Abstract
IgG4-related diseases (IgG4-RDs), such as autoimmune pancreatitis and IgG4-related Mikulicz disease, are often accompanied by intrathoracic lesions, which are called IgG4-related respiratory disease (IgG4-RRD). IgG4-RRD has few subjective symptoms, and is usually detected during workup of patients with extra-thoracic lesions of IgG4-RD. IgG4-RRD is characterized by various conditions, including masses, nodules, thickening, and infiltration at numerous sites in the thorax through lymphatic routes. Although elevated serum IgG4 concentrations and pathologic evidence of lymphoplasmacytic infiltrates with abundant IgG4-positive plasma cells are characteristic findings of IgG4-RD, other intrathoracic diseases, such as multicentric Castleman disease and malignancy, may present with similar findings. Developing diagnostic criteria for IgG4-RRD, including clinicoradiological and pathological characteristics, is necessary for its appropriate diagnosis.
Introduction
IgG4-related disease (IgG4-RD) is a recently recognized systemic disease, characterized by tumefactive lesions with abundant IgG4-positive (IgG4+) plasma cell infiltrates and elevated serum IgG4 concentrations [Citation1]. The lesions in IgG4-RD frequently involve multiple organs, including the lacrimal glands, salivary glands, lungs, pancreas, gall bladder, kidneys, and retroperitoneum. IgG4-RD encompasses numerous conditions, previously recognized as Mikulicz’s disease, sclerosing pancreatitis (autoimmune pancreatitis [AIP]), Kuttner’s disease, tubulointerstitial nephritis, inflammatory pseudotumor, and retroperitoneal fibrosis [Citation2]. Various conditions have been identified at numerous sites in the thoracic area, including the mediastinal lymph nodes, bronchial walls, peribronchovascular bundles, alveolar septa, and pleura [Citation3,Citation4]. All lesions in the thorax have been comprehensively classified as IgG4-related respiratory disease (IgG4-RRD) [Citation5]. IgG4-RRD is a frequent complication in patients with extra-thoracic lesions, such as IgG4-related sialadenitis or pancreatitis.
Clinical features of IgG4-RRD
IgG4-RRD is more frequently diagnosed in middle-aged to elderly males than in other age groups or in women, with a median age at diagnosis of about 60 years [Citation6]. Although the frequency of intrathoracic lesions in IgG4-RD has not yet been accurately determined, an assessment of 125 patients with IgG4-RD found lung involvement in 22 (17.6%) [Citation7]. Similarly, lung involvement was reported in 78 (23.4%) of 334 patients with IgG4-RD [Citation8]. Because lung involvement in these studies was defined as clinically or radiographically detectable manifestations in the bilateral pulmonary area, the rate of lung involvement may be higher when mediastinal/bronchial manifestations are included (see Image findings).
Generally, patients with IgG4-RRD have few respiratory symptoms, and IgG4-RRD has been found incidentally during workup for extra-thoracic lesions or as an abnormal lung shadow. Forty to fifty percent of patients with IgG4-RD have a history of allergic rhinitis and/or bronchial asthma [Citation6,Citation9] and some have asthmatic symptoms, such as cough and wheezing, at onset [Citation10,Citation11].
Laboratory findings
Laboratory characteristics of patients with IgG4-RRD include extremely elevated serum concentrations of IgG, to >3000 mg/dl, and IgG4, to >1000 mg/dl, concentrations higher than those observed in patients with sialadenitis or autoimmune pancreatitis alone. Serum concentrations of soluble interleukin 2-receptor (sIL-2R) are also high, around 1000 U/ml. About 40–60% of patients with IgG4-RRD are positive for autoantibodies such as rheumatoid factor and antinuclear antibodies, and about 50% have low serum complement (CH50) concentrations. White blood cell counts and serum concentrations of C-reactive protein (CRP) are frequently within normal limits. Assays of bronchoalveolar lavage fluid showed lymphocyte predominance, but the CD4/CD8 lymphocyte ratios were non-specific for IgG4-RRD [Citation12].
