rate of up to 50% among patients [Citation1]. The most current treatment for this disease is surgical repair [Citation2]. Hospital studies indicate an annual incidence of aortic dissection at 3–5 cases per 100,000 individuals [Citation3,Citation4]. Despite prompt surgical intervention, AAAD patients face a substantial risk of postoperative complications and mortality [Citation5,Citation6]. The International Registry of Acute Aortic Dissection (IRAD) reports an in-hospital mortality rate of 22% for AAAD patients, with an operative mortality rate of 18% [Citation7]. Although surgical and anesthetic techniques have made remarkable improvements, the prognosis for patients dealing with AAAD remains poor [Citation8]. According to the 2018 International Registry of Acute Aortic Dissection, the in-hospital mortality rate for patients undergoing surgical repair of AAAD remains as high as 22% [Citation9]. Early identification of risk factors linked to poor prognosis is crucial in clinical practice. While some studies have systematically reviewed factors like advanced age, cardiac tamponade, hypotension, and myocardial ischemia in relation to AAAD, these factors still fall short of meeting the evolving demands of modern clinical practice [Citation10].
Recent studies have confirmed a close association between SIH and the progression and prognosis of cardiovascular diseases. A recognized diagnostic criterion for SIH is defining admission blood glucose (ABG) levels as >7.8 mmol/L [Citation11]. Therefore, ABG reflecting blood glucose levels during hospitalization has been a focal point of in-depth research on this topic. Early studies have shown that patients who undergo cardiac surgery with hyperglycemia during and after the procedure have a less favorable prognosis [Citation12]. This correlation can be elucidated by multiple pathophysiological mechanisms, which include oxidative stress [Citation13], insulin resistance, and increased free fatty acid levels [Citation14]. Free fatty acids in excessive quantities could impart toxic effects on infarcted and ischemic myocardium. Upon admission, endothelial dysfunction in hyperglycemic patients may serve as another mechanism [Citation9]. The pathophysiological changes of AAAD create a strong stress response due to its critical nature, which may destabilize the body’s intracellular environment and lead to disruptions in the patient’s glucose metabolism. Nevertheless, there is no report on how hyperglycemia upon admission influences the prognosis of AAAD patients.
This study aimed to assess the predictability of admission hyperglycemia on the unfavorable prognosis of patients who undergo surgery for AAAD.
2. Materials and methods
2.1. Study participants
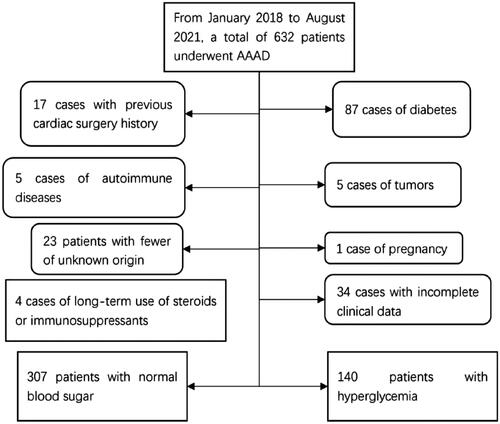
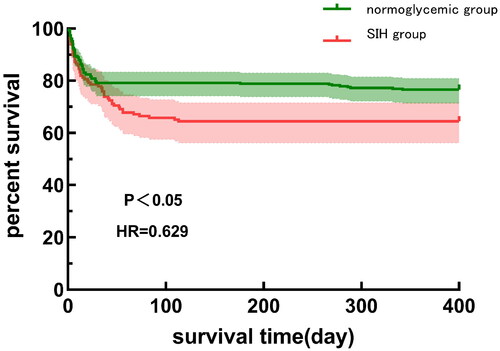
This retrospective observational study collected consecutive data from 632 patients who underwent AAAD surgery between January 2018 and August 2021 at the Asian Heart Hospital in Wuhan, Hubei Province. Excluded patient types included patients with diabetes (N = 69), Hba1c ≥ 6.5% [Citation15] despite the absence of a medical history of diabetes (N = 18), patients with a history of previous cardiac surgery (N = 17), patients with autoimmune disorders (N = 5), patients with neoplasms (N = 5), patients with unexplained fever (N = 23), patients with pregnancy (N = 1), and patients with long-term use of steroids or immunosuppressants (N = 4). In addition, (N = 34) patients were excluded due to incomplete medical records. Finally, 456 patients were included in the study. The detailed procedure is shown in . Patients were divided into two groups based on admission blood glucose: the normoglycemic group (blood glucose ≤ 7.8 mmol/L), and the SIH group (blood glucose > 7.8 mmol/L). The survival curves of the two groups of patients are shown in . The Ethics Committee of the Wuhan Asian Heart Hospital approved the study (Approval No. 2023-B022) which adhered to the ethical principles of the Declaration of Helsinki. Informed consent was not required as this was a retrospective study.
