Abstract
Objectives: To evaluate efficacy/safety of baricitinib for rheumatoid arthritis (RA) in Japanese subpopulations from four phase 3 studies, and assess whether results in these subpopulations are consistent with the overall study populations.
Methods: Subgroup analyses (394 patients) of four phase 3 randomized controlled trials: RA-BEGIN [no or limited treatment with disease-modifying antirheumatic drugs (DMARDs)], RA-BEAM [inadequate response (IR) to methotrexate], RA-BUILD [IR to conventional synthetic DMARDs (csDMARDs)], and RA-BEACON (IR to tumor necrosis factor inhibitors receiving csDMARDs).
Results: For American College of Rheumatology 20% improvement (ACR20) response rate, Japanese patients receiving baricitinib 4-mg showed similar improvement compared to methotrexate at Week 24 (72 versus 69%; RA-BEGIN), and greater improvement compared with placebo at Week 12 (67 versus 34%; RA-BEAM). Japanese patients receiving baricitinib 4-mg also showed greater improvement compared with placebo at Week 12 in RA-BUILD and RA-BEACON. Across all studies, baricitinib was well-tolerated, with no deaths and one malignancy. In RA-BEGIN and RA-BEAM, herpes zoster rates were higher for Japanese patients than for overall populations; all events were mild/moderate.
Conclusion: Data for baricitinib, with/without methotrexate, in Japanese subpopulations across all stages of the RA treatment continuum accord with the efficacy/safety profile in overall study populations. Baricitinib appears to be similarly effective in Japanese patients.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease that causes joint swelling, joint tenderness and progressive destruction of joints, which can lead to severe disability. Targeted synthetic disease-modifying antirheumatic drugs (tsDMARDs) and biologic disease-modifying antirheumatic drugs (bDMARDs) are used to treat RA, alone or in combination with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). The most commonly prescribed agent for RA is the csDMARD methotrexate, but inadequate efficacy, delayed onset of benefit, poor tolerability or contraindications limit its use in some patients [Citation1–3]. Newer agents that have been approved for patients with RA who have an inadequate response to csDMARDs include various injectable bDMARDs – for example, tumor necrosis factor (TNF) inhibitors [Citation4–6]. However, not all patients benefit from these agents [Citation7,Citation8], and other therapeutic options with alternative mechanisms of action are needed.
Janus kinase (JAK)-mediated cell signalling has been implicated in the pathogenesis of RA [Citation9,Citation10]. One tsDMARD that has been approved for patients with RA who have an inadequate response to csDMARDs is the JAK inhibitor tofacitinib – a small molecule that is a more potent inhibitor of JAK1 and JAK3 than JAK2, and can be taken orally [Citation11,Citation12]. Baricitinib is a JAK inhibitor that can also be taken orally, but it differs from tofacitinib in that it is selective for JAK1 and JAK2 [Citation13–16].
The efficacy and safety of baricitinib (1-mg, 2-mg, 4-mg, 8-mg) in Japanese patients with moderate-to-severe active adult-onset RA despite stable treatment with methotrexate have been assessed in a phase 2 study [Citation17]. In this study, 4-mg and 8-mg baricitinib showed earlier and/or greater improvements in most measures of disease activity than the 1-mg and 2-mg doses over 12 weeks of treatment when compared with placebo [Citation17]. Baricitinib was well-tolerated in this study; the safety profile of 4-mg appeared to offer some advantages over the 8-mg dose, and was similar to that of the lower doses.
Four global phase 3 trials of baricitinib that cover all stages of the RA treatment continuum, from patients who were DMARD-naïve to those who had failed multiple csDMARDs or bDMARDs, have been conducted: RA-BEGIN, RA-BEAM, RA-BUILD, and RA-BEACON [Citation18–21]. In RA-BEGIN, the study population was patients with no or minimal prior csDMARD and no prior bDMARD or tsDMARD treatment. In the overall study population, baricitinib 4-mg monotherapy was superior to methotrexate monotherapy at Week 24, shown by a higher American College of Rheumatology 20% (ACR20) response rate (77 versus 62%, p = .003). Similar results were observed for the baricitinib + methotrexate combination as for baricitinib monotherapy. Compared with the methotrexate group, the rate of progressive radiographic joint damage [van der Heijde modified total Sharp score (mTSS)] was lower in the baricitinib and baricitinib + methotrexate groups; the difference was statistically significant for the baricitinib + methotrexate group. In RA-BEAM, the study population was patients who had an inadequate response to methotrexate. In the overall study population, baricitinib 4-mg was superior to placebo and to adalimumab in terms of ACR responses at Week 12 [ACR20 response rates: 70% for baricitinib versus 40% for placebo (p ≤ .001) and versus 61% for adalimumab (p = .014)], and statistically significant improvements in mTSS were seen for baricitinib versus placebo at Week 24. In RA-BUILD and RA-BEACON, baricitinib 2-mg and 4-mg once daily showed improved efficacy compared with placebo, with the most rapid and robust improvements generally seen for the 4-mg dose. The safety profile of baricitinib seen in these studies appeared acceptable in the context of the observed efficacy, and the known safety profiles of approved DMARDs.
