?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The clinical impact of heart rate (HR) in heart failure with preserved ejection fraction (HFpEF) is a matter of debate. Among those with HFpEF, chronotropic incompetence (CI) has emerged as a pathophysiological mechanism linked to the severity of the disease. In this study, we sought to evaluate whether admission heart rate in acute heart failure differs along left ventricular ejection fraction (LVEF).
Methods
We included retrospectively 3,712 consecutive patients admitted for acute heart failure (AHF) in the Cardiology department of a third level center. HR values were assessed at presentation. LVEF was assessed by transthoracic echocardiogram during the index admission and stratified into four categories: reduced ejection fraction (40%), mildly reduced ejection fraction (41–49%), preserved ejection fraction (50–64%) and supranormal ejection fraction (
65%). The association between HR and LVEF was assessed by multivariate linear and multinomial regression analyses.
Results
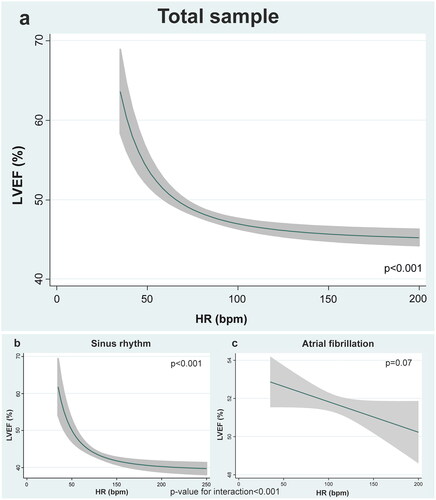
The mean age of the sample was 73,9 ± 11.3 years, 1,734 (47,4%) were women, and 1,214 (33,2%), 570 (15,6%), 1,229 (33,6%) and 648 (17,7%) patients showed LVEF 40%, 41–49%, 50–64%, and ≥65% respectively. The median HR at admission was 95 (IQR 78–120) beats per minute and 1,653 were on atrial fibrillation (45.2%). There was an inverse relationship between HR at admission and LVEF. Lower HR was significantly associated with a higher LVEF in the whole sample (p < 0,001). This inverse relationship was found in sinus rhythm but not in patients with atrial fibrillation.
Conclusion
HR at admission for AHF is a predictor of LVEF but only in patients with sinus rhythm.
Introduction
High heart rate (HR) is a well-known prognostic marker and therapeutic target in patients with chronic heart failure with reduced ejection fraction (HFrEF) [Citation1]. Indeed, -blockers are a class Ia drugs for these patients [Citation2,Citation3]. However, the clinical implication of HR in patients with HF and preserved ejection fraction (HFpEF) remains not totally defined. In these latter patients, both extremes of HR (lower and higher) have been associated with worse functional status, and a higher risk of adverse events [Citation4,Citation5]. Likewise, among patients with HFpEF, recent data indicate that those in the upper range of left ventricular ejection fraction (LVEF) show a differential clinical and pathophysiological profile [Citation6]. Current data suggests lower chronotropic response may be more frequent at higher LVEF [Citation7]. However, data is scarce and not conclusive. In this study, we sought to evaluate whether HR on admission differs along the continuum of LVEF and whether this association is modulated by the presence of atrial fibrillation in patients with acute heart failure (AHF).
Methods
Study sample
A consecutive cohort of 3,712 patients admitted for AHF in the Cardiology department of a third level center (Hospital Clínico Universitario de Valencia, Spain) from July 2011 to July 2020 was examined retrospectively. We did not include pacemaker carriers (including implantable cardioverter defibrillator or resynchronization therapy) who were considered to be pacemaker dependent (n = 51). Patients with acute coronary syndrome active infections as primary diagnosis, and advanced forms of atrioventricular block (requiring with pacemaker implantation during the hospitalization) were excluded. The final study sample included 3,661 patients. AHF was defined as severe onset of signs and symptoms of heart failure (HF) requiring medical attention and included both new onset and decompensation of chronic HF. All patients received intravenous diuretics during hospitalization.