2.2. Definition
SIH diagnostic criteria: According to the 2008 American Heart Association (AHA) Scientific Statement on Hyperglycemia and Acute Coronary Syndromes, a recognized diagnostic criterion for SIH is an admission blood glucose (ABG) >7.8 mmol/L [Citation11].
AKI diagnostic criteria: According to the KDIGO guidelines [Citation16], postoperative AKI is defined as a rise in serum creatinine to more than 1.5 times the baseline value; or an increase from the baseline value to 26.5 μmol/L within 48 h; or urine ≤0.5 mL/kg/h within 6 h. In the present study, we used the patient’s serum creatinine level on the 1st day of hospitalization as the baseline value compared with that of a review of the serum creatinine in the first 48 h of the postoperative period, to determine whether the patient had developed AKI. Whether the patients developed AKI after surgery.
2.3. Data collection
this included recording baseline and preoperative characteristics, intraoperative details, and postoperative data. In addition, we recorded the length of stay in the intensive care unit and during hospitalization, as well as the occurrence of postoperative complications.
2.4. Follow-up
The primary endpoints evaluated in this study were patient all-cause mortality rates at 30 days and 1 year. A follow-up was conducted at 30 days and 1 year through telephone interviews, outpatient consultations, or medical record reviews to assess these endpoints. Specifically trained physicians assessed all events related to endpoints.
2.5. Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median (25–75%) and normal distribution was assessed using the Kolmogorov–Smirnov test. Depending on the distribution of continuous variables, differences between groups were analyzed using the Student’s independent t-test or the Kruskal–Wallis test. Categorical variables were expressed as numbers (percentages) and compared using the Chi-square test or Fischer’s exact test. Logistic regression analysis was performed to assess the association of stress hyperglycemia with baseline variables. To analyze independent risk factors leading to postoperative mortality, factors that may have an impact on mortality outcomes were grouped into separate groups of patients as independent variables using 30 days and 1 year postoperatively as time cut-offs, and the cumulative survival of the components was estimated using the Kaplan–Meier method and compared using the log-rank test. A multifactorial analysis of the possible risk factors screened by log-rank test (p < 0.05) was performed using the Cox risk-proportional model, and the corresponding RR values and their 95% confidence intervals were calculated. Data were processed using the Statistical Package for the Social Sciences (SPSS) version 25.0 from IBM-SPSS, New York, NY, USA.
3. Results
3.1. Baseline characteristics
Of the 456 patients with AAAD included in our study, 343 (75.2%) were males with a mean age of 58.0 years. The 30-day mortality and 1-year mortality rates were 21.1% (96/456) and 27.4% (125/456), respectively. presents the baseline data of the two groups of patients. In this study, a total of 307 patients (67.3%) were normoglycemic and 149 patients (32.7%) were hyperglycemic. Patients in the hyperglycemic group were on average older, had a higher BMI, and had a higher percentage of pericardial effusion and coronary artery involvement. Hyperglycemic patients had higher CPB time, ACC time, and ICU stay time than patients in the normoglycemic group. In terms of perioperative complications (), the incidence of AKI was significantly higher in patients in the hyperglycemic group (27.5%) than in the normoglycemic group (16.3%).