Image findings
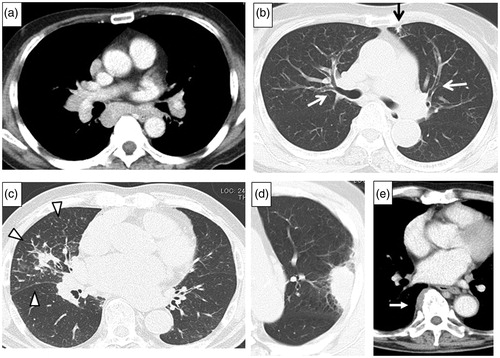
The most common findings on chest computed tomography (CT) are hilar and mediastinal lymphadenopathy, which are detected in 80% or more of patients with IgG4-RRD. Thickening of the bronchial wall and/or bronchovascular bundles are also common. Other characteristics on chest CT include nodules or consolidation, sometimes accompanied by thickening of the perilymphatic interstitium. Soft tissue masses in the paravertebral region are rarely observed but are characteristic of fibrosis in IgG4-RRD () [Citation12–14].
Figure 1. Chest CT scans of IgG4-RRD, showing. (a) Mediastinal lymphadenopathy. (b) Bronchial thickening in both upper lobes (white arrows), subpleural small consolidation in the left upper lobe (black arrow). (c) Interlobular thickening and infiltrative shadow in the right middle lobe (white arrowheads). (d) Mass-like consolidation in the left upper lobe. (e) Paravertebral thickening in the right lower lobe (white arrow).
Histopathologic findings
The histopathologic features of IgG4-RRD are identical to those of IgG4-RD, including dense lymphoplasmacytic and IgG4+ plasma cell infiltration; active fibrosis, typically a storiform pattern, and obliterative phlebitis. Obliterative arteritis has also been observed in patients with IgG4-RRD [Citation12,Citation15,Citation16]. Transbronchial lung biopsy (TBLB) samples are insufficient to detect these findings, whereas surgical lung biopsy specimens are needed for a definitive diagnosis [Citation17].
Clinical pulmonary manifestations
Various clinical conditions have been reported in patients with IgG4-RRD. Pulmonary lesions have been subclassified based on their intrathoracic compartments.
Airway manifestations
Airway manifestations may include rhino-sinusitis, asthmatic symptoms, bronchial tumors, and airway stenosis [Citation10,Citation18–20]. IgG4+ plasma cell infiltration has been observed in biopsies of the nasal mucosa of IgG4-RD patients with rhino-sinusitis and biopsies of the bronchial mucosa of IgG4-RD patients with asthmatic symptoms. These findings suggest a possible association between IgG4-RD and airway allergies [Citation10,Citation21].
Mediastinal manifestations
Hilar and mediastinal lymphadenopathy are the most common findings in IgG4-RRD. Mediastinal fibrosis may be associated with IgG4-RD and may be accompanied by systemic fibrosis such as retroperitoneal fibrosis [Citation22,Citation23].
Pulmonary parenchymal manifestations
Thickening of the perilymphatic interstitium, with or without subpleural and/or peribronchovascular consolidation, is very common because lung involvement spreads through lymphatic routes. Chest CT therefore frequently shows various manifestations, such as ground glass opacity (GGO), nodules, and dense irregular infiltrates. Mass-like lesions with high-grade accumulation of 18-fluorodeoxyglicose, mimicking lung cancer, have been observed in some patients [Citation24]. Lesions previously described as ‘inflammatory pseudotumors in the lung (plasma cell type)’, characterized by histopathologically active fibrosis and massive lymphoplasmacytic infiltration by IgG4+ plasma cells, are now regarded as being lesions of IgG4-RRD [Citation25].
Nonspecific interstitial pneumonia may be a manifestation of IgG4-RRD [Citation26], but diagnosis is difficult in the absence of extra-thoracic lesions of IgG4-RD [Citation27–29]. Organizing pneumonia also has been reported to occur in IgG4-RD [Citation30,Citation31].
