Abstract
Objectives: To evaluate the efficacy and safety of adalimumab in psoriatic arthritis (PsA) patients in Japan.
Methods: In this open-label, single-arm study conducted at six sites from October 2014 to June 2016 (UMIN000016543), PsA patients (≥20 years old) with inadequate response to nonsteroidal anti-inflammatory drugs received adalimumab subcutaneously (80 mg initially, then 40 mg every other week; 24 weeks total). Primary endpoint was American College of Rheumatology 20% improvement (ACR20) response rate at week 12.
Results: Of 42 enrolled patients, 37 were treated (mean (SD) age, 56.2 (13.0) years; male, 27 (73.0%)). ACR20, ACR50, and ACR70 response rates were 40.5%, 24.3%, and 16.2% at week 12 and increased to 45.9%, 37.8%, and 21.6% at week 24, respectively. Psoriasis Area and Severity Index (PASI) 50 response rates were unchanged at weeks 12 and 24 (73%), but PASI75 and PASI90 increased from 40.5% and 21.6% to 59.5% and 40.5%, respectively. Other indices such as Physician’s Global Assessment score, C-reactive protein-based disease activity score in 28 joints, Bath Ankylosing Spondylitis Disease Activity Index, and serum biomarker levels were significantly improved. No unexpected adverse events were reported.
Conclusion: Similar to the global population, adalimumab was efficacious and well tolerated in Japanese treatment-experienced PsA patients.
Introduction
Psoriatic arthritis (PsA) is a chronic, inflammatory arthritis that occurs in individuals with psoriasis and is characterized by synovitis, enthesitis, dactylitis, and spondylitis [Citation1,Citation2]. Globally, the prevalence of PsA among patients with psoriasis ranges from 6% to 42%, depending on the population and the method of diagnosis [Citation3]. Notably, the prevalence of PsA among Japanese patients with psoriasis is lower (10–14%) [Citation4,Citation5] than that in Western countries (9–41%) [Citation6,Citation7], possibly because of differences in genotypic backgrounds between Japanese and Caucasian populations. Disease onset typically occurs between 30 and 55 years of age and affects men and women equally [Citation2]. In most (approximately 75–80%) patients, PsA is diagnosed about 7–12 years after psoriasis develops [Citation1].
Early treatment with disease-modifying antirheumatic drugs (DMARDs) can potentially slow disease progression and lessen deterioration of patient quality of life (QoL) [Citation1]. Conventional treatments used to manage psoriasis such as methotrexate, sulfasalazine, and leflunomide are also used for PsA. However, these agents are often inadequately effective, provide temporary benefit, and may be associated with substantial safety concerns [Citation1].
Tumor necrosis factor-α (TNF-α) is pivotal to the chronic inflammation and aberrant immune responses associated with rheumatoid arthritis (RA), psoriasis, and PsA, and is present in higher concentrations in the joints and skin of patients with PsA than those without [Citation8,Citation9]. In addition to the aforementioned genotypic differences, population differences exist with regard to an association between TNF-α gene polymorphisms and PsA; for example, an association was not found in Japanese studies but was observed, especially with regard to joint erosion in early PsA, in studies involving Caucasians [Citation10]. Biologic agents that inhibit TNF-α (e.g. etanercept, infliximab, and adalimumab) are efficacious in the treatment of RA, psoriasis, and PsA either as monotherapy or in combination with methotrexate [Citation1,Citation2,Citation11–16]. In a study in a clinic setting, methotrexate was associated with a significant increase in radiographic damage compared to TNF-α blockers in patients with erosive PsA [Citation17]. Moreover, according to the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for PsA, TNF-α inhibitors are strongly recommended in patients with inadequate response to DMARDs or other biologics, regardless of clinical manifestation (peripheral PsA, axial PsA, enthesitis, dactylitis, psoriasis, and nail psoriasis) [Citation18].
In addition to TNF-α, the interleukin (IL)-23/IL-17 axis is also involved in the pathogenesis of PsA. Therefore, other biologics, including anti-IL-17 and anti-IL-12/IL-23 agents such as secukinumab, brodalumab, ixekizumab, and ustekinumab, are increasingly being used to treat PsA when TNF-α inhibitors are ineffective or not tolerated [Citation1,Citation19]. Based on the guidelines by the American College of Rheumatology (ACR) and the National Psoriasis Foundation [Citation20], TNF-α inhibitors are preferred over DMARDs or other biologics for treatment-naïve patients with active PsA. In patients with an inadequate response to DMARDs, a TNF-α inhibitor should be used and, when active disease persists, a switch to an IL-17 or IL-12/IL-23 inhibitor should be considered.