Table 1. Baseline data grouped according to admission blood glucose levels.
Table 2. Postoperative data stratified by glucose category.
3.2. Log-rank analysis of postoperative mortality in AAAD patients
Grouped by different characteristics of AAAD patients, intergroup differences in 30-day and 1-year postoperative mortality were analyzed. The results showed that grouped by hyperglycemia, there was no difference in 30-day mortality between the two groups (p = 0.35); The one-year mortality rate of patients in the SIH group was increased compared with the normal group (p < 0.05) when categorized by the seven factors: hyperglycemia, pericardial effusion, aortic coarctation involving coronary artery, renal arteries, superior mesenteric artery, iliac arteries, and perioperative myocardial ischemia.
3.3. Cox multifactorial regression analysis of postoperative mortality in AAAD patients
Seven factors (hyperglycemia, pericardial effusion, aortic coarctation involving coronary, renal, superior mesenteric, and iliac arteries, and perioperative myocardial ischemia) screened by log-rank univariate analysis were included in a multivariate regression analysis of the Cox proportional risk model, and it was found that the results revealed that hyperglycemia (RR = 1.505, 95% CI: 1.046–2.165, p = 0.028), aortic coarctation involving renal arteries (RR = 3.330, 95% CI: 2.237–4.957, p < 0.001), aortic coarctation involving superior mesenteric arteries (RR = 1.611, 95% CI: 1.056–2.455, p = 0.027), and aortic coarctation involving iliac arteries (RR = 2.034, 95% CI: 1.364–3.035, p = 0.001) were independent influences on 1-year postoperative mortality in AAAD patients.
4. Discussion
This study is the first to evaluate the relationship between SIH on admission and postoperative mortality in patients with AAAD. The results showed that patients with SIH and AAAD on admission had an increased risk of death 1 year after surgery. After adjusting for other factors, combined SIH was still independently associated with 1-year mortality. We compared the differences in postoperative outcomes between the two groups and found that the 1-year mortality rate of AAAD patients in the SIH group was significantly higher than that of the normoglycemic group. The mortality rate of AAAD patients in this study was 21.1%, which is consistent with the results of other studies [Citation7,Citation17].
Many previous studies have pointed out that SIH is strongly associated with increased perioperative mortality from critical cardiovascular disease [Citation18,Citation19]. One study also used admission blood glucose of 7.8 mmol/L as the diagnostic criterion for SIH to group 100 STEMI patients. The results showed that the in-hospital mortality rate in the hyperglycemia group was significantly increased [Citation20]. Similarly, Khalfalah et al. conducted a study including 660 patients with ST-segment elevation myocardial infarction who underwent surgery to elucidate the adverse consequences of SIH. Results showed that patients with SIH developed contrast-induced nephropathy, cardiogenic shock, and higher in-hospital mortality [Citation21].
Consistent with the findings of Lin et al. [Citation22], our study aligns with demonstrating that SIH does not impact in-hospital mortality among patients with AAAD. Initially, two pivotal pathologies of aortic dissection involve increased aortic wall stress and damage to the inner layer [Citation23]. Interestingly, hyperglycemia has been proven to confer a protective effect on aortic wall stress by enhancing the collagen network’s stability, primarily through the augmentation of aortic wall thickness [Citation24,Citation25]. Simultaneously, inflammation and the degradation of the extracellular matrix serve as principal mechanisms in the pathogenesis of aortic dissection. Aortic neovascularization and macrophage infiltration, crucial stimulating factors in aortic disease progression, exhibit significant suppression in a hyperglycemic environment [Citation26]. Notably, hyperglycemia, as observed in a mouse wound healing model, impedes new blood vessel formation by disrupting the activation of hypoxia-inducible factor 1 (HIF-1) and HIF-related nuclear hypoxia response element (HRE) [Citation27–29]. This interference results in diminished vascular endothelial growth factor expression and angiogenic responses, thereby decelerating the acute phase progression of aortic dissection. From this standpoint, it can be inferred that stress-induced hyperglycemia may play a protective role in the early stages of AAAD patients.