We registered demographic data, medical history, vital signs, 12-lead electrocardiogram, and laboratory (including natriuretic peptides) data at admission. Vital signs were assessed at presentation in emergency room. HR was obtained from the electrocardiogram. For patients in AF, HR was measured by counting the number of QRS complexes in a 6-second strip and multiplying the result by 10. LVEF was assessed by 2D-echocardiography during the index hospitalization using the biplane Simpson’s method.
The protocol was approved by the ethical committee of our center, following the principles of the Declaration of Helsinki and national regulations.
Exposures and endpoint
The exposure of the study was heart rate on admission and the dependent variable was LVEF. This was evaluated as continuous and stratified in four categories: reduced ejection fraction (40%), mildly reduced ejection fraction (41–49%), preserved ejection fraction (50–64%) and supranormal ejection fraction (SnLVEF) (
65%).
Statistical analysis
Continuous variables are presented as mean ± 1 standard deviation or median and interquartile range (IQR) for parametric and nonparametric distributions, respectively. They were compared using t test or Wilcoxon rank-sum test when appropriate. Discrete variables were presented as percentages and compared with the Chi-squared test. The correlation between HR and LVEF in the whole sample and among subgroups was assessed by Pearson correlation analyses. The relationship between HR on admission and LVEF as continuous variable was assessed by multivariate linear regression analysis. The association between HR and LVEF categories was examined through multinomial logistic regression. Candidate covariates included in the initial and final multivariate models were selected based on the biological plausibility. The final multivariate estimates were adjusted for age, sex, diabetes mellitus, smoking status, history of ischemic cardiomyopathy, New York Heart Association (NYHA) class before admission, cerebrovascular accident, chronic obstructive pulmonary disease, branch block, atrial fibrillation (AF), edema, previous treatment with diuretics or -blockers, systolic and diastolic blood pressure, hematocrit, C reactive protein, creatinine and estimated glomerular filtration rate at admission, end-diastole left ventricular volume, posterior wall thickness, tricuspid annular plane systolic excursion (TAPSE), tricuspid regurgitation grade, N-terminal pro B-type natriuretic peptide (NT-proBNP) and plasma antigen carbohydrate 125.
Estimates are presented as hazard ratios with their respective 95% confidence intervals. A 2-sided p value of <0.05 was considered statistically significant for all analyses. All analyses were performed using STATA 18.0.
Results
The mean age of the sample was 73.9 ± 11.3 years and 1,734 (47.4%) were women. The mean HR at admission was 99 ± 28 beats per minute (bpm) and the mean LVEF was 49 ± 15%. According to LVEF categories, 1,214 patients (33.2%) had reduced ejection fraction (LVEF 40%); 570 patients (15.6%) mildly reduced ejection fraction (41–49%); 1,229 patients (33.3%) preserved ejection fraction (50–64%) and 648 (17.7%) LVEF
65%.
show basal characteristics according to HR quartiles and LVEF categories, respectively. Overall, patients with lower HR at admission had more cardiovascular (CV) risk burden, more frequent history of coronary artery disease, worse functional class prior to admission, more signs of fluid overload at admission, and were more frequently treated with β-blockers and digoxin. Conversely, patients with higher HR were more frequently on AF. Antiarrhythmics were more frequently used in both the lower and upper categories of HR. Regarding LVEF, those in the upper category were older, more frequently women, had smaller ventricle size, higher prevalence of AF, and higher use of digoxin. Supplementary Tables 1–4 show basal characteristics according to HR quartiles and LVEF categories across the heart rhythm (sinus rhythm -SR- or AF).
Table 1. Baseline characteristics across heart rate quartiles.
Table 2. Baseline characteristics across LVEF categories.
Association between admission heart rate and LVEF as continuous
In the whole sample, HR was significant, inverse, and weakly correlated with LVEF (r = –0.08, p < 0.001). The magnitude of these inverse correlation was greater in those in SR on admission (r = –0.23, p < 0.001) and in those without β-blockers on admission (r = –0.10, p < 0.001). The correlation was still negative but weaker in those with AF (r = −0.07, p = 0.006) and even not significant in those on β-blockers (r = –0.05, p = 0.068).
Multivariate analysis confirmed the inverse association between HR and LVEF (). In this case the association was not linear revealing an exponential increase of risk (). The lower HR, especially lower than 60–50 bpm, the higher LVEF. In those with HR > 70–80 bpm the association remained mostly flat ().
