Abstract
Systemic sclerosis (SSc) is a connective tissue disease, the pathogenesis of which is thought to involve interleukin-6 (IL-6), an inflammatory cytokine. This is based on findings of its concentration in patient serum, the results of an IL-6 suppression experiment in an animal model, and the results of a pilot study using IL-6 receptor antibody. However, it appears that a number of factors are involved in the pathology of SSc depending on the state of disease progression. In addition, the degree of involvement of IL-6 differs depending on the difference of organs within particular severe symptoms. Based on the findings from measurements of patient serum, the influence of IL-6 on the pathogenesis of SSc is greater in patients at a relatively early phase of the disease and in patients with lung lesions. Interleukin-13 (IL-13) is one of pro-fibrotic factors, and it is afraid that SSc patients with higher IL-13 have already lost the influence of IL-6. Therefore, although a clinical trial using the anti-IL-6 receptor antibody tocilizumab is underway, it is important to recognize the state of SSc patients prior to selecting treatment.
Interleukin-6
Interleukin (IL)-6 is a cytokine with diverse physiological activities. It was identified by Kishimoto et al. as B-lymphocyte stimulating factor in 1983 [Citation1]. Its cDNA was cloned by Hirano et al. in 1986 [Citation2]. It is the same molecule as interferon-β2, hybridoma-plasmacytoma growth factor [Citation3].
Physiologically, IL-6 is thought to be involved in the maturation of B lymphocytes and platelet production [Citation4]. It also promotes the proliferation of kidney mesangial cells and skin keratinocytes, as shown by a study on IL-6 transgenic mice [Citation4]. IL-6 is thought to be produced locally in inflammation. It is believed to be involved in the ‘swelling,’ ‘redness,’ and ‘pain’ of tissues, which are the pathological features of inflammation. IL-6 receptor (IL-6R) is expressed on hepatocytes, and IL-6 produced locally binds to these receptors on the liver and produces acute-phase proteins such as C-reactive protein (CRP) and serum amyloid A (SAA) [Citation5]. It also induces the production of fibrinogen in the liver, and an increase of fibrinogen in peripheral blood is expressed as an elevation of the sedimentation ratio. After the binding of IL-6 with IL-6R on hepatocytes, the signal-transducing molecule gp130 is phosphorylated and transduces a signal to the nucleus via the phosphorylation of signal transducer and activator of transcription 3 (STAT3) [Citation6,Citation7]. The soluble form of IL-6 receptor (sIL-6R) is present in serum and the binding of IL-6 with sIL-6R can also stimulate gp130, which induces a signal that promotes IL-6 function. Most viable cells in humans express gp130, so most cells can be stimulated by IL-6 [Citation8].
IL-6 is also known to be involved in the pathogenesis of various diseases. Castleman disease is a benign tumor in which the symptoms are chronic fever and increases of CRP, SAA level, platelet cell count, and γ-globulins. These symptoms are explainable in view of the functions of IL-6. Indeed, the level of IL-6 in patients with Castleman disease was reported to be high, but decreased after resection of the focal lymph node. Immunohistochemical analysis also revealed positivity for IL-6 staining in the germinal center of the resected lymph node [Citation9]. Rheumatoid arthritis (RA) shows swelling and pain in the vicinity of inflammation, and CRP elevation or blood sediment increase has been observed in blood tests in patients with this condition. These phenomena are thought to be caused by the biological functions of IL-6 [Citation10].
Systemic sclerosis
Systemic sclerosis (SSc) is a connective tissue disease characterized by fibrotic changes in the skin and internal organs. It is also representative of diseases in which IL-6 is involved in the pathogenesis. There are two types of SSc, limited cutaneous systemic sclerosis (lcSSc) and diffuse cutaneous systemic sclerosis (dcSSc), which are classified according to the area of skin affected [Citation11]. Patients with lcSSc usually show sclerotic changes on the fingers, dorsum of the hands, and forearms, while most patients with dcSSc show sclerotic changes on not only peripheral areas but also the trunk and the face. Serologically, anti-topoisomerase I antibody, which is also called anti-Scl-70 antibody, is often detected in the sera of patients with dcSSc; alternatively, anti-RNA polymerase III antibody is sometimes detected. Anti-centromere antibody is often detected in the sera of patients with lcSSc [Citation12,Citation13].