Since being approved for use in RA by the United States Food and Drug Administration more than 15 years ago, adalimumab has been approved in more than 90 countries for 14 indications [Citation21]. In Japan, adalimumab was first approved in 2008 for RA and in 2010 for PsA [Citation22]. The efficacy of adalimumab vs. placebo in patients with moderate to severe active PsA and a history of inadequate response to nonsteroidal anti-inflammatory drugs (NSAIDs) was demonstrated in the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT; N = 315) [Citation2,Citation23]. At week 12, the primary endpoint of the study was met; American College of Rheumatology 20% improvement (ACR20) response rate was significantly higher among adalimumab-treated than among placebo-treated patients (58% vs. 14%, p < .001). Adalimumab significantly improved joint and skin manifestations, inhibited structural changes on radiographs, lessened disability due to joint damage, and improved QoL in patients with moderate to severe active PsA.
Although there is ample evidence for the efficacy of adalimumab for the treatment of PsA worldwide [Citation24,Citation25], there are limited data in Japan. We conducted a study to confirm the efficacy and safety of adalimumab in patients with PsA and inadequate response to NSAIDs in Japan.
Patients and methods
Study design
This prospective, open-label, single-arm study was conducted at six sites from October 2014 to June 2016 (UMIN ID 000016543). The study was conducted in accordance with the Declaration of Helsinki. The study was approved by local ethics committees at all participating sites and was registered in the University Hospital Medical Information Network database (UMIN000016543). Written informed consent was provided by all patients before study start.
Patients
Inclusion criteria
Patients aged 20 years or older diagnosed with PsA in accordance with ClASsification criteria for Psoriatic ARthritis (CASPAR) criteria and inadequate response to treatment with NSAIDs were eligible for inclusion. Patients being treated with NSAIDs, DMARDs, or oral corticosteroids must have received treatment at a stable dose for at least 4 weeks before study start.
Exclusion criteria
Patients with other active skin disease (possibly interfering with the evaluation of psoriasis-related skin symptoms), serious infection, active tuberculosis, a history of hypersensitivity to any component of the study drug, a history of or currently having demyelinating disease (e.g. multiple sclerosis), or congestive heart failure were excluded. Patients who received intra-articular or intratendinous steroid injection within 4 weeks, infliximab within 8 weeks, ustekinumab within 12 weeks, or other biologics (including adalimumab) within 24 weeks of study start, or who were receiving ultraviolet radiation therapy or taking cyclosporin or etretinate at study start were excluded. Patients judged by investigators as inappropriate for study participation were also excluded.
Treatment
Adalimumab was administered subcutaneously at an initial dose of 80 mg, then every other week at a dose of 40 mg for a total of 24 weeks. Administering an 80-mg dose after the initial dose was avoided to match the dosing in ADEPT [Citation2]. Concomitant use of NSAIDs, DMARDs, and oral corticosteroids was permitted if the dose was kept constant from 4 weeks before the study start to completion. Intra-articular or intratendinous steroid injection and use of other biologics, ultraviolet radiation therapy, cyclosporin, or etretinate after study start was prohibited.
Assessments and endpoints
Patients’ background information was collected at baseline. All other assessments were performed at baseline, week 12, week 24, and/or upon discontinuation.
Efficacy
The primary endpoint was ACR20 response rate at week 12. Secondary endpoints included ACR20 response rate at week 24; ACR50/70 and PASI50/75/90 response rates at weeks 12 and 24; and change from baseline to weeks 12 and 24 in C-reactive protein (CRP), CRP-based disease activity score in 28 joints (DAS-28CRP), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Physician’s Global Assessment (PGA) score, and biomarker (anti-cyclic citrullinated peptide (CCP) antibody, serum amyloid protein A (SAA), and matrix metalloproteinase (MMP-3)) levels. All assessments requiring articular manifestation, including ACR20 and DAS-28CRP, were performed by dermatologists with experience in those assessments (e.g. participating in multiple clinical trials for PsA, hosting PsA workshops, and having a training certificate from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis).
QoL
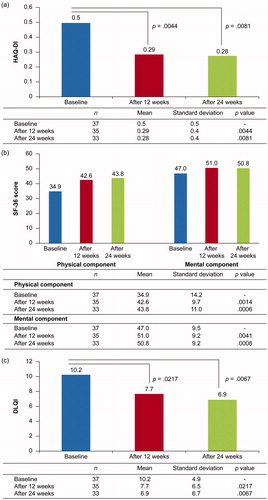
Change from baseline in the HAQ-Disability Index (HAQ-DI), short form-36 (SF-36), and Dermatology Life Quality Index (DLQI) scores at weeks 12 and 24 was assessed.
Safety
Incidence of adverse events (AEs) was monitored throughout the study.
Statistical analysis
The analysis population comprised all patients enrolled in the study, except for those with significant violation of ethics, not treated with adalimumab, and for whom efficacy was not evaluated.
Summary statistics, represented as mean (standard deviation; SD) of baseline characteristics, as well as ACR and PASI response rates with 95% confidence intervals (CIs), were calculated. Summary statistics of PGA, BASDAI, HAQ-DI, SF-36, and DLQI scores, DAS-28CRP, and CRP, SAA, MMP-3, and anti-CCP antibody levels were calculated, and changes from baseline to weeks 12 and 24 were assessed using the paired t test.