Japanese patients were included in the RA-BEGIN, RA-BEAM, RA-BUILD, and RA-BEACON studies. We aimed to evaluate the efficacy and safety of baricitinib in these patients with active RA across all stages of the RA treatment continuum and also assess whether the results are consistent with those in the overall study populations by conducting subgroup analyses of the Japanese patients participating in these studies.
Patients and methods
Analysis plan
We conducted analyses of the subgroups of Japanese patients enrolled in four studies of baricitinib for the treatment of RA. Results for the overall populations of each study are published elsewhere [Citation18–21]. The studies were phase 3, multicenter, randomized, double-blind, placebo/active-controlled trials. The ClinicalTrials.gov identifiers and study dates (first enrollment to completion) were:
RA-BEGIN: NCT01711359, 02 January 2013 to 26 August 2015
RA-BEAM: NCT01710358, 23 October 2012 to 29 September 2015
RA-BUILD: NCT01721057, 10 January 2013 to 19 December 2014
RA-BEACON: NCT01721044, 23 January 2013 to 02 September 2014
The studies were sponsored by Eli Lilly and Company and Incyte Corporation, and designed by Eli Lilly and Company in consultation with Incyte Corporation and academic advisory boards of people not employed by either study sponsor. All the studies were conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and approved by each participating center’s institutional review board or ethics committee. All patients provided written informed consent before participating in any study-related procedures.
Study population
Patients in all the studies had moderately-to-severely active RA. Key inclusion and exclusion criteria for each study are described briefly in Supplementary Table S1.
Treatment protocols
RA-BEGIN was a 52-week study in which patients with no or minimal prior csDMARDs and naïve to bDMARDs were randomized in a 4:3:4 ratio to receive methotrexate, baricitinib 4-mg once daily, or baricitinib 4-mg once daily + methotrexate. The RA-BEGIN protocol specified that patients treated with methotrexate would initially be given 10-mg methotrexate per week and then have their dose increased by 5-mg every 4 weeks to a maximum of 20-mg per week. However, since the maximum approved dose in Japan is 16-mg, Japanese patients (and other patients requiring a lower dose) treated with methotrexate started at 7.5-mg once weekly and had their dose increased by 2.5-mg every 4 weeks to a maximum of 12.5-mg per week.
RA-BEAM was a 52-week study in which bDMARD-naïve patients who had an inadequate response to methotrexate were randomly assigned in a 3:3:2 ratio to receive placebo (switched to baricitinib after 24 weeks), baricitinib 4-mg once daily, or the anti-TNF-α monoclonal antibody, adalimumab 40-mg biweekly, in addition to their existing background therapy (including methotrexate).
RA-BUILD was a 24-week study in which bDMARD-naïve patients who had an inadequate response or intolerance to ≥1 csDMARDs were randomly assigned in a 1:1:1 ratio to receive baricitinib 2-mg or 4-mg once daily or placebo.
RA-BEACON was a 24-week study in which patients with an inadequate response or intolerance to one or more previous TNF inhibitors were randomly assigned in a 1:1:1 ratio to receive baricitinib 2-mg or 4-mg once daily or placebo.
In all four studies, as required by the protocol, patients who reported symptomatic herpes zoster were discontinued.
Outcome measures
Each study had a primary objective that was based on ACR20. For RA-BEGIN, it was the proportion of patients achieving ACR20 at Week 24 for baricitinib monotherapy versus methotrexate. For RA-BEAM, RA-BUILD and RA-BEACON, it was the proportion achieving ACR20 at Week 12 for baricitinib 4-mg versus placebo.