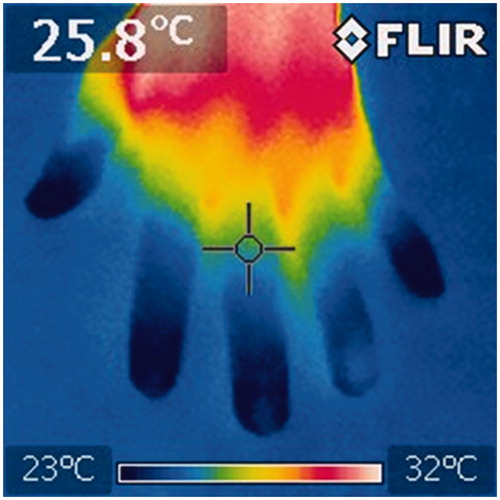
Raynaud’s phenomenon is the most common first symptom of SSc. Raynaud’s phenomenon has been believed to occur as a result of a spasm of blood vessels, resulting in ischemia (). Capillaroscopy analyzes have revealed that the shape of peripheral blood vessels become deformed and the number of them decreases [Citation14]. Disorders of peripheral vessels appear in the form of Raynaud’s phenomenon that arises in SSc [Citation15]. In such cases, cold stimulation causes the color of the patients’ fingers to change to white, purple, and then red. Since the supply of blood to the fingertips is insufficient, fingertip ulcers and fingerpad atrophy can occur there [Citation16].
Figure 1. Thermography image of the hand of an SSc patient. Patients with SSc have poor peripheral blood flow and have a constantly low hand temperature. In this picture, the skin temperature is under 26 °C in the area where the cross cursor is pointed.
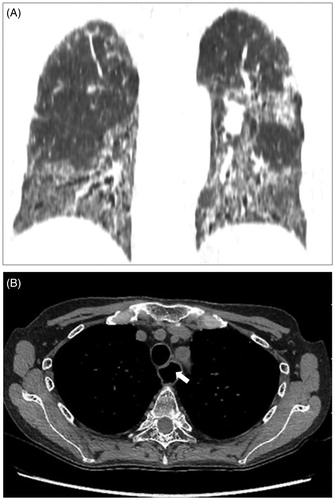
Fibrosis of the skin and subcutaneous tissue occurs, which results in hardening of the extremities. This limits the range of motion of each joint and impairs the activities of daily living [Citation17]. Tissue hardening is also observed in the lungs. Since the alveolar septal wall becomes fibrotic and thickened, inspiration becomes difficult and a restrictive lung disorder can develop. Moreover, this thickening of the alveolar septal wall results in a decrease in the gas diffusing ability. Therefore, decreases in the percent vital capacity (%VC) and in the percent diffusing capacity for carbon monoxide (%DLco) are observed when a respiratory function test is performed [Citation18]. In chest CT images, an elevation of density in the lung field is observed below the pleural area on the dorsal side of the lower lung field, and traction bronchial ectasia due to lung tissue contraction is observed (). Lungs have numerous capillary vessels, and damage to the lung capillaries results in disorder of pulmonary arterial circulation. This disorder can lead to the development of pulmonary hypertension. In SSc, the origin of pulmonary hypertension is complicated. This is because impaired hypoxemia associated with pulmonary fibrosis and cardiac wall motion accompanying myocardial fibrosis can also be causes of pulmonary hypertension.
Figure 2. Chest CT images of a patient with SSc. The coronal view presents sclerotic change, especially in the lower lung field (A). The axial mediastinum setting presents enlarged esophagus on the left side of the trachea (white arrow) (B).
Hardening and atrophy of the submucosal tissue and muscular layer of esophagus occur, which impair flexible movement of the esophagus [Citation19]. In addition, the esophageal diameter is increased due to the decrease of contractile force associated with fibrosis of the muscular layer. In chest CT in such cases, we can observe expansion of the esophageal lumen on the left side of the trachea (). In addition, owing to expansion and atrophic change, the long-axis diameter of the esophagus is shortened, inevitably resulting in esophageal hiatal hernia [Citation20]. Sclerosis of the lower esophageal sphincter makes it difficult to discharge undigested food into the stomach, and the digested food easily flows back to the esophageal side [Citation21]. The esophageal mucosa is constantly exposed to strong acids due to the backflow of gastric juice, and structural reflux esophagitis occurs. The fibrotic change of the lower intestinal tract reduces the abilities to perform digestion and absorption. Overall, patients with SSc have problems with their nutritional status [Citation22]. Owing to movement disturbances in the lower digestive tract, bowel movements tend to be stagnant. Some patients can develop pseudo-intestinal obstruction, called chronic intestinal pseudo-obstruction (CIPO), due to diminished peristalsis [Citation23]. This diminished peristalsis decreases the amount of bacteria excreted with the stools, and the associated excess proliferation of bacteria causes the retention of gas in the intestinal tract. This phenomenon is called small intestinal bacterial overgrowth (SIBO) () [Citation24].
Pathogenic factors deduced from pathological conditions of SSc
Many factors associated with the pathology of SSc have been reported. A representative cytokine among these factors is IL-1α. Kawaguchi et al. cultured the fibroblasts of an SSc patient and found an abnormally high level of pre-IL-1α production in the nucleus [Citation25].
Transforming growth factor (TGF)-β is also known as a stimulator of the synthesis of collagen by fibroblasts. Many studies have revealed the elevation of TGF-β level in bronchoalveolar lavage fluid [Citation26] and in tissue isolated from patients with SSc. Falanga et al. reported that cultured dermal fibroblasts from patients with SSc produced a higher amount of glycosaminoglycan than normal fibroblasts after stimulation by TGF-β [Citation27]. Given that Kubo et al. revealed the upregulation of TGF-β receptor on dermal fibroblasts isolated from SSc patients, it is possible that the fibroblasts of such patients exhibit an excessive response to TGF compared with that in healthy individuals [Citation28].