All statistical analyses were performed using SAS version 9.4 (Cary, NC).
Results
Demographics and baseline characteristics
Of 42 enrolled patients, 39 were included in the study (). Three patients were excluded as they did not meet the inclusion criteria (not meeting the CASPAR criteria, n = 1; not having received treatment at a stable dose of NSAIDs, DMARDs, or oral corticosteroids for at least 4 weeks before study start, n = 2). Of these, 37 patients were treated (analysis population), and 33 completed the study. Among the analysis population, mean (SD) age was 56.2 (13.0) years and most (73.0%) patients were male (). Mean duration of PsA was 119.4 (99.8) months, and mean number of tender and swollen joints was 10.7 (12.3) and 10.8 (11.1), respectively. Twenty-nine (78.4%) patients had comorbidities. All patients used concomitant medications, most commonly NSAIDS (22 (59.5%)), followed by DMARDs (10 (27.0%)) and biologics (9 (24.3%)). According to the Moll and Wright classification [Citation26], many patients had symmetric polyarthritis (11 (29.7%)), distal interphalangeal arthropathy (9 (24.3%)), or asymmetric oligoarthritis and spondylitis (8 (21.6%) each). Only one (2.7%) patient had arthritis mutilans. More than half of patients had inflammation of fingers (right, 20 (54.1%); left, 21 (56.8%)), spondylitis (20 (54.1%)), and inflammation of cervical spine (20 (54.1%)). In patients with previous use of biologics (n = 9), the mean (SD) tender and swollen joint counts were numerically lower than those in patients without previous use of biologics (n = 28): 7.9 (7.9) and 8.4 (6.2), respectively, vs. 11.6 (13.3) and 11.5 (12.2), respectively. Mean (SD) duration of PsA was comparable between patients with and without previous use of biologics: 128.3 (69.1) vs. 116.5 (108.7) months.
Figure 1. Patient disposition. Three patients were excluded as they did not meet the inclusion criteria (not meeting the CASPAR criteria, n = 1; not having received treatment at a stable dose of NSAIDs, DMARDs, or oral corticosteroids for at least 4 weeks before study start, n = 2). CASPAR: ClASsification criteria for Psoriatic Arthritis; DMARDs: disease-modifying antirheumatic drugs; NSAIDs: nonsteroidal anti-inflammatory drugs.
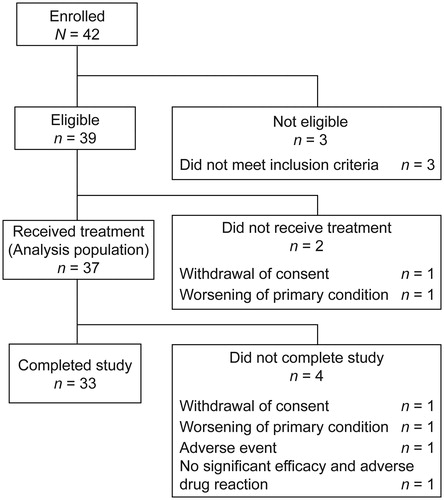
Table 1. Patient demographics and baseline characteristics (FAS population).
Efficacy
ACR20 response rate (95% CI) was 40.5% (24.8–57.9) at week 12 (primary endpoint) and increased to 45.9% (29.5–63.1) at week 24 (). Similarly, ACR50 and ACR70 response rates increased from 24.3% and 16.2% at week 12 to 37.8% and 21.6% at week 24, respectively (). Based on the subanalysis of ACR response rate by previous use of biologics, no notable differences were observed in ACR20, ACR50, and ACR70 response rates between patients with and without previous use of biologics at both weeks 12 and 24 (). Of note, statistical comparison was not feasible due to the insufficient number of patients in each subgroup. PASI50 response rate did not change from week 12 to 24 (73.0%) (). However, PASI75 and PASI90 response rates increased from 40.5% and 21.6% at week 12 to 59.5% and 40.5% at week 24, respectively (). Subanalysis of PASI response rate by previous use of biologics also showed that the rates were similar between patients with and without previous use of biologics ().
Figure 2. Efficacy outcomes during treatment of psoriatic arthritis with adalimumab (FAS population). (a) Percentage of patients with psoriatic arthritis who met the ACR20, ACR50, and ACR70 response criteria at weeks 12 and 24. (b) Subanalysis of the percentage of patients with psoriatic arthritis who met the ACR20, ACR50, and ACR70 response criteria at weeks 12 and 24 by previous use of biologics. (c) Percentage of patients with psoriatic arthritis who met the PASI50, PASI75, and PASI90 response criteria at weeks 12 and 24. (d) Subanalysis of the percentage of patients with psoriatic arthritis who met the PASI50, PASI75, and PASI90 response criteria at weeks 12 and 24 by previous use of biologics. (e) PGA scores in patients with psoriatic arthritis at weeks 12 and 24. ACR: American College of Rheumatology; CI: confidence interval; FAS: full analysis set; PASI: Psoriasis Area and Severity Index; PGA: Physician’s Global Assessment.
