Abstract
Objectives: The treatment response according to patient disease activity during Iguratimod therapy for rheumatoid arthritis has not been sufficiently assessed. A post-hoc analysis of post-marketing surveillance was performed. The treatment effect was evaluated using the European League against Rheumatism (EULAR) response criteria.
Methods: Disease Activity Score (DAS) 28 was assessed at various time points. Patients showing a moderate or good response according to the EULAR response criteria at 24 weeks after the start of Iguratimod therapy were considered Responders. Propensity score matching was also performed, after which the factors with the greatest effect on the treatment evaluation were investigated.
Results: The mean DAS28 at the start of administration and after 24 weeks was 4.31 and 2.52, respectively, in the Responder and 3.48 and 3.48, respectively, in the Non-responder. After propensity score matching for patient characteristics, the primary factors found to be related to being a Responder were concomitant use of methotrexate (MTX) with Iguratimod, and prior treatment with MTX before the start of Iguratimod.
Conclusion: As factors related to the treatment effect, the concomitant use of MTX may contribute to achieving a better effect, and this study has shown that real-world are consistent with the results of clinical trials.
Introduction
In the treatment of rheumatoid arthritis, methotrexate (MTX) is started as an anchor drug. The patient’s status is then checked regularly, and a decision is made as to whether to continue the therapy or change drugs. According to the European League against Rheumatism (EULAR) recommendation, the treatment effect is generally evaluated at 3 months, and if improvement is not seen within 3 months from the start of treatment, or if the treatment targets are not achieved within 6 months, the treatment should be reconsidered [Citation1]. The recommendations in the Japan College of Rheumatology treatment guidelines are similar, and treatment is performed accordingly [Citation2].
Indices used in determining the treatment effect in clinical trials are the remission rate when patients with high disease activity have been treated with a certain therapeutic agent for a certain period and the rate of decrease in Disease Activity Score (DAS) 28 based on EULAR improvement criteria [Citation3]. DAS28 is also widely used in routine care. However, there are cases in which DAS28 does not completely reflect the disease activity of individual patients [Citation4], and DAS28 has not always increased in cases when signs of a reduced effect are observed and the treatment is changed. Therefore, identification of treatment effect predictors in routine care is difficult with factor analysis alone when there is a remission from high disease activity or there is low disease activity based on the results of a clinical trial. The EULAR response criteria were developed as an index suitable for assessing disease activity before and after treatment in individual patients [Citation5], and they have been used as an index of treatment response when investigating predictors of the treatment effect of MTX [Citation6].
Iguratimod is a conventional synthetic disease-modifying antirheumatic drug (csDMARD) developed in Japan. A post-marketing surveillance (PMS) study of Iguratimod was conducted with all patients in whom administration was started between September 2012 and April 2013 [Citation7,Citation8]. The factors that contributed to low disease activity after administration for 52 weeks in this PMS were disease duration of less than 5 years, no concomitant steroids, low DAS28 at the start of administration, and concomitant MTX (≤8 mg/week) at the start of administration [Citation9]. However, at the time Iguratimod was started, 23.3% of patients had DAS28 of ≤3.2 (low disease activity or remission), which is a factor related to low disease activity, and no investigation to assess disease response was done.
As a post-hoc analysis of PMS, this study investigated the treatment effect based on EULAR response criteria at 24 weeks, with reference to the time points for judging treatment effect in guidelines. Treatment effect predictors were also investigated by comparing patient characteristics.
Materials and methods
Patients
This study was a post-hoc analysis of PMS conducted at 2608 institutions in Japan between September 2012 and November 2015. The PMS was registered in JAPIC (JapicCTI-152782 and JapicCTI-132051) and ClinicalTrials.gov (NCT01850966), and the details have been previously reported.
Definition of Responder/Non-responder
The EULAR response criteria were used to judge the treatment effect. Patients who achieved a moderate or good response according to the EULAR response criteria for DAS28-C-reactive protein (DAS28-CRP) values at 24 weeks of administration were classified as Responders, while patients who had no response were classified as Non-responders [Citation5].