Platelet-derived growth factor (PDGF) is one of the factors discussed regarding a link to the pathogenesis of SSc, as it can stimulate the proliferation of fibroblasts and functions in wound healing [Citation29]. In 2006, Baroni et al. reported the presence of an agonistic antibody to PDGF receptor (PDGFR)-α and -β subunits [Citation30]. They asserted that the ligation of these receptors resulted in collagen production and myofibroblast conversion. Since PDGFR-α and -β are expressed on fibroblasts and it has been asserted that the activation of PDGFR-β in kidney mesangial cells is involved in the pathogenesis of glomerulosclerosis [Citation31], hyperactivity of PDGFR may be involved in the pathological state of fibrotic change of SSc. Thereafter, though the several reports which deny the existence of agonistic anti-PDGFR antibody have presented [Citation32,Citation33], the study through the inhibition of PDGF is continued.
Multiple factors have been suggested to be involved in the pathogenesis of SSc, as described above. However, to date, no successful treatments involving the inhibition of these factors have been reported. A multicenter, randomized, placebo-controlled phase I/II trial of anti-TGF-β1 antibody not only showed no effect on skin lesions, but also serious adverse events including 4 deaths occurred [Citation34]. Imatinib is a kinase inhibitor of both PDGFR and TGF-β, and a phase II randomized double-blind trial for imatinib did not identify a difference between the imatinib group and the placebo group [Citation35].
IL-6 and SSc
Patients with SSc show various symptoms, as described above, and it is reasonable to consider that multiple factors are responsible for their development. It is possible that various molecules establish a network to form such various symptoms, but another possibility is that the involved cytokines differ depending on the organs suffering the damage. A third possibility is that the main factors causing the pathological state change as the disease progresses. As described below, IL-6 may be involved in the pathogenesis of SSc in the early phase, and the lung may be the main target among the various symptoms.
Findings have suggested that IL-6 concentration is high in the sera of SSc patients. Before the establishment of the sandwich enzyme-linked immunosorbent assay (ELISA) system for IL-6, Needleman et al. applied bioassays to compare IL-6 levels in the sera of SSc patients to those of healthy people; they found that the sera of SSc patients often showed measurable levels of IL-6, while the IL-6 level in the sera of healthy people was not measurable by bioassays [Citation36]. Moreover, Giacomelli et al. measured IL-6 in sera from patients with SSc and healthy controls using a sandwich ELISA method. They found that IL-6 was detectable in 8 out of 20 SSc sera, but not in any healthy sera [Citation37]. Furthermore, Sato et al. analyzed 32 different cytokines and chemokines in sera from SSc patients; they found that IL-6 levels correlated with skin score, which represents the degree of skin involvement [Citation38].
One potential reason for the high IL-6 concentration is that its production may be high in local tissues. Feghali et al. conducted primary culture of the skin tissue isolated from SSc patients; they reported that their culture supernatant contained a high concentration of IL-6. They also analyzed the culture supernatant of skin fibroblasts isolated from an area of affected skin in a patient with SSc, which was shown to contain from 6- to 30-fold the concentration of IL-6 compared with unaffected skin or control skin fibroblasts [Citation39]. In contrast, Gurram et al. reported a higher amount of IL-6 production from peripheral mononuclear cells isolated from SSc patients than from healthy people. They cultured peripheral blood mononuclear cells obtained from patients with SSc and healthy controls, and found that the supernatant of peripheral blood mononuclear cells cultured with type I collagen contained a higher amount of IL-6 than that from controls [Citation40]. In the case of SSc, it is possible that IL-6 is produced even in tissues other than the damaged sclerotic tissue.
However, IL-6 is not high in the serum of all SSc patients, and it has been found that there is a difference in IL-6 concentration depending on the particular diseased organ and the disease duration. Hasegawa et al. analyzed the serum levels of IL-6, sIL-6R, and the soluble form of gp130 in cases categorized into early-phase (<3 years) dcSSc, early-phase lcSSc, late-phase (over 3 years) dcSSc, and late-phase lcSSc [Citation41]. They found that the serum samples from early-phase dcSSc contained higher levels of IL-6 than those from the other groups. Their report indicated that the serum IL-6 level differs depending on the disease duration, and IL-6 may act in the early phase of SSc.