Notably, another significant discovery in this study is that SIH serves as an independent predictor of 1-year mortality risk. Yan et al.'s study emphasized SIH as an indicator of the long-term risk of adverse events following cardiac surgery [Citation30–32]. Within our study, the SIH group exhibited a higher prevalence of advanced age, high Body Mass Index (BMI), and pericardial effusion—recognized high-risk factors associated with poor prognosis [Citation10,Citation21]. Additionally, the SIH group manifested a heightened prevalence of coronary artery, renal artery, and lower limb artery involvement, coupled with cardiac dysfunction. These factors contribute to inadequate perfusion of the heart, digestive system, and kidneys post-surgery, establishing them as crucial prognostic indicators [Citation33]. Notably, patients in the SIH group experienced prolonged cardiopulmonary bypass time, aortic cross-clamp time, and Intensive Care Unit (ICU) stay compared to those in the normoglycemic group. Duncan et al.'s research [Citation34] results underscored that prolonged cardiopulmonary bypass and aortic cross-clamp times significantly elevate the postoperative adverse events rate in patients.
Some studies have highlighted that Acute Kidney Injury (AKI) following AAAD surgery is an independent predictor of patients’ long-term survival post-surgery [Citation35]. Logistic regression results in our study demonstrated that combined SSIH was linked to a heightened risk of AKI. A prior investigation discovered that in diabetic patients with Acute Myocardial Infarction (AMI), the presence of stress hyperglycemia predicted AKI in acutely hospitalized patients [Citation36]. Furthermore, another study delved into the impact of admission blood glucose(ABG) values on AKI in hospitalized patients, revealing a notable association between high ABG and AKI as well as mortality in non-diabetic patients [Citation37]. Acute spikes in blood glucose can induce osmotic diuresis, resulting in volume depletion and dehydration. Additionally, the neuroendocrine dysregulation and inflammatory activation accompanying stress hyperglycemia can expedite renal damage [Citation38,Citation39].
Therefore, we have reason to suspect that SIH may not only lead to an increased risk of death in ATAAD patients but also serve as a biomarker reflecting disease severity and the body’s stress response.
Blood glucose was derived from serum samples that are routinely tested in clinical settings and are readily available. SIH, as a new biomarker, may help to early predict the prognosis of AAAD patients. At the same time, it may also play a certain role in predicting doctors’ postoperative decisions about patients. As an important supplement to prognostic indicators, it has important clinical significance.
It is widely acknowledged that optimal glycemic control is crucial for enhancing patient clinical outcomes [Citation34]. Despite several studies proposing methods for appropriate SIH management, debates persist regarding glycemic targets. There is still no consensus among healthcare providers on how to effectively handle SIH [Citation40]. ESC guidelines for acute coronary syndrome (ACS) and ST-segment elevation myocardial infarction (STEMI) suggest maintaining blood glucose levels <11.1 mmol/L as reasonable [Citation41,Citation42]. According to previous research, non-diabetic AAAD patients with SIH should actively control blood sugar and monitor its fluctuations. Furthermore, based on the outcomes of this study, ongoing exploration of high-risk factors leading to SIH occurrence is warranted.