The major secondary measures for each study included the change from baseline in Disease Activity Score in 28 joints using the high-sensitivity C-reactive protein level (DAS28-hsCRP), the change from baseline in Health Assessment Questionnaire-Disability Index (HAQ-DI) score, and remission according to the Simplified Disease Activity Index (SDAI); these are reported here for the Japanese subpopulations of all four studies. In addition, the major secondary measures for RA-BEGIN and RA-BEAM included change in mTSS, and the major secondary measures for RA-BEAM included patient-reported outcomes; these are reported here for the Japanese subpopulations. Other secondary measures reported here for the Japanese subpopulations of RA-BEGIN and RA-BEAM include ACR50 (American College of Rheumatology 50% improvement) and ACR70 (American College of Rheumatology 70% improvement) measures, SDAI low disease activity, Clinical Disease Activity Index (CDAI) low disease activity and remission.
Statistical analysis
Efficacy analyses for all four studies were conducted on the basis of a modified intent-to-treat (mITT) principle – they included all patients treated with one or more dose of assigned study drug. Analysis of structural progression (mTSS) was conducted on a subset of the mITT population that included all randomized patients who received at least one dose of study drug, and had non-missing baseline and at least one non-missing postbaseline mTSS measurement. Safety observations for all patients treated with one or more dose of assigned study drug were analysed by assigned treatment until time of rescue (all studies), time of switch (RA-BEAM only) or completion of the treatment period (all studies). Statistical analyses for the Japanese subgroups were conducted as previously described for the overall populations of each study [Citation18–21], but without adjustment for multiplicity. Importantly, none of the studies were designed to provide adequate power for between-treatment comparisons in any subgroup, including that of the subgroup of Japanese patients.
Results
Patient disposition
In RA-BEGIN, 36, 29 and 39 Japanese patients were randomly assigned to receive methotrexate, baricitinib 4-mg once daily, or baricitinib 4-mg once daily + methotrexate, respectively (Supplementary Figure S1). In RA-BEAM, 93, 93 and 63 Japanese patients were randomly assigned to receive placebo, baricitinib 4-mg once daily, or adalimumab 40-mg biweekly, respectively (Supplementary Figure S2). In RA-BUILD, eight, six and seven Japanese patients were randomly assigned to receive placebo, baricitinib 2-mg once daily, or baricitinib 4-mg once daily, respectively (Supplementary Figure S3). In RA-BEACON, six, six and eight Japanese patients were randomly assigned to receive placebo, baricitinib 2-mg once daily, or baricitinib 4-mg once daily, respectively (Supplementary Figure S4).
Demographic and baseline clinical characteristics
Patient demographics and disease activity at baseline of Japanese subpopulations were generally consistent across the four studies (). The key differences between the studies related to deliberate differences in study design for the different target populations.
Table 1. Patient demographics and disease activity at baseline of the Japanese subpopulations for studies RA-BEGIN, RA-BEAM, RA-BUILD, and RA-BEACON.
All four studies enrolled patients with moderately-to-severely active RA (). However, there were differences, as expected, in the median time from RA symptom onset for the different patient populations (e.g. 0.5 years from RA symptom onset for treatment-naïve patients in RA-BEGIN, and 8.5 years from RA symptom onset in patients who had an inadequate response or intolerance to TNF inhibitors in RA-BEACON).
In RA-BEGIN, 96% of the Japanese subpopulation were DMARD-naïve (). In RA-BEAM, 89% of the Japanese subpopulation (221/249) were receiving one csDMARD (i.e. methotrexate), and 11% (28/249) were receiving two csDMARDs (i.e. methotrexate + other). Similar baseline characteristics to RA-BEAM were seen in RA-BUILD and RA-BEACON, although as expected in RA-BEACON, almost all patients (523/527 patients in the overall population, and 20/20 patients in the Japanese subpopulation) had prior bDMARD therapy, so this study represented a patient population refractory to treatment with biologics.