Heart rate and LVEF across AF
This relationship was significantly modified by the presence of AF (p for interaction < 0.001). In SR the association between lower heart rates and higher LVEF was highly significant (). It remained nonlinear with a steeper increase of LVEF in those lower than 70 bpm (). At the contrary, when AF was present this association was no longer significant ().
Heart rate and LVEF across β-blocker treatment
Under the same multivariate setting, prior treatment with β-blockers did not significantly modify the inverse association between HR and LVEF (p value for interaction = 0.201). Thus, lower HR identified a patient with higher LVEF irrespective of prior treatment with β-blockers (Supplementary Figure 1a and 1b).
Association between admission heart rate and LVEF categories
Admission mean HR (bpm) was lower when moving from lower to upper LVEF categories with LVEF < 40%, 41–49%, 50–64%, and ≥65% (101 ± 26, 99 ± 27, 99 ± 29, and 94 ± 30, p value for trend < 0.001). This trend was also found in subjects with AF (111 ± 30, 106 ± 31, 109 ± 31, and 103 ± 34, p value for trend = 0.011) and in those with β blockers (97 ± 26, 91 ± 26, 93 ± 29, and 88 ± 29, p value for trend = 0.042).
Multivariate multinomial regression analysis confirmed how HR was associated with LVEF categories (). Increased HR identified patients at higher risk of presenting LVEF 40% (). In patients with mildly reduced LVEF (41-49%) and LVEF 50-64% the association was neutral (, respectively). At the upper side of LVEF, the lower HR, especially <60bpm, increased the odds of having LVEF ≥ 65% (). Similar findings were found when analyzing patients with SR () and AF () separately. Higher HR predicted LVEF
40% and lower HR was associated with higher odds of LVEF ≥ 65% regardless the rhythm.
Figure 2. Predicted probability for each category of LVEF according to heart rate at admission. a. Predicted probability for LVEF ≤40% according to heart rate at admission. b. Predicted probability for LVEF 41–49% according to heart rate at admission. c. Predicted probability for LVEF 50–59% according to heart rate at admission. d. Predicted probability for LVEF ≥65% according to heart rate at admission. Bpm: Beats per minute; HR: heart rate; LVEF: left ventricular ejection fraction.
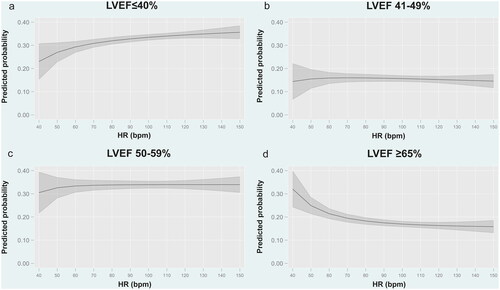
Figure 3. Predicted probability for each category of LVEF according to heart rate at admission in patients with sinus rhythm. a. Predicted probability for LVEF ≤40%. b. Predicted probability for LVEF 41–49%. c. Predicted probability for LVEF 50–59%. d. Predicted probability for LVEF ≥65%. Bpm: Beats per minute; HR: heart rate; LVEF: left ventricular ejection fraction.
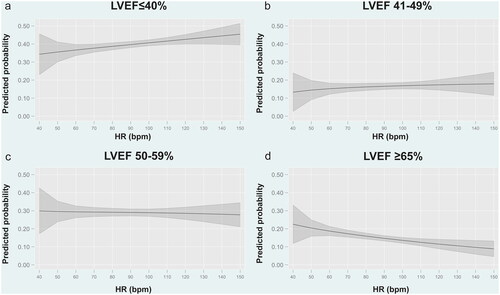
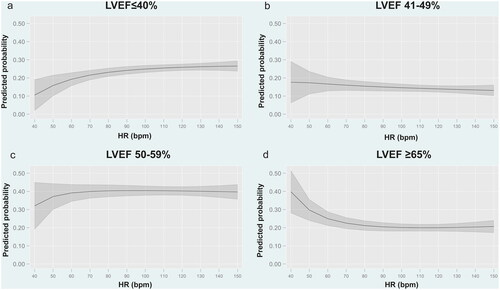
Figure 4. Predicted probability for each category of LVEF according to heart rate at admission in patients with atrial fibrillation. a. Predicted probability for LVEF 40%. b. Predicted probability for LVEF 41–49%. c. Predicted probability for LVEF 50–59%. d. Predicted probability for LVEF
65%. Bpm: Beats per minute; HR: heart rate; LVEF: left ventricular ejection fraction.