Alpha-smooth muscle actin-positive fibroblasts, called ‘myofibroblasts,’ are observed in normally healing granulation tissue and hypertrophic scars and are believed to be related to wound healing [Citation42]. They are also observed in SSc-involved tissue, and it is thought that the excessive production of collagen from myofibroblasts induces the sclerotic change that occurs in SSc [Citation43]. The number of these cells in dermal tissue was reported to be correlated with the degree of skin sclerosis [Citation44]. Melendez et al. demonstrated that IL-6 converted fibroblasts to myofibroblasts in cardiac tissue [Citation45]. Moreover, Gallucci et al. reported that IL-6 induces the expression of α-smooth muscle actin protein in dermal fibroblast culture [Citation46]. Therefore, the existence of α-smooth muscle actin-positive myofibroblasts appears to be related to the functions of IL-6. Indeed, when we administered anti-mouse IL-6 receptor antibody to bleomycin SSc model mice, the number of α-smooth muscle actin-positive cells decreased [Citation47]. Interestingly, pathological examination revealed that myofibroblasts disappeared from the tissue of atrophic-stage SSc, while they were detected in the dermis of progressive-stage SSc [Citation48]. Considering together the findings that IL-6 involves the production of myofibroblasts and that myofibroblasts are observed more frequently in the early stage of the disease, it is thought that IL-6 is involved in the pathogenesis of SSc at a relatively early stage.
Furthermore, the degree of involvement of IL-6 may differ depending on the involved visceral organs. Hasegawa et al. also reported an inverse correlation between serum IL-6 levels and %VC in early-stage dcSSc [Citation49], while Scala et al. reported similar results. Specifically, they measured IL-6 concentration in the sera from 20 SSc patients and found that the samples from diffuse cutaneous-type SSc with lung involvement had higher concentrations of IL-6 [Citation50]. These reports indicate the possibility of IL-6 being involved in lung fibrosis, which is an important symptom frequently observed in SSc patients.
The papers mentioned above provide a strong foundation for the assertion that IL-6 is involved in the pathogenesis of SSc. Furthermore, Kawaguchi et al. reported the data of an in vitro experiment with anti-IL-6 antibody. They cultured skin fibroblasts isolated from SSc patients with or without anti-IL-6 antibody. The concentrations of type I procollagen in culture supernatants cultured with anti-IL-6 antibody were lower than those without the antibody [Citation25]. Their report suggested that the production of sclerotic skin components, i.e. extra-cellular matrix, was influenced by IL-6 inhibition. Upon summarizing these reports, the findings suggest that IL-6 functions strongly in early-stage SSc patients or in SSc patients with pulmonary lesions.
Anti-IL-6 receptor antibody, tocilizumab
Tocilizumab (TCZ) is an IgG antibody capable of binding to the IL-6 receptor; it was developed by Chugai Pharmaceutical Co., Ltd., Japan [Citation51]. TCZ can inhibit the binding of IL-6 to the IL-6 receptor. Patients with Castleman disease treated with TCZ showed decreases of CRP, SAA, and immunoglobulins, and patients with RA showed improvements of joint swelling, decrease of CRP, and correction of their augmented platelet counts. In one study performed in 2001, 28 patients with Castleman disease received TCZ. All of them presented decreases of inflammatory parameters such as CRP, fibrinogen, and SAA, and increases of hemoglobin and serum albumin. Moreover, CRP and fibrinogen were completely normalized in 18 (64.3%) and 20 (71.4%) patients, respectively [Citation52]. Nishimoto et al. also reported a multicenter, randomized, controlled trial of TCZ for the treatment of RA. In that trial, 306 patients with active RA were divided into two groups, one treated with conventional synthetic disease-modified anti-rheumatic-drugs (csDMARDs) and the other treated with TCZ. After treatment for 52 weeks, the TCZ group showed statistically significantly less radiographic change regarding the total modified Sharp score than the csDMARD group [Citation53]. These results indicate that TCZ has the capacity to inhibit IL-6’s functions.
Given that IL-6 is thought to play a pathological role in SSc, we administered TCZ to patients with refractory SSc, who had not responded to conventional therapy including steroids [Citation54]. As reported previously, the skin scores were decreased after TCZ administration. We also measured the skin properties using a Vesmeter device, which can measure skin hardness, elasticity, and viscosity [Citation55] to provide an objective evaluation of the skin. Vesmeter hardness also showed a decrease after TCZ administration. One patient had severe joint stiffness due to sclerosis of the skin and subcutaneous tissue at the extremities without arthritis. We measured the range of joint motion (ROM) of each joint every 3 months. This patient showed not only skin softening but also ROM improvements [Citation56].
In another study performed in 2014, 87 patients with SSc were administered TCZ or placebo to examine the therapeutic effect of TCZ, as a phase II randomized controlled trial (faSScinate study, NCT01532869). In the faSScinate study, the entry criteria are patients within 60 months of onset, based on the understanding that the inflammatory stage of SSc occurs early in the disease. The least mean square change of the corrected skin score at 24 weeks of administration did not differ between the TCZ group (−3.92) and the placebo group (−1.22) [Citation57]. At 48 weeks, there was a smaller number of patients with a reduction of %VC in the TCZ group. Although no statistically significant difference was reached, a tendency for the skin score to decrease in the TCZ-treated group was observed, and pulmonary lesion progression was observed to be slight in the TCZ group.