Primary and secondary measures of efficacy
RA-BEGIN
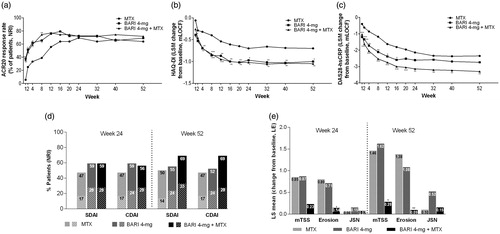
In the Japanese subpopulation who were naïve to DMARDs, baricitinib 4-mg monotherapy and baricitinib + methotrexate therapy showed similar improvement in the ACR20 response rate at Week 24, the primary measure, compared with methotrexate monotherapy (baricitinib 4-mg monotherapy: 72%, baricitinib + methotrexate: 72%, methotrexate monotherapy: 69%). With regards to major secondary measures at Week 24, improvements were observed in the DAS28-hsCRP and the HAQ-DI for baricitinib + methotrexate therapy and the HAQ-DI for baricitinib 4-mg monotherapy, compared with methotrexate monotherapy. Radiographic progression of structural joint damage at Week 52 was lower for baricitinib + methotrexate therapy, compared with methotrexate monotherapy. Improvements in ACR20 response rate, HAQ-DI, and DAS28-hsCRP were seen from as early as Week 1 for baricitinib 4-mg administered as monotherapy or in combination with methotrexate, as compared with methotrexate monotherapy (). Other efficacy measures, including ACR50, ACR70, CDAI, composite score components, and patient-reported outcomes were provided in Supplementary Table S2, Supplementary Figure S5, , Supplementary Figures S6 and S7.
Figure 1. Analyses of primary and secondary efficacy measures for Japanese patients in the RA-BEGIN study, conducted without adjustment for multiplicity. In Panel (d), tall bars indicate low disease activity (SDAI ≤11 and CDAI ≤10), and low (striped) bars indicate remission (SDAI ≤3.3 and CDAI ≤2.8). ***p ≤ .001, **p ≤ .01, *p ≤ .05, versus methotrexate. ACR20: American College of Rheumatology 20% improvement; BARI: baricitinib; CDAI: Clinical Disease Activity Index; DAS28-hsCRP: Disease Activity Score in 28 joints using the hsCRP level; HAQ-DI: Health Assessment Questionnaire-Disability Index; hsCRP: high-sensitivity C-reactive protein; JSN: joint space narrowing; LE: linear extrapolation; MTX: methotrexate; NRI: non-responder imputation; mTSS: modified total Sharp score; SDAI: Simplified Disease Activity Index.
RA-BEAM
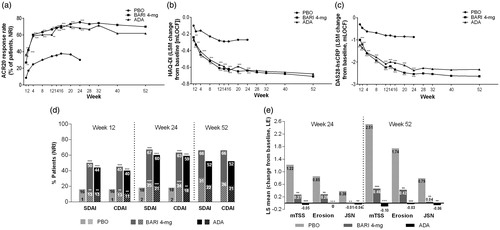
In the Japanese subpopulation who had active disease despite background methotrexate, baricitinib 4-mg and adalimumab showed greater improvements in the ACR20 response rate at Week 12, the primary measure, compared with placebo (baricitinib 4-mg: 67%, adalimumab: 60%, placebo: 34%). Greater improvements in baricitinib 4-mg compared with placebo were seen at Week 12 with respect to all major secondary measures, including HAQ-DI, DAS28-hsCRP, remission according to the SDAI (), duration and severity of morning joint stiffness, worst tiredness and worst joint pain (Supplementary Figure S10). Greater improvements in adalimumab compared with placebo were also seen at Week 12 in HAQ-DI, DAS28-hsCRP, remission according to the SDAI (), severity of morning joint stiffness and worst joint pain (Supplementary Figure S10). Baricitinib 4-mg and adalimumab inhibited radiographic progression of structural joint damage at Week 24 and 52, as compared with placebo. Improvements in ACR20 response, HAQ-DI, and DAS-28-hsCRP were seen from as early as Week 1 for both baricitinib 4-mg and adalimumab as compared with placebo (). Other efficacy measures including ACR50, ACR 70, CDAI, and composite score components were provided in Supplementary Table S3, Supplementary Figure S8, , and Supplementary Figure S9.