Discussion
In this large observational study with a wide representation of patients along the continuum of LVEF, we found an inverse association between HR on admission and LVEF assessed during the hospitalization. These findings confirmed the association between higher heart rates and left ventricular systolic dysfunction but expand to lower rates in patients with SnLVEF. Thus, these findings suggest that lower HR may play a role in the pathophysiology of patients with HF and SnLVEF.
Heart rate and left ventricular ejection fraction
To our knowledge, there are no previous studies showing an association between HR and LVEF. However, it has been consistently shown that HR has a differential prognostic impact when considering LVEF categories in HF. A higher HR has been repeatedly associated with worse prognosis in HFrEF [Citation1], and it is a well-known therapeutic target in these patients [Citation3,Citation8]. In a metanalysis from Kotecha et al. including 11 randomized clinical trials on HFrEF, β-blockers reduced mortality irrespective of basal HR [Citation9]. This is not true for HFpEF. In a substudy of the TOPCAT clinical trial (Spironolactone for heart failure with preserved ejection fraction) reductions in HR were not significantly associated with lower risk and β-blockers were associated with higher risk of HF-readmission [Citation10,Citation11]. More recently, in a large registry (435,897 patients with HF and LVEF ≥40%) -blocker use was associated with a higher risk of HF hospitalization as EF increased, with potential benefit in patients with HFmrEF and potential risk in patients with higher EF (particularly >60%) [Citation12].
Pathophysiology of HR response in AHF
Extreme HR (higher but also lower) are common triggers for HF decompensations [Citation13] in the setting of tachy and bradyarrhythmia, but not commonly reported in sinus rhythm. In these patients, under stress conditions (such as an episode of AHF), HR is expected to increase as a result of increased sympathetic activity. Thus, in sinus rhythm, increased HR is a compensatory mechanism aiming to increase cardiac output, and not a mechanism casually linked to worsening HF. At the opposite, blunted HR response in patients with AHF theoretically may identify those in which CI may be playing a pathophysiological role [Citation14]. Recent data shows CI is present in approximately 60% of patients with HFpEF and it may prevent the increase of cardiac output during stress situations in these patients that usually have stiff hearts with decreased compliance [Citation15]. Its potential causes include peripheral muscle dysfunction, imbalances in the autonomic nervous system, remodeling of the sinus node, and diminished density and responsiveness of β-receptors [Citation16–18]. CI has been positively associated with patient’s functional capacity [Citation19] and related with increased all-cause mortality and all-cause hospitalization in HFpEF, especially in SR [Citation20,Citation21].
Lower heart rate in heart failure with supranormal ejection fraction
SnLVEF is considered by most documents as a LVEF 65%. Epidemiological studies about this population show they are predominantly women, have less prevalence of ischemic heart disease, more non-CV comorbidity and show lower levels of NT-proBNP[Citation6]. Indeed, there is recent evidence showing that there is a U-shaped curve in terms of CV events and LVEF, meaning that this subgroup shows an increased risk of outcomes [Citation22,Citation23]. This risk is not fully understood but it seems to keep relation with the pathophysiological mechanisms of the disease, specifically with a smaller heart and a lower stroke volume [Citation22].
In our study we have shown how patients in the higher LVEF category showed smaller left ventricle end-diastolic diameter (LVEDD), greater thickness of both ventricular walls, and lower NT-proBNP. In addition, these patients presented with significantly lower HR at admission for AHF, even in comparison to those patients with LVEF ranging from 50-65% category. These results are in concordance to Maredziak et al. who found blunted heart rate reserve to a stress test with adenosine in women with snLVEF compared to normal LVEF, but not for basal HR or in men [Citation24].