4.1. Limitations
This study presents several constraints. Firstly, being a single-center retrospective study, it necessitates prospective multi-center studies with larger sample sizes to comprehensively assess the clinical applicability and constraints of our findings. Secondly, the research indicates a higher proportion of high-risk factors upon admission in the SIH group compared to the normoglycemic group, a crucial element influencing prognosis. While admission blood glucose(ABG) is closer to real clinical scenarios due to its simplicity and practical feasibility for AAAD patients, it is imperative to recognize that blood sugar issues encompass not only sustained high blood sugar but also fluctuations, including variations in elevation and decline [Citation43,Citation44]. This underscores the limitations of blood glucose indicators at a single time point, advocating the need for incorporating other markers or implementing dynamic blood glucose monitoring. Future research should delve into refining the optimal measurement method and control range for SIH in AAAD patients and execute extensive randomized clinical trials to enhance the clinical benefits for such patients.
4.2. Conclusion
In this study, about 40% of AAAD patients were complicated by SIH. These patients were more seriously ill when admitted and had a higher incidence of postoperative adverse events. SIH was identified as an independent predictor of 1-year mortality in AAAD patients and supports its use as a biomarker to assess the severity of admission to AAAD patients.
Ethical approval
The study was approved by the Ethics Committee of Wuhan Asian Heart Hospital (No. 2023-B022), all methods adhered to the Declaration of Helsinki, and the requirement for informed consent was waived by the Ethics Committee of Wuhan Asian Heart Hospital because the study was only a retrospective analysis of previous clinical data.
Consent for publication
Not applicable.
Authors contributions
All authors contributed significantly to this manuscript and endorsed the final version of it.
Acknowledgments
None.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data supporting the results of this study were available from the Wuhan Asian Heart Hospital. Still, the availability of these data was limited, and these data were used under license for this study and therefore not publicly available. However, information may be obtained from the corresponding author with permission from the Wuhan Asian Heart Hospital.
Additional information
Funding
References
- 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281.
- Roselli EE, Loor G, He J, et al. Distal aortic interventions after repair of ascending dissection: the argument for a more aggressive approach. J Thorac Cardiovasc Surg. 2015;149(2 Suppl):S117–S124.e3. doi: 10.1016/j.jtcvs.2014.11.029.
- Olsson C, Thelin S, Ståhle E, et al. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14 000 cases from 1987 to 2002. Circulation. 2006;114(24):2611–2618. doi: 10.1161/CIRCULATIONAHA.106.630400.
- Acosta S, Ogren M, Bengtsson H, et al. Increasing incidence of ruptured abdominal aortic aneurysm: a population-based study. J Vasc Surg. 2006;44(2):237–243. doi: 10.1016/j.jvs.2006.04.037.
- Bekkers JA, Bol Raap G, Takkenberg JJM, et al. Acute type A aortic dissection: long-term results and reoperations. Eur J Cardiothorac Surg. 2013;43(2):389–396. doi: 10.1093/ejcts/ezs342.
- Amano J, Kuwano H, Yokomise H. Thoracic and cardiovascular surgery in Japan during 2011: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2013;61(10):578–607. doi: 10.1007/s11748-013-0289-2.
- Pape LA, Awais M, Woznicki EM, et al. Presentation, diagnosis, and outcomes of acute aortic dissection. J Am Coll Cardiol. 2015;66(4):350–358. doi: 10.1016/j.jacc.2015.05.029.
- Elsayed RS, Cohen RG, Fleischman F, et al. Acute type A aortic dissection. Cardiol Clin. 2017;35(3):331–345. doi: 10.1016/j.ccl.2017.03.004.
- Kanaya AM, Dobrosielski DA, Ganz P, et al. Glycemic associations with endothelial function and biomarkers among 5 ethnic groups: the multi-ethnic study of atherosclerosis and the mediators of atherosclerosis in South Asians living in America studies. J Am Heart Assoc. 2013;2(1):e004283. doi: 10.1161/JAHA.112.004283.
- Evangelista A, Isselbacher EM, Bossone E, et al. Insights from the International Registry of Acute Aortic Dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137(17):1846–1860. doi: 10.1161/CIRCULATIONAHA.117.031264.
- Deedwania P, Kosiborod M, Barrett E, et al. Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2008;117(12):1610–1619. doi: 10.1161/CIRCULATIONAHA.107.188629.