The change of concomitant MTX dose
For patients concomitantly administered MTX, we calculated the MTX doses at the start of Iguratimod administration and at 24 weeks or 52 weeks, to categorize the dose changes from baseline as follows: ‘Increased’, ‘Constant’, and ‘Decreased’. Calculations were made only for the patients who used MTX at any of these points. If MTX was not used at one of these points, the dose at that point was taken to be zero mg. Those who received no MTX at any of these points were excluded from this section.
Statistical analysis
All analyses were done using SAS System version 9.3 (SAS Institute Inc., Cary, NC, USA). The level of significance was set at 0.05 in a two-tailed test.
For comparisons between the Responders and Non-responders in the assessment period, a two-sample t-test was used for DAS28-CRP measurements, and Fisher’s exact test was used for the efficacy rate. To identify treatment effect predictors, a logistic regression analysis was performed with the DAS28-CRP of Responders as the response variable. From past reports, ‘prior treatment with MTX’ and ‘concomitant MTX dose’ were forcibly included as the covariates, and other variable selection was done with a stepwise method. When selecting variables, the significance level was set at 20%. Other explanatory variables were as follows: sex, age, body weight, rheumatoid arthritis disease duration, Steinbrocker stage, Steinbrocker functional class, whether there was combination therapy for rheumatoid arthritis, whether steroids were concomitantly used, DAS28-CRP at the start of administration, concomitant NSAID use, and concomitant folic acid use.
Propensity score matching
For Responders/Non-responders after 24 weeks, a propensity score with Responders as 1 (Treated) and Non-responders as 0 (Control) was calculated from a logistic regression analysis with age (years) and DAS28-CRP at the start of administration as explanatory variables. Nearest neighbor matching of 1:1 was done with respect to the calculated propensity score. The caliper in matching was 0.2, and matching was done in the order of higher propensity scores. After adjustment, standardized difference was calculated for each characteristics statistics.
Results
DAS28 trends in Responders and Non-responders
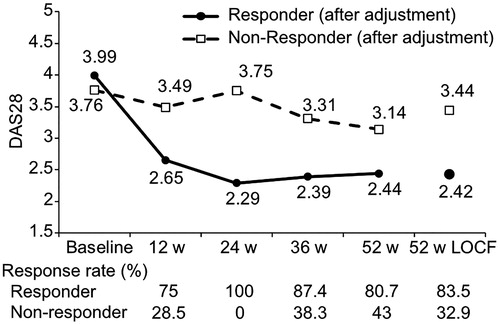
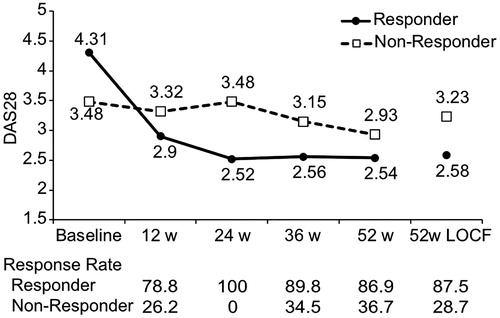
DAS28 was measured at the start of Iguratimod administration and after 24 weeks in 875 patients; 582 patients with a moderate or good response at 24 weeks according to the EULAR response criteria were classified as the Responder group, and 293 with no response were classified as the Non-responder group. The mean DAS28 at the start of administration and after 24 weeks was 4.31 and 2.52, respectively, in the Responder group, and 3.48 and 3.48, respectively, in the Non-responder group. DAS28 at 52 weeks was 2.54 in the Responder group and 2.93 in the Non-responder group ().
Figure 1. Trend in DAS28 at each observation point. Mean DAS28 values at each observation point and at 52 weeks with missing data supplemented are shown for Responders and Non-responders. The solid line is Responders, and the broken line is Non-responders. The EULAR response criteria effective rate at each observation point is shown at the bottom. LOCF, last observation carried forward.