Figure 2. Analyses of primary and secondary efficacy measures for Japanese patients in the RA-BEAM study, conducted without adjustment for multiplicity. In Panel (d), tall bars indicate low disease activity (SDAI ≤11 and CDAI ≤10), and low (striped) bars indicate remission (SDAI ≤3.3 and CDAI ≤2.8). ***p ≤ .001, **p ≤ .01, *p ≤ .05, versus placebo. ACR20: American College of Rheumatology 20% improvement; ADA: adalimumab; BARI: baricitinib; CDAI: Clinical Disease Activity Index; DAS28-hsCRP: Disease Activity Score in 28 joints using the hsCRP level; HAQ-DI: Health Assessment Questionnaire-Disability Index; hsCRP: high-sensitivity C-reactive protein; JSN: joint space narrowing; LE: linear extrapolation; NRI: non-responder imputation; PBO: placebo; mTSS: modified total Sharp score; SDAI: Simplified Disease Activity Index.
RA-BUILD and RA-BEACON
Although few Japanese patients were included in these studies, baricitinib demonstrated improvements in ACR20 response, HAQ-DI, and DAS28-hsCRP compared with placebo in each. The most robust improvements were seen for patients receiving the 4-mg once daily dose ().
Table 2. Primary and secondary efficacy measures at Weeks 12 and 24 for Japanese patients in studies RA-BUILD and RA-BEACON.
Safety and tolerability measures
RA-BEGIN
In RA-BEGIN, safety and tolerability were generally comparable across the three active treatment groups in the Japanese subpopulation (). One death was reported, due to drowning (methotrexate group). Adverse events leading to discontinuation occurred most commonly in the combination group; infections, most commonly herpes zoster [which required study discontinuation per protocol (see below)], contributed most to this pattern. No patients who received treatment with baricitinib had a major adverse cardiovascular event. Malignancies were reported for two patients: carcinoid tumor (methotrexate), and cervical carcinoma (baricitinib). Serious infection rates were balanced across groups. The incidence of herpes zoster was 6% for methotrexate, 10% for baricitinib, 8% for baricitinib + methotrexate; all cases were mild or moderate in severity.
Table 3. Safety and laboratory data for japanese patients in studies RA-BEGIN and RA-BEAM.
Mean changes from baseline and CTCAE grade increases in selected laboratory analytes in the Japanese subpopulation of RA-BEGIN are shown in and Supplementary Table S4. Increases in alanine aminotransferase levels were observed across groups; the largest was seen with baricitinib + methotrexate. Increases in creatine kinase levels were seen in both baricitinib groups. Levels of low-density lipoprotein cholesterol and high-density lipoprotein cholesterol also increased with baricitinib.
RA-BEAM
In the Japanese subpopulation of RA-BEAM, there were no deaths and few patients discontinued from the study because of an adverse event (). Compared with placebo, treatment-emergent adverse events (TEAEs) were reported more frequently in the baricitinib and adalimumab groups. No patients who received treatment with baricitinib had a major adverse cardiovascular event. One malignancy (ovarian cancer) and one lymphoproliferative disorder were reported, with placebo and adalimumab, respectively. Very few serious infections occurred. For Weeks 0–24, the incidence of herpes zoster in Japanese patients was 0% in patients receiving placebo, 4% in those receiving baricitinib, and 2% in those receiving adalimumab. For Weeks 0–52, the incidence was 5% in those receiving baricitinib and 3% in those receiving adalimumab. All cases were mild or moderate in severity.
Compared with placebo, small increases in haemoglobin and small decreases in neutrophil counts were seen for baricitinib and adalimumab, while platelet counts increased and decreased slightly with baricitinib and adalimumab, respectively (, Supplementary Table S4). Compared with placebo, small and comparable increases in alanine aminotransferase levels were observed for baricitinib and adalimumab; for one patient this increase was Grade 3 (baricitinib group). Increases from baseline in levels of creatinine, creatine kinase, low-density lipoprotein, and high-density lipoprotein cholesterol were also seen compared with placebo in both active treatment groups; the magnitudes were largest with baricitinib.
RA-BUILD and RA-BEACON
Too few Japanese patients were included in these studies to enable conclusion to be reasonably drawn for safety findings. For completeness, however, safety data are summarized in Supplementary Table S5.
Discussion
This is the first report of the efficacy and safety of baricitinib in Japanese patients with moderately-to-severely active RA enrolled in phase 3 studies assessing all stages of the RA treatment continuum. In these subgroup analyses of four phase 3 trials, which included a total of 394 Japanese patients, baricitinib (with or without methotrexate) demonstrated consistent efficacy with an acceptable safety and tolerability profile in Japanese patients who were DMARD-naïve or showed inadequate response to methotrexate, csDMARDs, or bDMARDs. These efficacy and safety results were generally consistent with results observed in the overall study population of each study.