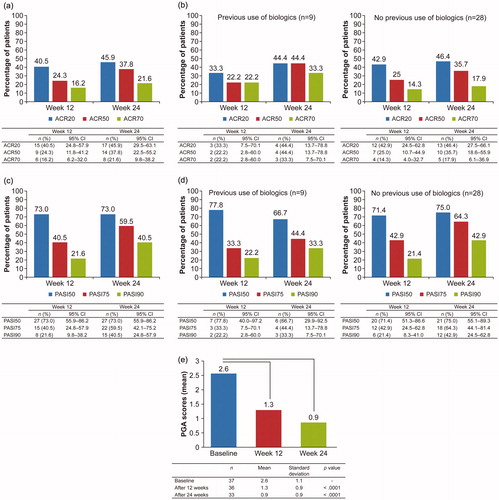
Mean PGA scores decreased from 2.6 at baseline to 1.3 and 0.9 at weeks 12 and 24, respectively (p < .0001), indicating improvement in disease severity (). Mean serum CRP levels decreased from 0.5 mg/dL at baseline to 0.1 mg/dL and 0.08 mg/dL at weeks 12 and 24 (p < .0001; ) and mean DAS-28CRP decreased from 3.9 at baseline to 2.4 and 2.1 at weeks 12 and 24, respectively (p < .0001; ), indicating reduced systemic inflammation. Patients experienced symptom alleviation as indicated by decreased BASDAI scores (3.8 at baseline to 2.3 at week 12 (p = .0046) and 1.7 at week 24 (p < .0001); ). Significant reductions in mediators of inflammation (SAA: from 104.5 µg/mL at baseline to 17.3 µg/mL at week 12 (p = .022) and 18.6 µg/mL at week 24 (p = .037); MMP-3: from 148.6 ng/mL at baseline to 70.1 ng/mL at week 12 (p = .0076) and 61.0 ng/mL at week 24 (p = .0083); ) also were observed. Anti-CCP antibody levels were comparable at all time points.
Figure 3. DAS-28CRP (a) and BASDAI (b) scores at weeks 12 and 24 (FAS population). BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; DAS-28CRP: Disease Activity Score-28 CRP; FAS: full analysis set.
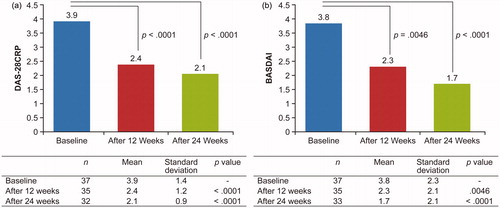
Table 2. Serum biomarker levels at weeks 12 and 24.
QoL
Mean HAQ-DI score decreased from 0.5 at baseline to 0.29 at week 12 (p = .0044) and 0.28 at week 24 (p = .0081) (), and SF-36 physical and mental component scores significantly increased (physical: from 34.9 at baseline to 42.6 at week 12 (p = .0014) and 43.8 at week 24 (p = .0006); mental: from 47.0 at baseline to 51.0 at week 12 (p = .0041) and 50.8 at week 24 (p = .0008); ). Mean DLQI score also significantly decreased from 10.2 at baseline to 7.7 at week 12 (p = .0217) and 6.9 at week 24 (p = .0067) (). Collectively, these findings indicate an improvement in QoL.
Safety
Overall, AEs were reported in 24 (64.9%) patients and treatment-related AEs in 19 (51.4%) patients. Most frequently (≥5%) reported AEs included elevated liver enzymes (5 (13.5%)), abnormal Krebs von den Lungen-6 (KL-6) glycoprotein (2 (5.4%)), lactate dehydrogenase (LDH) increase (2 (5.4%)), pharyngitis (2 (5.4%)), worsening of primary condition (2 (5.4%)), ringworm (2 (5.4%)), headache (2 (5.4%)), and fever (2 (5.4%)) (). Most frequently (≥5%) reported treatment-related AEs were abnormal KL-6 (2 (5.4%)), LDH increase (2 (5.4%)), pharyngitis (2 (5.4%)), elevated liver enzymes (5 (13.5%)), headache (2 (5.4%)), and fever (2 (5.4%)). A detailed list of AEs and ADRs is included as supplementary material. All AEs were mild or moderate in severity.
Table 3. Commonly (≥5%) reported adverse events.
Discussion
To our knowledge, this is the first prospective, multicenter study to evaluate the efficacy, QoL, and safety of adalimumab for PsA solely in Japanese psoriasis patients. We designed the present study to reflect clinical settings in Japan and to confirm results from ADEPT, which was a global study of adalimumab in patients with PsA [Citation2]. Patients were closely matched with those in ADEPT barring a few exceptions.