Treatment effect predictors
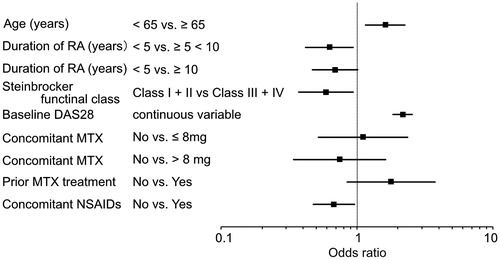
To investigate the characteristics of patients who are likely to be Responders, that is, for whom a treatment effect is anticipated at 24 weeks, a logistic regression analysis was performed with DAS28 improvement at 24 weeks as the response variable and patient characteristics as explanatory variables. MTX is considered to be an anchor drug in the drug treatment of rheumatoid arthritis [Citation1]. Therefore, whether MTX treatment has been performed prior to or concomitantly with the drug in this study is an important indicator. In variable selection, after forced choice of ‘prior treatment with MTX’ and ‘concomitant MTX dose’ variables were selected with a stepwise method.
Factors that increased the likelihood of being a Responder at 24 weeks were identified as age (≥65 years old), rheumatoid arthritis disease duration (<5 years), Steinbrocker functional class (I, II), DAS28 at the start of administration (continuous variable), and concomitant NSAIDs (no concomitant use) (). The change of concomitant MTX doses from the start of Iguratimod administration to at 24 weeks were calculated in order to examine the possible impact of concomitant MTX doses on the effects of Iguratimod treatment. The MTX doses used during the Iguratimod treatment were found to be constant in most of the patients (Supplementary Figure).
Figure 2. Treatment effect predictors. A logistic regression analysis was performed to analyze treatment effect factors. Odds ratios for the explanatory variables ultimately selected are shown. Explanatory variables were selected with a stepwise method and consideration of clinical significance. MTX, methotrexate; NSAID, non-steroidal anti-inflammatory drug.
Adjustment of patient characteristics (propensity score matching)
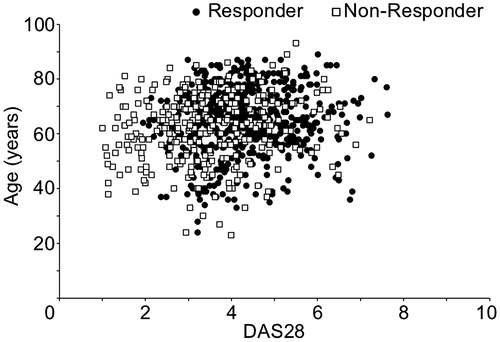
Since there were no correlations between the explanatory variables in the logistic regression analysis (variance inflation factor ≤3 for all variables), no factors were excluded prior to variable selection. It has been shown that, in Japan, the mean age of rheumatoid arthritis patients and the age of onset are both increasing [Citation10], and although there was no significant correlation, it should be considered that age and disease duration have effects. At the same time, it was thought that the higher the DAS at the start of administration, the likelier it is for a difference to appear in improvement as a treatment effect and for a patient to be a Responder. This item was also identified in a previous study to be a factor related to low disease activity after 52 weeks [Citation8]. Then, to identify factors other than age and DAS at the start of administration that affect treatment results at 24 weeks, these two factors were matched in the Responder group and Non-responder group. First, upon confirming the distribution of DAS28 and age at the start of administration in the Responder and Non-responder groups, there was found to be a large overlap in the groups (). A propensity score was then calculated with age and DAS at the start of administration as explanatory factors, and propensity score matching was done based on the calculated values. After adjustment, the number of patients was 255 in the Responder group and 255 in the Non-responder group. The mean DAS28 at the start of administration and at 24 weeks of administration was 3.99 and 2.29, respectively, after adjustment in the Responder group and 3.76 and 3.75, respectively, after adjustment in the Non-responder group (). DAS28 at 52 weeks was 2.44 after adjustment in the Responder group and 3.14 after adjustment in the Non-responder group. Even among Non-responders a moderate or good response was later seen in some patients. It was thus found to be possible for the treatment effect to appear at a later time.