Based on these findings, the U.S. Food and Drug Administration (FDA) granted breakthrough therapy designation (BTD) status to TCZ for SSc. Moreover, to determine the effect of TCZ on patients with SSc, an expanded study using TCZ was initiated. This new expanded double-blinded multicenter trial is being conducted in 27 countries (focuSSced study, NCT02453256). While this focuSSced study is ongoing, the results of the interim analysis will be reported in fiscal 2018.
Problems with clinical trials for SSc
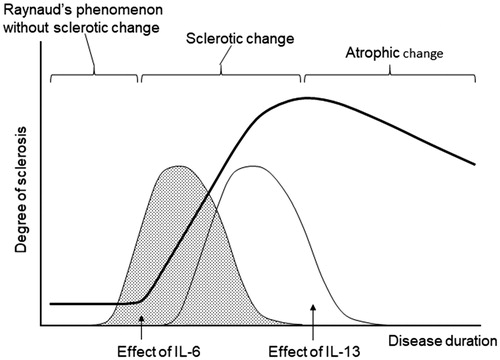
As mentioned above, IL-6 is thought to be a pathological factor of SSc. As such, it should be resolved why large-scale studies did not present distinct results, although the focuSSced study is still underway. Several problems remain to be resolved regarding clinical trials for SSc. The first problem is to determine what type of SSc patients are indicated for TCZ treatment. As indicated by the results from the previous studies, the response to TCZ varies. As a countermeasure against the first problem, it is conceivable to include a disease duration threshold in the inclusion criteria as in faSScinate study. However, as many clinicians are aware, the rate of disease progression differs from one patient to the next. To determine which patients are responsive to TCZ administration, we conducted an open-label study using TCZ [Citation58]. Fifteen SSc patients were divided into a group with TCZ supplementation and a group continuing with conventional therapy. TCZ was administered for 6 months, and changes of skin score were analyzed along with the baseline levels of cytokines, chemokines, adhesion molecules, and biochemical data for peripheral blood. In the results of this study, there were patients with a higher IL-6 level among those with a shorter disease duration, and the patients with a shorter disease duration and higher CRP had larger decreases in skin score. Furthermore, the decrease in skin score was negatively correlated with IL-13 and C–C motif chemokine ligand (CCL)5 (). IL-13 is a cytokine associated with post-inflammatory fibrosis, which is believed to be involved in the remodeling of bronchial tissue in patients with bronchial asthma [Citation59]. Hasegawa et al. reported increases in serum IL-4, IL-10, and IL-13 concentrations in SSc patients [Citation60], while Fuschiotti et al. found that increased serum IL-13 in SSc patients was correlated with skin fibrosis [Citation61]. Both studies demonstrated that serum IL-13 concentrations might depend on the extent of disease progression. In a survey of 13 cytokines, Gourh et al. reported increased serum IL-6 and IL-13 levels in SSc patients with pulmonary hypertension [Citation62]. SSc patients with high levels of serum IL-13 may be in the fibrotic stage and are considered to have lost reactivity to TCZ (). Therefore, it appears insufficient to set entry criteria of clinical trials based on the length of the medical history or the skin score, and it is desirable to determine whether trial entry is possible by measuring cytokines and chemokines that play roles in the pathology of SSc.
Figure 4. The relationship between disease duration and baseline IL-6 concentration, and between baseline IL-13 concentration and decreased count of mRSS after TCZ treatment. There were patients with a higher IL-6 level among those with a shorter disease duration. The patients showing higher decreases in mRSS counts during the TCZ treatment had lower levels of IL-13. mRSS: modified Rodnan skin score; IL-6: interleukin-6; IL-13: interleukin-13.
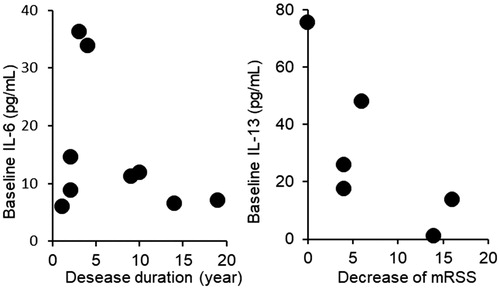
Figure 5. Schematic drawing of the correlation between sclerosis and the effect of IL-6. IL-6 plays a pathological role throughout the disease course in RA. However, pathological factors are substituted during the disease course of SSc.
The second problem is the evaluation methods used to determine the effects of TCZ. SSc is a systemic disease whose pathology is diverse, affecting regions such as the lungs, gastrointestinal tract, and blood vessels. However, most clinical trials have used the modified Rodnan skin score (mRSS) as the primary endpoint. Originally, in 1968, Rodnan presented a semi-quantitative evaluation method [Citation63]. The original method required skin evaluations at 26 areas, while the modified Rodnan skin score is simplified to 17 areas. The score is determined by examiners pinching the skin between their thumb and forefinger. It is clinically useful and does not require a specific device or electrical source, and its inter- and intra-observer variabilities are reported to be 17.7% and 20.7%, respectively [Citation64]. However, this evaluation method has the following problems: (a) there are only four different score levels for each skin area; (b) all areas are evaluated in the same manner, although the standard hardness of each area differs even among healthy people; (c) it has not been established whether the average or maximal (or minimal) score should be given when there is a mixture of different scores in the same area. Many researchers and clinicians are aware of these problems of using mRSS for evaluation in clinical trials, so various alternative devices have been proposed. The Durometer is one type of hardness gage [Citation65], and Cutometer is also available as a device to measure deformability [Citation66]. As an alternative to these, we have proposed a portable device called a Vesmeter, which can measure the skin viscosity, elasticity, and hardness [Citation55]. Unfortunately, none of these devices has become widely used due to problems such as high cost and a long time being required for measurement.