- Nam K, Jeon Y, Kim WH, et al. Intraoperative glucose variability, but not average glucose concentration, may be a risk factor for acute kidney injury after cardiac surgery: a retrospective study. Can J Anaesth. 2019;66(8):921–933. doi: 10.1007/s12630-019-01349-0.
- Kitano D, Takayama T, Nagashima K, et al. A comparative study of time-specific oxidative stress after acute myocardial infarction in patients with and without diabetes mellitus. BMC Cardiovasc Disord. 2016;16(1):102. doi: 10.1186/s12872-016-0259-6.
- Lazzeri C, Valente S, D'Alfonso MG, et al. Determinants of C-peptide levels and acute insulin resistance/sensitivity in nondiabetic STEMI role of Killip class. IJC Metab Endocr. 2014;2:35–38. doi: 10.1016/j.ijcme.2014.02.003.
- Hoelzel W, Weykamp C, Jeppsson JO, et al. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem. 2004;50(1):166–174. doi: 10.1373/clinchem.2003.024802.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789.
- Swoboda PP, Erhayiem B, Kan R, et al. Cardiovascular magnetic resonance measures of aortic stiffness in asymptomatic patients with type 2 diabetes: association with glycaemic control and clinical outcomes. Cardiovasc Diabetol. 2018;17(1):35. doi: 10.1186/s12933-018-0681-4.
- De Bernardis Murat C, Leão RM. A voltage‐dependent depolarization induced by low external glucose in neurons of the nucleus of the tractus solitarius: interaction with KATP channels. J Physiol. 2019;597(9):2515–2532. doi: 10.1113/JP277729.
- Wu XY, Zhuang YK, Cai Y, et al. Serum glucose and potassium ratio as a predictive factor for prognosis of acute intracerebral hemorrhage. J Int Med Res. 2021;49(4):030006052110096. doi: 10.1177/03000605211009689.
- Rylski B, Hoffmann I, Beyersdorf F, et al. Acute aortic dissection type A: age-related management and outcomes reported in the German Registry for Acute Aortic Dissection Type A (GERAADA) of over 2000 patients. Ann Surg. 2014;259(3):598–604. doi: 10.1097/SLA.0b013e3182902cca.
- Ohnuma T, Shinjo D, Fushimi K. Hospital mortality of patients aged 80 and older after surgical repair for type A acute aortic dissection in Japan. Medicine. 2016;95(31):e4408. doi: 10.1097/MD.0000000000004408.
- Lin L, Lin Y, Peng Y, et al. Admission hyperglycemia in acute type A aortic dissection predicts for a prolonged duration of mechanical ventilation. Int Heart J. 2022;63(1):106–112. doi: 10.1536/ihj.21-485.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. J Am Coll Cardiol. 2010;55(14):e27–e129. doi: 10.1016/j.jacc.2010.02.015.
- Golledge J, Karan M, Moran CS, et al. Reduced expansion rate of abdominal aortic aneurysms in patients with diabetes may be related to aberrant monocyte-matrix interactions. Eur Heart J. 2008;29(5):665–672. doi: 10.1093/eurheartj/ehm557.
- Astrand H, Rydén-Ahlgren A, Sundkvist G, et al. Reduced aortic wall stress in diabetes mellitus. Eur J Vasc Endovasc Surg. 2007;33(5):592–598. doi: 10.1016/j.ejvs.2006.11.011.
- Tedesco MM, Terashima M, Blankenberg FG, et al. Analysis of in situ and ex vivo vascular endothelial growth factor receptor expression during experimental aortic aneurysm progression. Arterioscler Thromb Vasc Biol. 2009;29(10):1452–1457. doi: 10.1161/ATVBAHA.109.187757.
- Choke E, Thompson MM, Dawson J, et al. Abdominal aortic aneurysm rupture is associated with increased medial neovascularization and overexpression of proangiogenic cytokines. Arterioscler Thromb Vasc Biol. 2006;26(9):2077–2082. doi: 10.1161/01.ATV.0000234944.22509.f9.