Overall, ACR20, ACR50, and ACR70 response rates were comparable to ADEPT, although ACR20 at week 12 was slightly higher in ADEPT than the current study (week 12, 58%, 36%, and 20% vs. 40.5%, 24.3%, and 16.2%, respectively; week 24: 57%, 39%, and 23% vs. 45.9%, 37.8%, and 21.6%, respectively). In contrast, PASI50, PASI75, and PASI90 response rates were generally similar in ADEPT and the present study (week 12: 72%, 49%, and 30% vs. 73.0%, 40.5%, and 21.6%, respectively; week 24: 75%, 59%, and 42% vs. 73.0%, 59.5%, and 40.5%, respectively). PASI is a tool that evaluates psoriatic lesions in terms of affected area and severity. In the present study, most patients visited a dermatologist primarily for treatment of skin manifestations rather than articular symptoms. Baseline mean (SD) PASI scores were lower in ADEPT than the current study: 7.4 (6.0) vs. 11.6 (13.4). In contrast, the mean (SD) tender joint count and swollen joint count, which are components of the ACR response criteria, were higher in ADEPT compared with the current study: 23.9 (17.3) vs. 10.7 (12.3) and 14.3 (12.2) vs. 10.8 (11.1), respectively. We speculate that the difference in patient background characteristics may have affected these responses.
ACR and PASI response rates were comparable between patients with and without previous use of biologics in the current study. These findings contrast with those from the 12-week, open-label ACCLAIM study (N = 127) [Citation27]. In that study, previous use of biologics did not affect ACR response rate but did significantly affect the PASI response rate. The fact that the current study included patients with an inadequate response or intolerance specifically to NSAIDs whereas ACCLAIM included patients with an inadequate response or intolerance to any prior therapies for PsA may possibly have contributed to the difference in the effect of previous use of biologics on ACR and PASI response. Therefore, further studies in larger populations are required to confirm the effects of previous biologic use on ACR and PASI responses.
In terms of QoL, improvements were comparable in ADEPT and the current study based on mean changes from baseline in HAQ-DI, SF-36, and DLQI scores. Mean HAQ-DI score decreased by 0.4 and 0.2, respectively. At week 12, the physical component of the SF-36 score had increased by 9.3 and 7.2, respectively; at week 24, the score had remained constant in ADEPT but increased by 9.0 in the current study. The DLQI score decreased by 6.1 in ADEPT and 3.3 in the current study at 24 weeks.
In addition to the above parameters, additional parameters were used to assess efficacy and QoL in the current study. Indicators of disease severity (PGA scores ()), systemic inflammation (CRP () and DAS28-CRP levels ()), and symptoms (BASDAI scores ()), as well as two of three inflammatory biomarker levels (SAA, MMP-3 ()), significantly decreased over time supporting the efficacy of adalimumab in our patient population. Mean PGA score decreased from 2.6 at baseline to 1.3 at week 12 and 0.9 at week 24; this result corresponded to improvement from a ‘mild-to-moderate’ to an ‘almost clear’ and ‘clear’ condition, respectively [Citation28]. In a study of predictors of a good clinical response to adalimumab, an association between serum CRP levels and achievement of ACR50 response at week 12 (p = .035) was found [Citation29]. Furthermore, mean (SD) serum CRP levels in the current study were lower than those reported in a retrospective study of Japanese patients with PsA successfully treated with adalimumab for at least 3 months: 12 weeks, 0.1 (5.4) mg/dL vs. 0.46 (0.81) mg/dL and 24 weeks, 0.08 (4.6) mg/dL vs. 0.47 (1.07) mg/dL. This may be attributed to the higher PASI levels at baseline in the aforementioned study vs. the current study, i.e. 12.2 (12.9) vs. 11.6 (13.4), because PASI levels of 12 or more were shown to be associated with significant elevation of serum CRP [Citation30]. In addition, reduction in DAS-28CRP levels at week 24 in the current study was comparable to that in an observational, real-world study in patients with PsA receiving anti-TNF treatment (1.9 vs. 1.7, respectively) [Citation31].
Finally, mean serum concentrations of SAA and MMP-3 decreased from 104.5 µg/mL to 18.6 µg/mL and from 148.6 ng/mL to 61.0 ng/mL at baseline and 24 weeks, respectively. SAA is involved in joint destruction through MMP induction and collagen cleavage, and the two biomarkers together are involved in TNF-α regulation [Citation32]. Since this is possibly the foremost study to assess SAA levels in patients with PsA treated with adalimumab, comparison of the current study with other studies may not be possible; however, it is important to note that SAA is a marker of matrix degradation and radiographic progression and may correlate better than erythrocyte sedimentation rate or CRP with disease activity [Citation33]. There is growing interest in MMP-3, a soluble biomarker involved in cartilage and bone metabolism; in a proof-of-concept study conducted in patients with PsA, there was significant decrease in median serum MMP-3 levels in adalimumab-treated patients although the decrease was slightly lesser than that in the current study (−24.7 ng/mL vs. −32.0 ng/mL) [Citation34].