Figure 3. Adjustment of patient characteristics. Propensity score matching was done to adjust for bias in patient characteristics. The distributions before adjustment of DAS28 and age at the start of administration are shown. The black circles are Responders, and the white squares are Non-responders.
Post-adjustment predictors
After propensity score matching, patient characteristics for which the component ratio differed by ≥10% in the Responder and Non-responder groups after adjustment were whether there was concomitant MTX, the concomitant MTX dose and MTX before administration of Iguratimod. The respective ratios were 59.6% and 47.1% for concomitant MTX and 63.1% and 51.0% for MTX pretreatment (). The standardized difference was 0.25 for each factor.
Table 1. Patient characteristics after adjustment.
Discussion
In this study, a post-hoc analysis was done from the perspective of treatment response. As shown in , the fact that disease activity at the start of treatment differed considerably is thought to be a problem in terms of interpretation. A re-analysis was done using propensity score matching, and the characteristics of the patient group (Responders) with a high treatment effect were examined.
After adjustment with propensity matching, the factors that increased the likelihood of being a Responder, in other words, treatment effect predictors, were MTX administration prior to Iguratimod therapy and concomitant MTX during Iguratimod therapy. As to intracellular signaling pathways, Iguratimod is considered to act downstream from I-κB degradation and to suppress NF-κB activation by inhibiting NF-κB translocation into the nucleus [Citation11]. With this mechanism, Iguratimod mainly inhibits production of IgG and IgM by B cells and the release of inflammatory cytokines by monocytes/macrophages and synovial cells [Citation12–14]. MTX inhibits nucleic-acid synthesis by blocking folic acid metabolic pathways [Citation15], and thereby has immunosuppressive and anti-inflammatory effects which are exerted via the inhibition of cytokines, as well as anti-proliferative actions on synovial cells and lymphocytes [Citation16,Citation17]. Because Iguratimod and MTX have different pharmacological mechanisms of action, anti-rheumatic effects of these drugs were exerted without the two interfering with each other, resulting in good patient responses to the treatment.
In a phase-III clinical trial of concomitant MTX and Iguratimod, the American College of Rheumatology response rate was about the same when observed 24 weeks after the addition of Iguratimod at 28 weeks of MTX treatment and when observed at 52 weeks of concomitant MTX and Iguratimod from the start of the trial [Citation18,Citation19]. The results of this study were similar to the results of the phase-III clinical trial, and they are thought to provide supportive evidence for the efficacy of the addition and concomitant use of Iguratimod with MTX treatment in real-world rheumatoid arthritis treatment. Furthermore, relatively high differences between the Responder group and the Non-responder group have been observed in the post-adjustment analysis, even with csDMARDs in combination treatment. From this, it can be concluded that Iguratimod can be expected to have strong effects when used in combination with other anti-rheumatic drugs including MTX. In the Responder group, many patients received 8 mg or lower doses of MTX. The proportion of patients who received >8 mg of MTX was nearly equal between the two groups, showing no clear trend. The doses of MTX were not changed in most of the patients during Iguratimod treatment (Supplementary Figure). As shown by the results for patients who received ≤8 mg of MTX, some in the Non-responder group were resistant to low-dose MTX. These patients may have shown poorer responses to other drugs, and thus also responded poorly to Iguratimod.
Patients in whom Iguratimod therapy was effective (good response or moderate response) on the EULAR response criteria at 24 weeks achieved a DAS28 level <2.6, the standard for remission, even at 52 weeks. Therefore, judgment at 24 weeks was also shown to be one option for the timing to predict the effect of Iguratimod.