Pleural manifestations
Pleural thickening and/or masses have been observed, with or without parenchymal lesions, and as well as pleural effusion, they are regarded as manifestations of IgG4-RRD. Pleural effusion may be unilateral or bilateral, with or without cardiac effusion [Citation32,Citation33]. Analysis of effusion fluid shows exudates containing lymphocytes and IgG4+ plasma cells. Generally, pleural lesions are accompanied by lung parenchymal lesions, but pleural lesions can occur alone.
Pulmonary vascular involvement
Pulmonary hypertension has been reported in several patients with IgG4-RD. Obliterative vasculitis with dense lymphoplasmacytic infiltrates may result in pulmonary hypertension [Citation34,Citation35].
Diagnostic criteria of IgG4-RRD
Comprehensive diagnostic criteria (CDC) for IgG4-RD were formulated in 2011 [Citation1]. Although these criteria include respiratory involvement, other diseases, including multicentric Castleman’s disease (MCD) meet these criteria and may be diagnosed as IgG4-RRD. To increase diagnostic specificity and to increase diagnostic sensitivity in patients without lung biopsy, diagnostic criteria have been proposed for IgG4-RRD [Citation5]. These include: (1) an abnormal shadow on chest CT; (2) serum IgG4 ≥ 135 mg/dl; (3) histopathologic features fulfilling CDC; and (4) the presence of extrathoracic lesions (). However, a typical storiform pattern indicative of fibrosis or obliterative vasculitis may be difficult to detect in TBLB samples, except for samples from mass lesions. To confirm a diagnosis of IgG4-RRD, clinico-radiological and pathological correlations are needed. The algorithm attached to the diagnostic criteria for IgG4-RRD may therefore be useful ().
Figure 2. Diagnostic algorithm.
CD: Castleman disease (plasma cell type); EGPA: eosinophilic granulomatosis with polyangiitis; SLE: systemic lupus erythematosus. *sclerosing dacryoadenitis・sialadenitis, autoimmune pancreatitis, IgG4-related sclerosing cholangitis, IgG4-related kidney disease, retroperitoneal fibrosis.
Reprinted permission from Reference [Citation5].
![Figure 2. Diagnostic algorithm. CD: Castleman disease (plasma cell type); EGPA: eosinophilic granulomatosis with polyangiitis; SLE: systemic lupus erythematosus. *sclerosing dacryoadenitis・sialadenitis, autoimmune pancreatitis, IgG4-related sclerosing cholangitis, IgG4-related kidney disease, retroperitoneal fibrosis.Reprinted permission from Reference [Citation5].](/cms/asset/0e8b287a-4bd7-4864-8f5a-7a693a02b200/imor_a_1548089_f0002_b.jpg)
Table 1. Summary of diagnostic criteria for IgG4-RRD.
Differential diagnosis
High serum IgG4 level and/or infiltrates of IgG4+ plasma cells occur not only in patients with IgG4-RRD but in patients with other pulmonary disorders. Major differential diagnoses include lung cancer, infection, collagen-vascular disease related interstitial pneumonia, malignant lymphoma, and lymphoproliferative disorders such as MCD ().
Table 2. Differential diagnosis for IgG4-RRD.
Some diseases, which are difficult to distinguish from IgG4-RRD, are described below.
Multicentric Castleman’s disease
MCD is a non-malignant lymphoproliferative disease with hyper IL-6 production. It may show mediastinal lymphadenopathy and GGO in bilateral lung fields with high concentrations of serum IgG4 and high levels of IgG4-positive lymphoplasmacytic infiltrates in lung tissues [Citation36,Citation37]. However, MCD may present with polyclonal hypergammopathy, with elevated serum CRP concentration and IL-6, and an absence of active fibrosis and lower amounts of eosinophil infiltrates in lung tissues unlike patients with IgG4-RRD.