In the present study, BASDAI scores significantly decreased from baseline to weeks 12 and 24. As PsA has peripheral and axial clinical manifestations [Citation35], the diagnosis of spinal involvement in PsA or axial PsA presents a clinical and classification challenge. BASDAI is used for measuring disease activity of axial diseases such as ankylosing spondylitis and axial PsA [Citation35–37], but not peripheral PsA; therefore, it is generally not considered useful for measuring PsA disease activity. Consequently, the observed decrease in BASDAI scores may have occurred because the percentage of patients with spondylitis (axial PsA) was high in the current study and higher than in ADEPT (21.6% in current study, 0.7% in ADEPT). Indices that measure only peripheral joint activity such as the DAS and ACR response criteria may be inadequate in capturing disease activity associated with axial PsA. Therefore, a tool such as BASDAI may be useful to assess complete disease activity. However, further studies are required to confirm the usefulness of BASDAI in measurement of PsA disease activity.
Lastly, AEs such as elevated liver enzymes, upper respiratory tract infection, psoriasis aggravation, headache, and rash reported in this study were in line with those observed in ADEPT [Citation2], as well as those previously reported for adalimumab [Citation38].
As mentioned, secukinumab, an anti-IL-17A monoclonal antibody, and ustekinumab, which targets IL-12 and IL-23, are being used to treat PsA when TNF-α inhibitors are ineffective. ACR response rates observed in the present study are in line with those reported from international, phase 3, randomized, double-blind, placebo-controlled studies of secukinumab (FUTURE; treatment-experienced patients with active PsA) and ustekinumab (PSUMMIT; treatment-experienced patients with active PsA) [Citation39,Citation40]. ACR20 response rates at week 24 were comparable between adalimumab (45.9%) and secukinumab (54%, 51%, and 29% for 300-, 150-, and 75-mg doses, respectively) and ustekinumab (42.4% and 49.5% with 45- and 90-mg doses, respectively) [Citation39,Citation40].
A few studies of biologics have been conducted in Japan, albeit with small numbers of patients. For example, in a study of 151 Japanese patients with moderate-to-severe plaque psoriasis, including 17 patients with PsA, who received brodalumab, an antibody against IL-17 receptor A, the ACR20 response rate at week 12 with the 140-mg dose (40%) was comparable to that for adalimumab in the present study (40.5%) [Citation41]. However, this comparison is marred because only approximately 15% of patients in the brodalumab study had PsA, and results specifically for PsA patients were not provided. In yet another study involving 78 Japanese patients with plaque psoriasis (including 11 patients with PsA), erythrodermic psoriasis, or generalized pustular psoriasis treated with ixekizumab, an anti-IL‐17A monoclonal antibody, four of five (80%) patients with PsA achieved ACR20 at week 12 and 100% at week 52 [Citation42]; however, we believe this population size is too small to be conclusive. In another study among treatment-experienced Japanese patients with plaque psoriasis, PsA, pustular psoriasis, or psoriatic erythroderma, a switch to infliximab (5 mg/kg at weeks 0, 2, and 6 and then every 8 weeks up to week 46) resulted in an ACR20 response rate of 66.7% at week 14 and 80.0% at week 46. However, a wash-out of existing psoriasis treatments was not required before switching to infliximab and, hence, the residual effects of the pretreatments may have influenced the results [Citation43].
This is first study to evaluate the effects of adalimumab on Japanese patients with PsA. While this study has some strengths such as evaluation of multiple efficacy, QoL, and safety parameters, we must acknowledge some limitations. The study was open-label and a control group was not included; however, the primary purpose of this study was to compare findings from ADEPT, a global study, with those from a Japanese population. The population size was smaller than ADEPT (37 vs. 153); however, the population size was in line with other Japanese studies of PsA treatments and provided adequate statistical power. Study duration was 24 weeks; long-term observation is required to confirm long-term efficacy and safety in the Japanese population.
Conclusion
In the current study of Japanese patients with PsA with prior inadequate response to NSAIDs, articular symptoms, skin manifestation, and QoL were improved after treatment with adalimumab. Adalimumab was well tolerated, and no new safety concerns were reported. Findings from this Japanese population were comparable to those from the global population in ADEPT, a double-blind, randomized, placebo-controlled trial.