Consistent with known clinical practices and the genetic and clinical characteristics of patients with RA in Japan [Citation22–24], there were some expected differences in patient characteristics at baseline for the Japanese subpopulations compared with the overall populations. For example, lower body weight and lower methotrexate doses were seen in Japan in all four studies [Citation18–21]; the maximum approved weekly methotrexate dose for RA in Japan is 16 mg [Citation25]. However, data reported here show treatment responses to baricitinib for Japanese subpopulations that were generally similar to those described in the analyses of the overall study populations.
The results of overall study populations in the phase 3 studies demonstrated baricitinib improved efficacy compared with placebo as well as to oral (methotrexate) and injectable (adalimumab on background methotrexate) standard of care DMARDs. Improvements were seen in a rapid and durable manner across relevant domains of efficacy including ACR responses, composite disease activity scores, the components of those scores, low disease activity rates, physical function, and patient-reported outcomes. Disease modification was demonstrated through reduction in radiographic progression. The most robust efficacy was seen for the 4-mg once daily dose [Citation18–21]. In the phase 3 studies, results obtained from Japanese subpopulations were generally consistent with results of the overall study populations. With respect to the primary measure, in study RA-BEGIN, ACR20 response at Week 24 was achieved by 72% of the Japanese patients in the baricitinib 4-mg monotherapy group, and by 72% in the baricitinib + methotrexate group versus 70% in the methotrexate monotherapy group, compared to 77, 78 and 62%, respectively, in the overall study population. In study RA-BEAM, the ACR20 response rate at Week 12 was achieved by 67% of the Japanese patients in the baricitinib 4-mg group, and by 60% in the adalimumab group versus 34% in the placebo group, compared to 70, 61 and 40%, respectively, in the overall study population. With regard to other efficacy measures in the four phase 3 studies, results obtained from Japanese subpopulations were also generally similar to those of overall study population.
In all four of the phase 3 studies, the safety and tolerability profile of baricitinib in Japanese patients appeared acceptable and generally consistent with results from the prior phase 2 study of baricitinib in Japan [Citation17], and with the overall study population data [Citation18–21]. In study RA-BEGIN, the incidence of TEAEs during 52 weeks was 97% of the Japanese patients in the baricitinib 4-mg monotherapy group, and 92% in the baricitinib + methotrexate group versus 83% in the methotrexate monotherapy group, compared to 71, 78 and 72%, respectively, in the overall study population. In study RA-BEAM, the incidence of TEAEs during 24 weeks was 86% of the Japanese patients in the baricitinib 4-mg group, and 83% in the adalimumab group versus 71% in the placebo group, compared to 71, 68 and 60%, respectively, in the overall study population. Among the Japanese patients in the baricitinib treatment arms, there were no death, one malignancy, no major adverse cardiovascular events, and few serious infections, and few patients discontinued from the studies because of adverse events. In studies RA-BEAM and RA-BEGIN, in which reasonable numbers of Japanese patients were enrolled, the rates of herpes zoster in the Japanese subpopulations were higher than the rates in the overall populations. In study RA-BEGIN, the incidence of herpes zoster during 52 weeks was 10% (3/29) of the Japanese patients in the baricitinib 4-mg group, and 8% (3/39) in the baricitinib + methotrexate group versus 6% (2/36) in the methotrexate monotherapy group, compared to 3, 2 and <1%, respectively, in the overall study population. In study RA-BEAM, the incidence of herpes zoster during 24 weeks was 4% of the Japanese patients in the baricitinib 4-mg group, and 2% in the adalimumab group versus 0% in the placebo group, compared to 1, 1 and <1%, respectively, in the overall study population. As per the protocol, all patients who had herpes zoster were discontinued from the study drug. However, most events were non-severe and manageable, and most patients recovered after withdrawal of the study drug and use of antiviral therapy (data not shown). The rates in the Japanese subpopulations were higher across all active treatment groups in RA-BEGIN and RA-BEAM, including methotrexate and adalimumab, which is consistent with other reports, including those in patients treated with tofacitinib [Citation26]. In an analysis of patients with RA who were treated with tofacitinib, the highest rates of herpes zoster were observed in Japan and Korea, and lower rates were seen in Southeast Asia [Citation26]. The reasons underlying this pattern are presently unclear and will require further study.