Clinical implications
According to the current findings, we speculate that in patients with AHF, the inability to properly increase HR as expected may be a surrogate of chronotropic incompetence. Therefore, lower HR response may be helpful for early recognition of patients with HFpEF, and especially SnLVEF, avoiding therapeutic measures with no evidence or even harmful. In this regard, in a recent randomized clinical trial, Palau et al. demonstrated that betablockers cessation in patients with HFpEF and chronotropic incompetence led to a short-term increase in functional capacity evaluated by peak VO2 [Citation25]. However, the evidence is scarce and inconsistent when it comes to find any therapeutic measure in this regard. For instance, the RAPID-HF trial (rate-adaptive atrial pacing in diastolic heart failure) has shown no benefit on rate-adaptative atrial pacing for HFpEF and chronotropic incompetence [Citation26]. We believe the debate is open and more studies are needed.
Limitations
Our study has several limitations that need to be addressed. First, it is a retrospective analysis of a single center. Second, in this observational study there are potential unmeasured confounders that may modify the reposted association. Third, we did not assess crucial echocardiographic parameters that may better disentangle these findings. Fourth, assessment of heart rates in subsequent days following hospitalization (and their changes) were not performed, precluding to examine the association between different timepoints of HR and LVEF. Finally, in the current study we cannot infer the mechanism behind this association and its clinical implications.
Conclusion
In patients with AHF, HR at presentation was inversely related to LVEF. This association was significantly found in sinus rhythm but not in patients with AF.
Supplementry Table 4.docx
Download MS Word (21.9 KB)Supplementary Table 1.docx
Download MS Word (21.6 KB)Supplementary Table 2.docx
Download MS Word (21.6 KB)Supplementry Table 3.docx
Download MS Word (22.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Castagno D, Skali H, Takeuchi M, et al. Association of heart rate and outcomes in a broad spectrum of patients with chronic heart failure. J Am Coll Cardiol. 2012;59(20):1785–1795. doi: 10.1016/j.jacc.2011.12.044.
- Lan WR, Lin SI, Liao FC, et al. Effect of reducing heart rate on outcomes in patients with reduced ejection fraction. Am J Cardiol. 2021;150:77–81. doi: 10.1016/j.amjcard.2021.03.050.
- Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376(9744):875–885. doi: 10.1016/S0140-6736(10)61198-1.
- Bristow MR, Altman NL. Heart rate in preserved ejection fraction heart failure. JACC Heart Fail. 2017;5(11):792–794. doi: 10.1016/j.jchf.2017.09.004.
- Maeder MT, Kaye DM. Differential impact of heart rate and blood pressure on outcome in patients with heart failure with reduced versus preserved left ventricular ejection fraction. Int J Cardiol. 2012;155(2):249–256. doi: 10.1016/j.ijcard.2010.10.007.
- Van Essen BJ, Tromp J, Ter Maaten JM, et al. Characteristics and clinical outcomes of patients with acute heart failure with a supranormal left ventricular ejection fraction. Eur J Heart Fail. 2023;25(1):35–42. doi: 10.1002/ejhf.2695.
- Meyer M, LeWinter MM. Heart rate and heart failure with preserved ejection fraction: time to slow β-blocker use? Circ Heart Fail. 2019;12(8):e006213. doi: 10.1161/CIRCHEARTFAILURE.119.006213.
- Rienstra M, Damman K, Mulder BA, et al. Beta-blockers and outcome in heart failure and atrial fibrillation. JACC Heart Fail. 2013;1(1):21–28. doi: 10.1016/j.jchf.2012.09.002.
- Kotecha D, Flather MD, Altman DG, et al. Heart rate and rhythm and the benefit of beta-blockers in patients with heart failure. J Am Coll Cardiol. 2017;69(24):2885–2896. doi: 10.1016/j.jacc.2017.04.001.
- Vazir A, Claggett B, Pitt B, et al. Prognostic importance of temporal changes in resting heart rate in heart failure and preserved ejection fraction. JACC Heart Fail. 2017;5(11):782–791. doi: 10.1016/j.jchf.2017.08.018.