Conflict of interest
In relation to the submitted work, Akimichi Morita had received honorarium from AbbVie GK, Boehringer Ingelheim Japan, Inc., Celgene K.K., Eisai Co., Ltd., Eli Lilly Japan K.K., Inforward, Inc, Janssen Pharmaceutical K.K., Kyowa Hakko Kirin Co., Ltd., Maruho Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nichi-Iko Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Novartis Pharma K.K., Sun Pharmaceutical Industries Ltd., Taiho Pharmaceutical Co., Ltd., Torii Pharmaceutical Co., Ltd, and Ushio INC. Additionally, Akimichi Morita had received funding from AbbVie GK, Eisai Co., Ltd., Eli Lilly Japan K.K., Kyowa Hakko Kirin Co., Ltd., LEO Pharma KK, Maruho Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Novartis Pharma K.K., and Torii Pharmaceutical Co., Ltd. Outside the submitted work, Akimichi Morita reports consulting fee from AbbVie GK, Boehringer Ingelheim Japan, Inc., Celgene K.K., Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., Kyowa Hakko Kirin Co., Ltd., Maruho Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nichi-Iko Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Novartis Pharma K.K., and Sun Pharmaceutical Industries Ltd. Norito Katoh received research funding from Abbvie. Chiharu Tateishi received research and travel funding from Eisai. Emi Nishida received honoraria from AbbVie GK, Kyowa Hakko Kirin Co., Ltd., Celgene K.K., Taiho Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Novartis Pharma K.K., Maruho Co., Ltd., Janssen Pharmaceutical K.K., Inforward, Inc, Eisai Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Aichi Medical Association, and Kissei Pharmaceutical Co., Ltd., and consulting fees from Abbvie GK and Maruho Co., Ltd. Shohei Nishimoto and Kenzo Muramoto are employees of Eisai Co. Ltd. Daisuke Tsuruta received honoraria from Maruho Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Torii Pharmaceutical Co., Ltd., and Taiho Pharmaceutical Co., Ltd., and research funding from Maruho Co., Ltd. and Eli Lilly Japan K.K. Ryuhei Okuyama, Eisaku Ogawa, Koji Masuda, Toshifumi Komorie, Takamitsu Makino, and Hironobu Ihn have nothing to disclose.
Supplemental Material
Download MS Word (22.1 KB)Additional information
Funding
References
- Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74(4):423–41.
- Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52(10):3279–89.
- Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl. 2):ii14–7.
- Ohara Y, Kishimoto M, Takizawa N, Yoshida K, Okada M, Eto H, et al. Prevalence and clinical characteristics of psoriatic arthritis in Japan. J Rheumatol. 2015;42(8):1439–42.
- Yamamoto T, Ohtsuki M, Sano S, Igarashi A, Morita A, Okuyama R, et al. Epidemiological analysis of psoriatic arthritis patients in Japan. J Dermatol. 2016;43(10):1193–6.
- Khraishi M, Chouela E, Bejar M, Landells I, Hewhook T, Rampakakis E, et al. High prevalence of psoriatic arthritis in a cohort of patients with psoriasis seen in a dermatology practice. J Cutan Med Surg. 2012;16(2):122–7.
- Ogdie A, Langan S, Love T, Haynes K, Shin D, Seminara N, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford). 2013;52(3):568–75.
- Ettehadi P, Greaves MW, Wallach D, Aderka D, Camp RD. Elevated tumour necrosis factor-alpha (TNF-alpha) biological activity in psoriatic skin lesions. Clin Exp Immunol. 2008;96(1):146–51.
- Partsch G, Steiner G, Leeb BF, Dunky A, Broll H, Smolen JS. Highly increased levels of tumor necrosis factor-alpha and other proinflammatory cytokines in psoriatic arthritis synovial fluid. J Rheumatol. 1997;24:518–23.
- Tanaka Y. Psoriatic arthritis in Japan: difference in clinical features and approach to precision medicine. Clin Exp Rheumatol. 2016;34(4 Suppl. 98):49–52.
- Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10:iii–v, xi–xiii, 1–229.
- Goulabchand R, Mouterde G, Barnetche T, Lukas C, Morel J, Combe B. Effect of tumour necrosis factor blockers on radiographic progression of psoriatic arthritis: a systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis. 2014;73(2):414–9.
- Lemos LL, de Oliveira Costa J, Almeida AM, Junior HO, Barbosa MM, Kakehasi AM, et al. Treatment of psoriatic arthritis with anti-TNF agents: a systematic review and meta-analysis of efficacy, effectiveness and safety. Rheumatol Int. 2014;34(10):1345–60.
- Ma X, Xu S. TNF inhibitor therapy for rheumatoid arthritis. Biomed Rep. 2013;1(2):177–84.
- Mantravadi S, Ogdie A, Kraft WK. Tumor necrosis factor inhibitors in psoriatic arthritis. Expert Rev Clin Pharmacol. 2017;10(8):899–910.
- Rein P, Mueller RB. Treatment with biologicals in rheumatoid arthritis: an overview. Rheumatol Ther. 2017;4(2):247–61.
- Eder L, Thavaneswaran A, Chandran V, Gladman DD. Tumour necrosis factor α blockers are more effective than methotrexate in the inhibition of radiographic joint damage progression among patients with psoriatic arthritis. Ann Rheum Dis. 2014;73(6):1007–11.
- Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta-Felquer M, Armstrong AW, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060–71.
- Suzuki E, Mellins ED, Gershwin ME, Nestle FO, Adamopoulos IE. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13(4–5):496–502.
- Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, et al. American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Rheumatol. 2019;71(1):5–32.
- Abbvie. AbbVie’s HUMIRA® (adalimumab) receives CHMP positive opinion to treat adolescents with hidradenitis suppurativa, a chronic inflammatory skin disease. 2016. Available from: https://news.abbvie.com/news/abbvies-humira-adalimumab-receives-chmp-positive-opinion-to-treat-adolescents-with-hidradenitis-suppurativa-chronic-inflammatory-skin-disease.htm
- Eisai Co., Ltd. Fully human monoclonal anti-TNF-α antibody HUMIRA® receives approval as Japan's first biological agent for psoriasis. Available from: http://www.eisai.com/news/news201005.html
- Gladman DD, Mease PJ, Ritchlin CT, Choy EH, Sharp JT, Ory PA, et al. Adalimumab for long-term treatment of psoriatic arthritis: forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trial. Arthritis Rheum. 2007;56(2):476–88.
- Salvarani C, Pipitone N, Catanoso M, Chiarolanza I, Boiardi L, Caruso A, et al. Adalimumab in psoriatic arthritis. J Rheumatol Suppl. 2012;89:77–81.
- Saraceno R, Bavetta M, Zangrilli A, Chiricozzi A, Potenza C, Chimenti S, et al. Adalimumab in the treatment of plaque-type psoriasis and psoriatic arthritis. Expert Opin Biol Ther. 2013;13(9):1325–34.
- Moll JM, Wright V. Familial occurrence of psoriatic arthritis. Ann Rheum Dis. 1973;32(3):181–201.
- Gladman DD, Sampalis JS, Illouz O, Guerette B. Responses to adalimumab in patients with active psoriatic arthritis who have not adequately responded to prior therapy: effectiveness and safety results from an open-label study. J Rheumatol. 2010;37(9):1898–906.
- Pascoe VL, Enamandram M, Corey KC, Cheng CE, Javorsky EJ, Sung SM, et al. Using the Physician Global Assessment in a clinical setting to measure and track patient outcomes. JAMA Dermatol. 2015;151(4):375–81.
- Van den Bosch F, Manger B, Goupille P, McHugh N, Rodevand E, Holck P, et al. Effectiveness of adalimumab in treating patients with active psoriatic arthritis and predictors of good clinical responses for arthritis, skin and nail lesions. Ann Rheum Dis. 2010;69(2):394–9.
- Asahina A, Umezawa Y, Yanaba K, Nakagawa H. Serum C-reactive protein levels in Japanese patients with psoriasis and psoriatic arthritis: long-term differential effects of biologics. J Dermatol. 2016;43(7):779–84.
- Heiberg MS, Kaufmann C, Rodevand E, Mikkelsen K, Koldingsnes W, Mowinckel P, et al. The comparative effectiveness of anti-TNF therapy and methotrexate in patients with psoriatic arthritis: 6 month results from a longitudinal, observational, multicentre study. Ann Rheum Dis. 2007;66(8):1038–42.
- Connolly M, Mullan RH, McCormick J, Matthews C, Sullivan O, Kennedy A, et al. Acute-phase serum amyloid A regulates tumor necrosis factor alpha and matrix turnover and predicts disease progression in patients with inflammatory arthritis before and after biologic therapy. Arthritis Rheum. 2012;64(4):1035–45.
- Cunnane G, Grehan S, Geoghegan S, McCormack C, Shields D, Whitehead AS, et al. Serum amyloid A in the assessment of early inflammatory arthritis. J Rheumatol. 2000;27(1):58–63.
- van Kuijk AW, DeGroot J, Koeman RC, Sakkee N, Baeten DL, Gerlag DM, et al. Soluble biomarkers of cartilage and bone metabolism in early proof of concept trials in psoriatic arthritis: effects of adalimumab versus placebo. PLoS One. 2010;5(9):e12556.
- Taylor WJ, Harrison AA. Could the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) be a valid measure of disease activity in patients with psoriatic arthritis? Arthritis Rheumat. 2004;51(3):311–5.
- Fernández-Sueiro JL, Willisch A, Pértega-Diaz S, Tasende JA, Fernández-López JC, Villar NO, et al. Validity of the Bath Ankylosing Spondylitis Disease Activity Index for the evaluation of disease activity in axial psoriatic arthritis. Arthritis Care Res. 2010;62:78–85.
- Mease PJ. Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT‐F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI). Arthritis Care Res. 2011;63(Suppl. 11):S64–S85.
- HUMIRA® (adalimumab) package insert. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125057s0276lbl.pdf
- McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780–9.
- McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(9999):1137–46.
- Nakagawa H, Niiro H, Ootaki K. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81(1):44–52.
- Saeki H, Nakagawa H, Nakajo K, Ishii T, Morisaki Y, Aoki T, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52‐week, open‐label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44(4):355–62.
- Torii H, Nakagawa H. Long-term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol. 2011;38(4):321–34.