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis
Major ANCA-associated vasculitis mimicking IgG4-RRD includes granulomatosis with polyangiitis (GPA) and eosinophilic granulomatosis with polyangiitis (EGPA) [Citation38–40]. Lung lesions in patients with GPA may show infiltration of IgG4+ plasma cells with vasculitis and characteristic parenchymal necrosis, whereas patients with EGPA may present with asthmatic symptoms and high serum IgG4 concentrations at onset. Patients with IgG4-RD are usually negative for serum ANCAs.
Sarcoidosis
Sarcoidosis is a systemic noncaseating granulomatous disorder that frequently affects the lungs, eyes, and skin. Serologically, serum angiotensin converting enzyme may increase, but elevation of serum IgG4 is rarely observed in patients with sarcoidosis. Imaging findings include mediastinal lymphadenopathy and thickening of peribronchovascular bundles. Clinically, sarcoidosis responds well to corticosteroid therapy or may show spontaneous remission. The clinical course of sarcoidosis resembles that of IgG4-RD, although noncaseating granuloma has not been observed in the latter [Citation41].
Lung cancer
Patients with IgG4-RD have been reported to be at high risk of malignancy, especially within one year after onset, suggesting that IgG4-RD is a para-neoplastic syndrome [Citation42]. An analysis of 334 patients found 67 malignancies in 57 patients, with lung cancer being the most frequent, being present in 12 patients [Citation8]. Additionally, an analysis of 294 patients with non-small cell lung cancer who underwent surgical resection found >20 IgG4+ plasma cells per high power field in 35 patients, with six of these 35 patients having an IgG4/IgG ratio >40% [Citation43]. These reports, suggesting a strong association between IgG4-RD and lung cancer, indicate the importance of strictly ruling out lung cancer at the diagnosis of IgG4-RRD, as well as subsequently monitoring these patients for the development of malignancy.
Nonspecific interstitial pneumonia
Idiopathic nonspecific interstitial pneumonia or collagen vascular disease related interstitial pneumonia may be characterized by elevated serum IgG4 and infiltration of IgG4+ plasma cells into lung tissues [Citation15,Citation27]. Differentiating these conditions from IgG4-RRD is very difficult, especially in patients with solitary IgG4-RD lesions in the lung. Careful diagnosis by a multidisciplinary team is required.
Rosai-Dorfman disease (RDD)
RDD, a disease characterized by non-clonal S100 positive histiocyte proliferation, shows a lymphangitic distribution on imaging and IgG4+ plasma cell infiltrates in tissues [Citation44]. Overlapping histopathologic findings of IgG4-RD and RDD have been observed in some patients [Citation45,Citation46]. Although RDD is rare, its relationship to IgG4-RD requires further study.
Treatment and prognosis
In IgG4-RRD, the goal of treatment is to maintain pulmonary function. Lung fibrosis and bronchial asthma may cause pulmonary dysfunction. Though there is no current consensus on the indication of treatment for IgG4-RRD, systemic corticosteroid therapy is recommended for patients with symptoms and with high disease activity. For asthma symptoms, inhaled corticosteroids may be selected, but the effect is limited [Citation10,Citation47].
The treatment of IgG4-RRD complies with Japanese consensus guidelines for the treatment of AIP. Initial treatment with 0.6 mg/kg/day prednisolone is recommended, followed by a gradual tapering to a maintenance dosage of 5 mg/day, depending on clinical improvements, biochemical markers, and imaging [Citation48]. Recommended biochemical markers include serum IgG4 and sIL2-R concentrations [Citation49].
An interim analysis of a prognostic survey of 77 cases by the respiratory working group of the Ministry of Health, Labor, and Welfare of Japan (MHLWJ) found that 86% of patients with IgG4-RRD had extrathoracic lesions and corticosteroid therapy was selected for 75%, with 90% of these patients showing improvement (Reports for MHLWJ 2014, unpublished in English). These findings suggest that the diagnosis be reviewed in patients with steroid-resistant disease.