Recently, the Clinical Trials in Early Diffuse Cutaneous Systemic Sclerosis (CRISS) index was developed for a clinical trial of dcSSc [Citation67]. This index was developed with the aim of assessing whether the symptoms of SSc were improved when performing the intervention trial. It is composed of two steps. In step 1, the subject is asked about the occurrence of a new onset of renal crisis, new onset or worsening of lung fibrosis, new onset of pulmonary arterial hypertension, or new onset of left ventricular failure during the trial. If these are present, the patient is judged as being ‘not improved.’ The remaining cases move to step 2, in which the changes of mRSS, FVC%, patient’s global assessment, physician’s global assessment, and Health Assessment Questionnaire Disability Index (HAQ-DI) are calculated. If the result calculated in step 2 is 0.60 or more, the case is determined to be ‘improved.’ Since the CRISS index is an index determined by whether a kidney lesion appears or a lung lesion deteriorates during the observation period, it is not a value like DAS 28 in RA, which is used for judging disease activity in routine clinical practice. For clinical trials on SSc to be conducted in the near future, the CRISS index is supposed to be indispensable, but on the other hand, it is also necessary to create a composite measure to be used in daily clinical practice, or necessary to develop new medical devices that can be easily and conveniently used at low cost.
Conflict of interest
Y.S. received medicines from Chugai Pharmaceutical Co., Ltd. when conducting the multicenter trial described in Shima et al. [Citation58] and also received consultation fees from Genentech Inc./F. Hoffmann-La Roche Ltd. when the focuSSced study was planned.
References
- Okada M, Sakaguchi N, Yoshimura N, Hara H, Shimizu K, Yoshida N, et al. B cell growth factors and B cell differentiation factor from human T hybridomas. Two distinct kinds of B cell growth factor and their synergism in B cell proliferation. J Exp Med. 1983;157(2):583–90.
- Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces Bc lymphocytes to produce immunoglobulin. Nature. 1986;324(6092):73–6.
- Van Damme J, Opdenakker G, Simpson RJ, Rubira MR, Cayphas S, Vink A, et al. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin 1 and tumor necrosis factor. J Exp Med. 1987;165(3):914–9.
- Suematsu S, Matsusaka T, Matsuda T, Ohno S, Miyazaki J, Yamamura K, et al. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc Natl Acad Sci USA. 1992;89(1):232–5.
- Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci USA. 1987;84(20):7251–5.
- Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, et al. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58(3):573–81.
- Minami M, Inoue M, Wei S, Takeda K, Matsumoto M, Kishimoto T, et al. STAT3 activation is a critical step in gp130-mediated terminal differentiation and growth arrest of a myeloid cell line. Proc Natl Acad Sci USA. 1996;93(9):3963–6.
- Yawata H, Yasukawa K, Natsuka S, Murakami M, Yamasaki K, Hibi M, et al. Structure-function analysis of human IL-6 receptor: dissociation of amino acid residues required for IL-6-binding and for IL-6 signal transduction through gp130. Embo J. 1993;12(4):1705–12.
- Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, Aozasa K, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989;74(4):1360–7.
- Hirano T, Matsuda T, Turner M, Miyasaka N, Buchan G, Tang B, et al. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988;18(11):1797–801.
- LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15(2):202–5.
- Reveille JD, Solomon DH. Evidence-based guidelines for the use of immunologic tests: anticentromere, Scl-70, and nucleolar antibodies. Arthritis Rheum. 2003;49(3):399–412.
- Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35(1):35–42.
- Cutolo M, Herrick AL, Distler O, Becker MO, Beltran E, Carpentier P, et al. Nailfold videocapillaroscopic features and other clinical risk factors for digital ulcers in systemic sclerosis: a multicenter, prospective cohort study. Arthritis Rheumatol. 2016;68(10):2527–39.
- Flavahan NA. A vascular mechanistic approach to understanding Raynaud phenomenon. Nat Rev Rheumatol. 2015;11(3):146–58.
- Hachulla E, Clerson P, Launay D, Lambert M, Morell-Dubois S, Queyrel V, et al. Natural history of ischemic digital ulcers in systemic sclerosis: single-center retrospective longitudinal study. J Rheumatol. 2007;34(12):2423–30.
- Poole JL, Steen VD. The use of the Health Assessment Questionnaire (HAQ) to determine physical disability in systemic sclerosis. Arthritis Care Res. 1991;4(1):27–31.