- Ceradini DJ, Yao D, Grogan RH, et al. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008;283(16):10930–10938. doi: 10.1074/jbc.M707451200.
- Onuta G, Westerweel PE, Zandvoort A, et al. Angiogenic sprouting from the aortic vascular wall is impaired in the BB rat model of autoimmune diabetes. Microvasc Res. 2008;75(3):420–425. doi: 10.1016/j.mvr.2007.11.006.
- Hao Y, Lu Q, Li T, et al. Admission hyperglycemia and adverse outcomes in diabetic and non-diabetic patients with non-ST-elevation myocardial infarction undergoing percutaneous coronary intervention. BMC Cardiovasc Disord. 2017;17(1):6. doi: 10.1186/s12872-016-0441-x.
- Zhang JW, Zhou YJ, Cao SJ, et al. Impact of stress hyperglycemia on in-hospital stent thrombosis and prognosis in nondiabetic patients with ST-segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron Artery Dis. 2013;24(5):352–356. doi: 10.1097/MCA.0b013e328361a942.
- Landenhed M, Engström G, Gottsäter A, et al. Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: a prospective cohort study. J Am Heart Assoc. 2015;4(1):e001513. doi: 10.1161/JAHA.114.001513.
- Li Y, Yang N, Duan W, et al. Acute aortic dissection in China. Am J Cardiol. 2012;110(7):1056–1061. doi: 10.1016/j.amjcard.2012.05.044.
- Duncan AE, Abd-Elsayed A, Maheshwari A, et al. Role of intraoperative and postoperative blood glucose concentrations in predicting outcomes after cardiac surgery. Anesthesiology. 2010;112(4):860–871. doi: 10.1097/ALN.0b013e3181d3d4b4.
- Helgason D, Helgadottir S, Ahlsson A, et al. Acute kidney injury after acute repair of type A aortic dissection. Ann Thorac Surg. 2021;111(4):1292–1298. doi: 10.1016/j.athoracsur.2020.07.019.
- Gao S, Liu Q, Chen H, et al. Predictive value of stress hyperglycemia ratio for the occurrence of acute kidney injury in acute myocardial infarction patients with diabetes. BMC Cardiovasc Disord. 2021;21(1):157. doi: 10.1186/s12872-021-01962-2.
- Gorelik Y, Bloch-Isenberg N, Hashoul S, et al. Hyperglycemia on admission predicts acute kidney failure and renal functional recovery among inpatients. J Clin Med. 2021;11(1):54. doi: 10.3390/jcm11010054.
- Rangaswami J, Bhalla V, Blair JEA, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019;139(16):e840–e878. doi: 10.1161/CIR.0000000000000664.
- Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36(23):1437–1444. doi: 10.1093/eurheartj/ehv010.
- Vedantam D, Poman DS, Motwani L, et al. Stress-induced hyperglycemia: consequences and management. Cureus. 2022;14(7):e26714. doi: 10.7759/cureus.26714.
- Keykhaei M, Ashraf H, Rashedi S, et al. Differences in the 2020 ESC versus 2015 ESC and 2014 ACC/AHA guidelines on the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Curr Atheroscler Rep. 2021;23(12):77. doi: 10.1007/s11883-021-00976-7.
- Thiele H, Barbato E, Barthelemy O, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. 2020.
- Sbarouni E, Georgiadou P, Analitis A, et al. High neutrophil to lymphocyte ratio in type A acute aortic dissection facilitates diagnosis and predicts worse outcome. Expert Rev Mol Diagn. 2015;15(7):965–970. doi: 10.1586/14737159.2015.1042367.
- DI Marco L, Leone A, Murana G, et al. Acute type A aortic dissection: rationale and outcomes of extensive repair of the arch and distal aorta. Int J Cardiol. 2018;267:145–149. doi: 10.1016/j.ijcard.2018.05.111.