Conclusion
Many lung diseases present with high serum IgG4 levels and high IgG4+ plasma cell infiltration. Both of these increases, however, are non-specific phenomena, resulting from an inflammatory reaction or a defensive reaction against an in vivo trigger. Because these phenomena are also observed in lung cancer, bronchial asthma and other conditions, careful observation is indispensable for the diagnosis of IgG4-RRD.
Conflict of interest
None.
Acknowledgements
The author thanks Dr. Hiroshi Yamamoto, Dr. Tomohiro Handa, Dr. Yuko Waseda, and Dr. Seijiro Minamoto for their contribution to the study of IgG4-RRD by the research teams from the Ministry of Health, Labor, and Welfare of Japan.
Additional information
Funding
References
- Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22(1):21–30.
- Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, et al. Research Program for Intractable Disease by Ministry of Health, Labor and Welfare (MHLW) Japan G4 Team. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22(1):1–14.
- Ryu JH, Sekiguchi H, Yi ES. Pulmonary manifestations of immunoglobulin G4-related sclerosing disease. Eur Respir J. 2012;39(1):180–6.
- Inoue D, Zen Y, Abo H, Gabata T, Demachi H, Kobayashi T, et al. Immunoglobulin G4-related lung disease: CT findings with pathologic correlations. Radiology 2009;251(1):260–70.
- Matsui S, Yamamoto H, Minamoto S, Waseda Y, Mishima M, Kubo K. Proposed diagnostic criteria for IgG4-related respiratory disease. Respir Investig. 2016;54(2):130–2.
- Matsui S, Taki H, Shinoda K, Suzuki K, Hayashi R, Tobe K, et al. Respiratory involvement in IgG4-related Mikulicz's disease. Mod Rheumatol. 2012;22(1):31–9.
- Wallace ZS, Deshpande V, Mattoo H, Mahajan VS, Kulikova M, Pillai S, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67(9):2466–75.
- Yamada K, Yamamoto M, Saeki T, Mizushima I, Matsui S, Fujisawa Y, et al. New clues to the nature of immunoglobulin G4-related disease: a retrospective Japanese multicenter study of baseline clinical features of 334 cases. Arthritis Res Ther. 2017;19(1):262.
- Masaki Y, Dong L, Kurose N, Kitagawa K, Morikawa Y, Yamamoto M, et al. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis. 2009;68(8):1310–5.
- Ito S, Ko SBH, Morioka M, Imaizumi K, Kondo M, Mizuno N, et al. Three cases of bronchial asthma preceding IgG4-related autoimmune pancreatitis. Allergol Int. 2012;61(1):171–4.
- Sekiguchi H, Horie R, Aksamit TR, Yi ES, Ryu JH. Immunoglobulin G4-related disease mimicking asthma. Can Respir J. 2013;20(2):87–9.
- Matsui S, Hebisawa A, Sakai F, Yamamoto H, Terasaki Y, Kurihara Y, et al. Immunoglobulin G4-related lung disease: clinicoradiological and pathological features. Respirology 2013;18(3):480–7.
- Inoue D, Yoshida K, Yoneda N, Ozaki K, Matsubara T, Nagai K, et al. IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore). 2015;94(15):e680.
- Fujinaga Y, Kadoya M, Kawa S, Hamano H, Ueda K, Momose M, et al. Characteristic findings in images of extra-pancreatic lesions associated with autoimmune pancreatitis. Eur J Radiol. 2010;76(2):228–38.
- Zen Y, Inoue D, Kitao A, Onodera M, Abo H, Miyayama S, et al. IgG4-related lung and pleural disease: a clinicopathologic study of 21 cases. Am J Surg Pathol. 2009;33(12):1886–93.
- Shrestha B, Sekiguchi H, Colby TV, Graziano P, Aubry MC, Smyrk TC, et al. Distinctive pulmonary histopathology with increased IgG4-positive plasma cells in patients with autoimmune pancreatitis: report of 6 and 12 cases with similar histopathology. Am J Surg Pathol. 2009;33(10):1450–62.