- Steen VD, Conte C, Owens GR, Medsger TA. Jr., Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994;37(9):1283–9.
- Thoua NM, Bunce C, Brough G, Forbes A, Emmanuel AV, Denton CP. Assessment of gastrointestinal symptoms in patients with systemic sclerosis in a UK tertiary referral centre. Rheumatology. 2010;49(9):1770–5.
- Ebert EC. Esophageal disease in scleroderma. J Clin Gastroenterol. 2006;40(9):769–75.
- Weston S, Thumshirn M, Wiste J, Camilleri M. Clinical and upper gastrointestinal motility features in systemic sclerosis and related disorders. Am J Gastroenterol. 1998;93(7):1085–9.
- Caporali R, Caccialanza R, Bonino C, Klersy C, Cereda E, Xoxi B, et al. Disease-related malnutrition in outpatients with systemic sclerosis. Clin Nutr. 2012;31(5):666–71.
- Nunokawa T, Yokogawa N, Ohtsuka H, Shimada K, Sugii S. Transgastric long tube placement following percutaneous endoscopic gastrostomy for severe chronic intestinal pseudo-obstruction related to systemic sclerosis. Mod Rheumatol. 2015;25(6):958–61.
- Marie I, Ducrotté P, Denis P, Menard JF, Levesque H. Small intestinal bacterial overgrowth in systemic sclerosis. Rheumatology (Oxford). 2009;48(10):1314–9.
- Kawaguchi Y, Hara M, Wright TM. Endogenous IL-1alpha from systemic sclerosis fibroblasts induces IL-6 and PDGF-A. J Clin Invest. 1999;103(9):1253–60.
- Ludwicka A, Ohba T, Trojanowska M, Yamakage A, Strange C, Smith EA, et al. Elevated levels of platelet derived growth factor and transforming growth factor-beta 1 in bronchoalveolar lavage fluid from patients with scleroderma. J Rheumatol. 1995;22(10):1876–83.
- Falanga V, Tiegs SL, Alstadt SP, Roberts AB, Sporn MB. Transforming growth factor-beta: selective increase in glycosaminoglycan synthesis by cultures of fibroblasts from patients with progressive systemic sclerosis. J Invest Dermatol. 1987;89(1):100–4.
- Kubo M, Ihn H, Yamane K, Tamaki K. Upregulated expression of transforming growth factor-beta receptors in dermal fibroblasts of skin sections from patients with systemic sclerosis. J Rheumatol 2002;29(12):2558–64.
- Kaplan DR, Chao FC, Stiles CD, Antoniades HN, Scher CD. Platelet alpha granules contain a growth factor for fibroblasts. Blood. 1979;53(6):1043–52.
- Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354(25):2667–76.
- Floege J, Burns MW, Alpers CE, Yoshimura A, Pritzl P, Gordon K, et al. Glomerular cell proliferation and PDGF expression precede glomerulosclerosis in the remnant kidney model. Kidney Int. 1992;41(2):297–309.
- Classen JF, Henrohn D, Rorsman F, Lennartsson J, Lauwerys BR, Wikström G, et al. Lack of evidence of stimulatory autoantibodies to platelet-derived growth factor receptor in patients with systemic sclerosis. Arthritis Rheum. 2009;60(4):1137–44.
- Loizos N, Lariccia L, Weiner J, Griffith H, Boin F, Hummers L, et al. Lack of detection of agonist activity by antibodies to platelet-derived growth factor receptor alpha in a subset of normal and systemic sclerosis patient sera. Arthritis Rheum. 2009;60(4):1145–51.
- Denton CP, Merkel PA, Furst DE, Khanna D, Emery P, Hsu VM, et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2007;56(1):323–33.
- Prey S, Ezzedine K, Doussau A, Grandoulier AS, Barcat D, Chatelus E, et al. Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol. 2012;167(5):1138–44.
- Needleman BW, Wigley FM, Stair RW. Interleukin-1, interleukin-2, interleukin-4, interleukin-6, tumor necrosis factor alpha, and interferon-gamma levels in sera from patients with scleroderma. Arthritis Rheum. 1992;35(1):67–72.
- Giacomelli R, Cipriani P, Danese C, Pizzuto F, Lattanzio R, Parzanese I, et al. Peripheral blood mononuclear cells of patients with systemic sclerosis produce increased amounts of interleukin 6, but not transforming growth factor beta 1. J Rheumatol. 1996;23(2):291–6.
- Sato S, Hasegawa M, Takehara K. Serum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosis. J Dermatol Sci. 2001;27(2):140–6.
- Feghali CA, Bost KL, Boulware DW, Levy LS. Mechanisms of pathogenesis in scleroderma. I. Overproduction of interleukin 6 by fibroblasts cultured from affected skin sites of patients with scleroderma. J Rheumatol. 1992;19(8):1207–11.
- Gurram M, Pahwa S, Frieri M. Augmented interleukin-6 secretion in collagen-stimulated peripheral blood mononuclear cells from patients with systemic sclerosis. Ann Allergy. 1994;73(6):493.