The main strengths of these four subgroup analyses are that they are based on global phase 3 randomized controlled trials, and that these trials included patients with active RA across all stages of the RA treatment continuum. The numbers of Japanese patients in each study are a relevant limitation, particularly in RA-BUILD and RA-BEACON, which had fewer than 10 Japanese patients in each study arm; this limits the comparison of efficacy and safety for the 2-mg baricitinib and 4-mg baricitinib groups with the placebo group. However, much larger numbers of Japanese patients were included in RA-BEGIN and in particular in the methotrexate inadequate responder RA-BEAM study. In comparison to the phase 3 development programs of other approved therapies, the sample of approximately 400 Japanese patients enrolled in these global studies is comparatively large. The study durations (24–52 weeks) are too short to allow a robust evaluation of safety for rare events or those with long latency; however, a large majority (328 patients) of Japanese patients completing these studies enrolled in an ongoing long-term extension study (RA-BEYOND) which will enable these evaluations (Supplementary Figures S1–S4).
In conclusion, these data show that the efficacy and safety of baricitinib in Japanese patients who participated in its global phase 3 RA development program was generally consistent with that observed in the overall populations of RA-BEGIN, RA-BEAM, RA-BUILD, and RA-BEACON, which collectively included patients with RA who were methotrexate-naïve or showed inadequate response to methotrexate, csDMARDs, or bDMARDs. Overall, these data indicate that baricitinib has a favourable benefit–risk balance and may thus be considered as a potential treatment option for Japanese patients with moderately-to-severely active RA.
Conflict of interest
Y.T. has received research grants from Mitsubishi Tanabe Pharma, Takeda Pharmaceutical Company, Daiichi Sankyo Company, Chugai Pharmaceutical Company, Bristol-Myers Squibb, MSD, Astellas Pharma, Abbvie, and Eisai Company, and has received speaking fees and/or honoraria from Abbvie, Chugai Pharmaceutical Company, Daiichi Sankyo Company, Bristol-Myers Squibb, Mitsubishi Tanabe Pharma, Astellas Pharma, Takeda Pharmaceutical Company, Pfizer, Teijin Pharma, Asahi Kasei Corporation, YL Biologics, Sanofi, Janssen, Eli Lilly and Company, and GlaxoSmithKline. T.A. has participated in consultancies and advisory panels for Pfizer, UCB Japan Co, GlaxoSmithKline K.K., Integrated Development Associates, Daiichi Sankyo Company, Eli Lilly Japan K.K., and Sanofi K.K. K.A. has participated in consultancies and advisory panels for Pfizer Japan Inc., and is a member of the baricitinib advisory board for Eli Lilly Japan K.K. M.H. has participated in consultancies and advisory panels for Bristol-Myers Squibb K.K., Eisai Company, Ono Pharmaceuticals, Takeda Pharmaceutical Company, Mitsubishi Tanabe Pharma Co., and Eli Lilly and Company, and is the baricitinib advisor for Eli Lilly Japan K.K. T.I. and T.P.R. are employees of and own stock in Eli Lilly and Company. O.K. and N.A. are employees of Eli Lilly and Company. T.T. has received: grants from Astellas Pharma Inc, Bristol–Myers K.K., Chugai Pharmaceutical Co, Ltd., Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd., Teijin Pharma Ltd., AbbVie GK, Asahikasei Pharma Corp., Mitsubishi Tanabe Pharma Co., Pfizer Japan Inc., and Taisho Toyama Pharmaceutical Co., Ltd., Eisai Co., Ltd., AYUMI Pharmaceutical Corporation; speaking fees from AbbVie GK, Bristol–Myers K.K., Chugai Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Pfizer Japan Inc., Astellas Pharma Inc., and Diaichi Sankyo Co., Ltd.; and consultant fees Astra Zeneca K.K., Eli Lilly Japan K.K., Novartis Pharma K.K., Mitsubishi Tanabe Pharma Co., Abbivie GK, Nipponkayaku Co. Ltd., Janssen Pharmaceutical K.K., Astellas Pharma Inc., and Taiho Pharmaceutical Co., Ltd.
Role of the sponsor
Eli Lilly and Company was involved in the study design, data collection, data analysis, and preparation of the article.