- Otani K, Inoue D, Itoh T, Zen Y. Transbronchial lung biopsy for the diagnosis of IgG4-related lung disease. Histopathology 2018;73(1):49–58.
- Yamamoto H, Yasuo M, Nomura Y, Agatsuma T, Ushiki A, Yokoyama T, et al. IgG4-related airway involvement which developed in a patient receiving corticosteroid therapy for autoimmune pancreatitis. Intern Med. 2011;50(24):3023–6.
- Ito M, Yasuo M, Yamamoto H, Tsushima K, Tanabe T, Yokoyama T, et al. Central airway stenosis in a patient with autoimmune pancreatitis. Eur Respir J. 2009;33(3):680.
- Moteki H, Yasuo M, Hamano H, Uehara T, Usami S. IgG4-related chronic rhinosinusitis: a new clinical entity of nasal disease. Acta Otolaryngol. 2011;131(5):518–26.
- Yamamoto M, Takahashi H, Shimizu Y, Yajima H, Suzuki C, Naishiro Y, et al. Seasonal allergies and serial changes of serum levels of IgG4 in cases treated with maintenance therapy for IgG4-related disease. Mod Rheumatol. 2016;26(1):161–2.
- Zen Y, Sawazaki A, Miyayama S, Notsumata K, Tanaka N, Nakanuma Y. A case of retroperitoneal and mediastinal fibrosis exhibiting elevated levels of IgG4 in the absence of sclerosing pancreatitis (autoimmune pancreatitis). Hum Pathol. 2006;37(2):239–43.
- Inoue M, Nose N, Nishikawa H, Takahashi M, Zen Y, Kawaguchi M. Successful treatment of sclerosing mediastinitis with a high serum IgG4 level. Gen Thorac Cardiovasc Surg. 2007;55(10):431–3.
- Kitada M, Matuda Y, Hayashi S, Ishibashi K, Oikawa K, Miyokawa N, et al. IgG4-related lung disease showing high standardized uptake values on FDG-PET: report of two cases. J Cardiothorac Surg. 2013;8:160.
- Zen Y, Kitagawa S, Minato H, Kurumaya H, Katayanagi K, Masuda S, et al. IgG4-positive plasma cells in inflammatory pseudotumor (plasma cell granuloma) of the lung. Hum Pathol. 2005;36(7):710.
- Tanaka K, Nagata K, Tomii K, Imai Y. A case of isolated IgG4-related interstitial pneumonia: a new consideration for the cause of idiopathic nonspecific interstitial pneumonia. Chest 2012;142(1):228–30.
- Takato H, Yasui M, Ichikawa Y, Fujimura M, Nakao S, Zen Y, et al. Nonspecific interstitial pneumonia with abundant IgG4-positive cells infiltration, which was thought as pulmonary involvement of IgG4-related autoimmune disease. Intern Med. 2008;47(4):291.
- Umeda M, Fujikawa K, Origuchi T, Tsukada T, Kondo A, Tomari S, et al. A case of IgG4-related pulmonary disease with rapid improvement. Mod Rheumatol. 2012;22(6):919–23.
- Ikeda S, Sekine A, Baba T, Okudela K, Iwasawa T, Sakai F, et al. Abundant immunoglobulin (Ig)G4-positive plasma cells in interstitial pneumonia without extrathoracic lesions of IgG4-related disease: is this finding specific to IgG4-related lung disease? Histopathology 2017;70(2):242–52.
- Taniguchi T, Hamasaki A, Okamoto M. A case of suspected lymphocytic hypophysitis and organizing pneumonia during maintenance therapy for autoimmune pancreatitis associated with autoimmune thrombocytopenia. Endocr J. 2006;53(4):563–6.
- Duvic C, Desrame J, Lévêque C, Nedelec G. Retroperitoneal fibrosis, sclerosing pancreatitis and bronchiolitis obliterans with organizing pneumonia. Nephrol Dial Transplant. 2004;19(9):2397.