- Silverman DM, Plante TB, Infeld M, et al. Association of β-blocker use with heart failure hospitalizations and cardiovascular disease mortality among patients with heart failure with a preserved ejection fraction: a secondary analysis of the TOPCAT trial. JAMA Netw Open. 2019;2(12):e1916598. doi: 10.1001/jamanetworkopen.2019.16598.
- Arnold SV, Silverman DN, Gosch K, et al. Beta-blocker use and heart failure outcomes in mildly reduced and preserved ejection fraction. JACC Heart Fail. 2023;11(8 Pt 1):893–900. doi: 10.1016/j.jchf.2023.03.017.
- Oliva F, Sormani P, Contri R, et al. Heart rate as a prognostic marker and therapeutic target in acute and chronic heart failure. Int J Cardiol. 2018;253:97–104. doi: 10.1016/j.ijcard.2017.09.191.
- Wang J, Fang F, Yip GWK, et al. Importance of chronotropic response and left ventricular long-axis function for exercise performance in patients with heart failure and preserved ejection fraction. Int J Cardiol. 2016;202:339–343. doi: 10.1016/j.ijcard.2015.09.029.
- Zweerink A, Van Der Lingen ALCJ, Handoko ML, et al. Chronotropic incompetence in chronic heart failure: a state-of-the-art review. Circ Heart Fail. 2018;11(8):e004969. doi: 10.1161/CIRCHEARTFAILURE.118.004969.
- Sanders P, Kistler PM, Morton JB, et al. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004;110(8):897–903. doi: 10.1161/01.CIR.0000139336.69955.AB.
- Messias LR, Messias ACNV, De Miranda SMR, et al. Abnormal adrenergic activation is the major determinant of reduced functional capacity in heart failure with preserved ejection fraction. Int J Cardiol. 2016;203:900–902. doi: 10.1016/j.ijcard.2015.10.224.
- Mesquita T, Zhang R, Cho JH, et al. Mechanisms of sinoatrial node dysfunction in heart failure with preserved ejection fraction. Circulation. 2022;145(1):45–60. doi: 10.1161/CIRCULATIONAHA.121.054976.
- Domínguez E, Palau P, Núñez E, et al. Heart rate response and functional capacity in patients with chronic heart failure with preserved ejection fraction. ESC Heart Fail. 2018;5(4):579–585. doi: 10.1002/ehf2.12281.
- Magrì D, Corrà U, Di Lenarda A, et al. Cardiovascular mortality and chronotropic incompetence in systolic heart failure: the importance of a reappraisal of current cut-off criteria: cardiovascular mortality and chronotropic incompetence. Eur J Heart Fail. 2014;16(2):201–209. doi: 10.1002/ejhf.36.
- Benes J, Kotrc M, Borlaug BA, et al. Resting heart rate and heart rate reserve in advanced heart failure have distinct pathophysiologic correlates and prognostic impact. JACC Heart Fail. 2013;1(3):259–266. doi: 10.1016/j.jchf.2013.03.008.
- Shah S, Segar MW, Kondamudi N, et al. Supranormal left ventricular ejection fraction, stroke volume, and cardiovascular risk. JACC: Heart Fail. 2022;10(8):583–594. doi: 10.1016/j.jchf.2022.05.007.
- Wehner GJ, Jing L, Haggerty CM, et al. Routinely reported ejection fraction and mortality in clinical practice: where does the nadir of risk lie? Eur Heart J. 2020;41(12):1249–1257. doi: 10.1093/eurheartj/ehz550.
- Abidov A, Hachamovitch R, Hayes SW, et al. Prognostic impact of hemodynamic response to adenosine in patients older than age 55 years undergoing vasodilator stress myocardial perfusion study. Circulation. 2003;107(23):2894–2899. doi: 10.1161/01.CIR.0000072770.27332.75.
- Palau P, Seller J, Domínguez E, et al. Effect of β-blocker withdrawal on functional capacity in heart failure and preserved ejection fraction. J Am Coll Cardiol. 2021;78(21):2042–2056. doi: 10.1016/j.jacc.2021.08.073.
- Reddy YNV, Koepp KE, Carter R, et al. Rate-adaptive atrial pacing for heart failure with preserved ejection fraction: the RAPID-HF randomized clinical trial. JAMA. 2023;329(10):801–809. doi: 10.1001/jama.2023.0675.