MORH_1392057_Supplementary_Materials_akashi.docx
Download MS Word (597.1 KB)Acknowledgements
The authors would like to thank the patients and investigators for their participation in the study.
Additional information
Funding
References
- MTX clinical practice guidelines development subcommittee, Japan College of Rheumatology. Methotrexate (MTX) in the treatment of rheumatoid arthritis: clinical practice guidelines, 2011 edition (in Japanese). Available from: http://www.ryumachi-jp.com/info/img/MTX2011kanni.pdf [last assessed 24 Apr 2017].
- Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheum. 2016;68:1–26.
- European Medicines Agency. Guideline on clinical investigation of medicinal products other than NSAIDS for treatment of rheumatoid arthritis (draft); 2015. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/06/WC500187583.pdf [last accessed 24 Apr 2017].
- Koike R, Takeuchi T, Eguchi K, Miyasaka N. Update on the Japanese guidelines for the use of infliximab and etanercept in rheumatoid arthritis. Mod Rheumatol. 2007;17:451–8.
- Koike T, Harigai M, Ishiguro N, Inokuma S, Takei S, Takeuchi T, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of the first 3,000 patients. Mod Rheumatol. 2012;22:498–508.
- Miyasaka N, Takeuchi T, Eguchi K. Guidelines for the proper use of etanercept in Japan. Mod Rheumatol. 2006;16:63–7.
- Rubbert-Roth A, Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther. 2009;11(Suppl. 1):S1.
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–38.
- O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl. 2):ii111–15.
- Nakayamada S, Kubo S, Iwata S, Tanaka Y. Recent progress in JAK inhibitors for the treatment of rheumatoid arthritis. BioDrugs. 2016;30:407–19.
- Yamanaka H, Tanaka Y, Takeuchi T, Sugiyama N, Yuasa H, Toyoizumi S, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open-label, long-term extension study. Arthritis Res Ther. 2016;18:34.
- Meyer DM, Jesson MI, Li X, Elrick MM, Funckes-Shippy CL, Warner JD, et al. Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm. 2010;7:41.
- Fridman JS, Scherle PA, Collins R, Burn TC, Li Y, Li J, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol. 2010;184:5298–307.
- Norman P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin Investig Drugs. 2014;23:1067–77.
- Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509.
- Kubo S, Nakayamada S, Tanaka Y. Baricitinib for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol. 2016;12:911–19.
- Tanaka Y, Emoto K, Cai Z, Aoki T, Schlichting D, Rooney T, et al. Efficacy and safety of baricitinib in Japanese patients with active rheumatoid arthritis receiving background methotrexate therapy: a 12-week, double-blind, randomized placebo-controlled study. J Rheumatol. 2016;43:504–11.
- Dougados M, van der Heijde D, Chen YC, Greenwald M, Drescher E, Liu J, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis. 2017;76:88–95.
- Fleischmann R, Schiff M, van der Heijde D, Ramos-Remus C, Spindler A, Stanislav M, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheum. 2017;69:506–17.
- Genovese MC, Kremer J, Zamani O, Ludivico C, Krogulec M, Xie L, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243–52.
- Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376:652–62.
- Kochi Y, Suzuki A, Yamada R, Yamamoto K. Ethnogenetic heterogeneity of rheumatoid arthritis-implications for pathogenesis. Nat Rev Rheumatol. 2010;6:290–5.
- Ogasawara M, Tamura N, Onuma S, Kusaoi M, Sekiya F, Matsudaira R, et al. Observational cross-sectional study revealing less aggressive treatment in Japanese elderly than nonelderly patients with rheumatoid arthritis. J Clin Rheumatol. 2010;16:370–4.
- Yamanaka H, Tanaka Y, Inoue E, Hoshi D, Momohara S, Hanami K, et al. Efficacy and tolerability of tocilizumab in rheumatoid arthritis patients seen in daily clinical practice in Japan: results from a retrospective study (REACTION study). Mod Rheumatol. 2011;21:122–33.
- Koike T, Kentaro I. How can the treatment of rheumatoid arthritis be improved in Japan? Int J Clin Rheumatol. 2015;10:235–44.
- Winthrop KL, Yamanaka H, Valdez H, Mortensen E, Chew R, Krishnaswami S, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheum. 2014;66:2675–84.