- Hasegawa M, Sato S, Fujimoto M, Ihn H, Kikuchi K, Takehara K. Serum levels of interleukin 6 (IL-6), oncostatin M, soluble IL-6 receptor, and soluble gp130 in patients with systemic sclerosis. J Rheumatol. 1998;25(2):308–13.
- Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 2003;73(6):713–21.
- Sappino AP, Masouyé I, Saurat JH, Gabbiani G. Smooth muscle differentiation in scleroderma fibroblastic cells. Am J Pathol. 1990;137(3):585–91.
- Kissin EY, Merkel PA, Lafyatis R. Myofibroblasts and hyalinized collagen as markers of skin disease in systemic sclerosis. Arthritis Rheum. 2006;54(11):3655–60.
- Meléndez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56(2):225–31.
- Gallucci RM, Lee EG, Tomasek JJ. IL-6 modulates alpha-smooth muscle actin expression in dermal fibroblasts from IL-6-deficient mice. J Invest Dermatol. 2006;126(3):561–8.
- Kitaba S, Murota H, Terao M, Azukizawa H, Terabe F, Shima Y, et al. Blockade of interleukin-6 receptor alleviates disease in mouse model of scleroderma. Am J Pathol. 2012;180(1):165–76.
- Rajkumar VS, Howell K, Csiszar K, Denton CP, Black CM, Abraham DJ. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther. 2005;7(5):R1113–23.
- Hasegawa M, Fujimoto M, Matsushita T, Hamaguchi Y, Takehara K, Sato S. Serum chemokine and cytokine levels as indicators of disease activity in patients with systemic sclerosis. Clin Rheumatol. 2011;30(2):231–7.
- Scala E, Pallotta S, Frezzolini A, Abeni D, Barbieri C, Sampogna F, et al. Cytokine and chemokine levels in systemic sclerosis: relationship with cutaneous and internal organ involvement. Clin Exp Immunol. 2004;138(3):540–6.
- Mihara M, Nishimoto N, Ohsugi Y. The therapy of autoimmune diseases by anti-interleukin-6 receptor antibody. Expert Opin Biol Ther. 2005;5(5):683–90.
- Nishimoto N, Kanakura Y, Aozasa K, Johkoh T, Nakamura M, Nakano S, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood 2005;106(8):2627–32.
- Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66(9):1162–7.
- Shima Y, Kuwahara Y, Murota H, Kitaba S, Kawai M, Hirano T, et al. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology. 2010;49(12):2408.
- Kuwahara Y, Shima Y, Shirayama D, Kawai M, Hagihara K, Hirano T, et al. Quantification of hardness, elasticity and viscosity of the skin of patients with systemic sclerosis using a novel sensing device (Vesmeter): a proposal for a new outcome measurement procedure. Rheumatology. 2008;47(7):1018–24.
- Shima Y, Hosen N, Hirano T, Arimitsu J, Nishida S, Hagihara K, et al. Expansion of range of joint motion following treatment of systemic sclerosis with tocilizumab. Mod Rheumatol. 2015;25(1):134–7.
- Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet. 2016;387(10038):2630–40.
- Shima Y, Kawaguchi Y, Kuwana M. Add-on tocilizumab versus conventional treatment for systemic sclerosis, and cytokine analysis to identify an endotype to tocilizumab therapy. Mod Rheumatol. 2019;29(1)134–139.
- Doucet C, Brouty-Boyé D, Pottin-Clémenceau C, Canonica GW, Jasmin C, Azzarone B. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma. J Clin Invest. 1998;101(10):2129–39.
- Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol. 1997;24(2):328–32.
- Fuschiotti P, Medsger TA, Jr, Morel PA. Effector CD8+ T cells in systemic sclerosis patients produce abnormally high levels of interleukin-13 associated with increased skin fibrosis. Arthritis Rheum. 2009;60(4):1119–28.
- Gourh P, Arnett FC, Assassi S, Tan FK, Huang M, Diekman L, et al. Plasma cytokine profiles in systemic sclerosis: associations with autoantibody subsets and clinical manifestations. Arthritis Res Ther. 2009;11(5):R147.
- Rodnan GP, Lipinski E, Luksick J. Skin thickness and collagen content in progressive systemic sclerosis and localized scleroderma. Arthritis Rheum. 1979;22(2):130–40.
- Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22(7):1281–5.
- Merkel PA, Silliman NP, Denton CP, Furst DE, Khanna D, Emery P, et al. Validity, reliability, and feasibility of durometer measurements of scleroderma skin disease in a multicenter treatment trial. Arthritis Rheum. 2008;59(5):699–705.
- Nikkels-Tassoudji N, Henry F, Piérard-Franchimont C, Piérard GE. Computerized evaluation of skin stiffening in scleroderma. Eur J Clin Invest. 1996;26(6):457–60.
- Khanna D, Berrocal VJ, Giannini EH, Seibold JR, Merkel PA, Mayes MD, et al. The American College of Rheumatology provisional composite response index for clinical trials in early diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 2016;68(2):299–311.