- Rossi G, Marchioni A, Guicciardi N, Cadioli A, Cavazza A. Recurrent pleural and pericardium effusions in a white woman with IgG4-related syndrome. Am J Surg Pathol. 2009;33(5):802–3.
- Sekiguchi H, Horie R, Utz JP, Ryu JH. IgG4-related systemic disease presenting with lung entrapment and constrictive pericarditis. Chest 2012;142(3):781.
- Ishida M, Miyamura T, Sato S, Kimura D, Suematsu E. Pulmonary arterial hypertension associated with IgG4-related disease. Intern Med. 2014;53(5):493–7.
- Fukihara J, Kondoh Y, Taniguchi H, Kimura T, Kataoka K, Matsuda T, et al. Pulmonary hypertension associated with obliterative phlebitis in IgG4-related lung disease. Eur Respir J. 2015;45(3):842–5.
- Terasaki Y, Ikushima S, Matsui S, Hebisawa A, Ichimura Y, Izumi S, et al. Comparison of clinical and pathological features of lung lesions of systemic IgG4-related disease and idiopathic multicentric Castleman's disease. Histopathology 2017;70(7):1114–24.
- Otani K, Inoue D, Fujikura K, Komori T, Abe-Suzuki S, Tajiri T, et al. Idiopathic multicentric Castleman's disease: a clinicopathologic study in comparison with IgG4-related disease. Oncotarget 2018;9:6691–706.
- Chang SY, Keogh KA, Lewis JE, Ryu JH, Cornell LD, Garrity JA, et al. IgG4-positive plasma cells in granulomatosis with polyangiitis (Wegener's): a clinicopathologic and immunohistochemical study on 43 granulomatosis with polyangiitis and 20 control cases. Hum Pathol. 2013;44(11):2432–7.
- Yamamoto M, Tabeya T, Naishiro Y, Yajima H, Ishigami K, Shimizu Y, et al. Value of serum IgG4 in the diagnosis of IgG4-related disease and in differentiation from rheumatic diseases and other diseases. Mod Rheumatol. 2012;22(3):419–25.
- Perez Alamino R, Martínez C, Espinoza LR. IgG4-associated vasculitis. Curr Rheumatol Rep. 2013;15(8):348.
- Tsushima K, Tanabe T, Yamamoto H, Koizumi T, Kawa S, Hamano H, et al. Pulmonary involvement of autoimmune pancreatitis. Eur J Clin Invest. 2009;39(8):714–22.
- Shiokawa M, Kodama Y, Yoshimura K, Kawanami C, Mimura J, Yamashita Y, et al. Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol. 2013;108(4):610–7.
- Fujimoto M, Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Koyanagi I, et al. Stromal plasma cells expressing immunoglobulin G4 subclass in non-small cell lung cancer. Hum Pathol. 2013;44(8):1569–76.
- Roberts SS, Attanoos RL. IgG4+ Rosai-Dorfman disease of the lung. Histopathology 2010;56(5):662–4.
- de Jong WK, Kluin PM, Groen HM. Overlapping immunoglobulin G4-related disease and Rosai-Dorfman disease mimicking lung cancer. Eur Respir Rev. 2012;21(126):365–7.
- Hasegawa M, Sakai F, Okabayashi A, Katsura H, Kamata T, Koh E, et al. Rosai-Dorfman disease of the lung overlapping with IgG4-related disease: the difficulty in its differential diagnosis. Intern Med. 2017;56(8):937–41.
- To M, Kono Y, Soeda S, Hara H, Araki K, Kishi H, et al. A case of IgG4-related bronchial disease successfully treated with inhaled corticosteroids. J Allergy Clin Immunol Pract. 2016;4(1):168–70.
- Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, et al. Standard steroid treatment for autoimmune pancreatitis. Gut 2009;58(11):1504–7.
- Handa T, Matsui S, Yoshifuji H, Kodama Y, Yamamoto H, Minamoto S, et al. Serum soluble interleukin-2 receptor as a biomarker in immunoglobulin G4-related disease. Mod Rheumatol. 2018;28(5):838–44.