In the EULAR recommendation, the criterion for treatment effect at 3 months from the start of treatment is change to a better disease activity state or showing an improvement of at least 50% [Citation1]. In this study, the mean DAS28 from the start of Iguratimod therapy in the Responder group decreased in a range from moderate disease activity to low disease activity, satisfying this criterion. After 6 months of administration, the patients achieved the standard for remission (DAS28 < 2.6), and they also satisfied the standard in the guidelines of achieving treatment targets within 6 months from the start of treatment. When making decisions earlier, i.e. at 12 weeks, for predicting treatment effects, 28% of the patients in the Non-responder group showed a good/moderate response. At 24 weeks, however, they no longer showed a response. In these patients, treatment effects might be short-lived, possibly diminishing over time. At the same time, it is reported that, when looking at individual patients, there are some in whom the treatment effect is confirmed after the 24th week [Citation20], but while there were patients in the Non-responder group who showed a delayed response, becoming Responders at 36 weeks or 52 weeks from the start of Iguratimod therapy, the percentage of patients with a delayed response was not large. Thus, setting the timing for decisions on the effect of Iguratimod therapy at 24 weeks is appropriate, in line with both Japanese and international guidelines, and is thought to be acceptable.
The limitations of this study are that the observation period was 52 weeks, and that longer-term observation was not done. The results of an investigation of the effect of Iguratimod therapy over 3 years have also recently been published, and there are reports contradicting the finding that concomitant MTX is a predictor of the achievement of remission or low disease activity [Citation21]. Differences in patient characteristics and research designs may have played a role, but the fact that the observation period was limited to 52 weeks should also be considered. Longer-term observations may be necessary. This study was a post-hoc analysis of all-case surveillance conducted as PMS. All-case surveillance studies are observational studies that include even patients in whom efficacy has not been evaluated. Patients in whom there was discontinuation before 24 weeks, the timing at which the treatment effect is judged, were not included in this investigation. It is possible, therefore, that the patient characteristics of Non-responders were not shown. In addition, because this study was based on all-case surveillance study data, it has missing data, such as patient background items and clinical tests. Therefore, there is a possibility of unmeasured confounding factors that affected the results. To resolve these problems, further study with the primary purpose of estimating treatment effect predictors is needed.
In conclusion, addition and concomitant use of MTX are thought to be factors eliciting a greater treatment effect, and this study showed that real-world results are consistent with those in clinical trials. It was also shown that, in judging the effect of Iguratimod therapy, patients who achieve a moderate response or good response on the EULAR response criteria at 24 weeks of administration can fully anticipate a remission at 52 weeks with continuation.
Author contributions
N.I. contributed to acquisition and interpretation of the data, as well as both writing and critically reviewing the manuscript. K.S., A.Y., S.I., and M.I. contributed to the design and conduct of the study, as well as analysis of the results, and either writing or critically reviewing the manuscript. All authors approved the final version of this manuscript for submission.
Conflict of interest
N. Ishiguro received research grants or speaker’s fees from Astellas Pharma Inc., AbbVie GK, Asahi Kasei Corp., Bristol-Myers Squibb Company, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., Kaken Pharmaceutical Co., Ltd., Medical Corporation SANJINKAI, Medical Corporation TOUKOUKAI, Mitsubishi Tanabe Pharma Corp., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Taisho Toyama Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Zimmer Biomet G.K. and consulting fees from Ono Pharmaceutical Co., Ltd. K. Shibata, A. Yoshimura, S. Ikeuchi and M. Ishii are employees of Eisai Co., Ltd.
Supplemental Material
Download GIF Image (41.9 KB)Acknowledgements
The authors thank the doctors and medical staff nationwide in Japan who cooperated in the post-marketing surveillance study. The authors also thank the PMS Committee of the Japan College of Rheumatology for their advice on safety in conducting the survey and to Dr. Kazuhiko Yamamoto for his medical advice. A word of thanks also goes to CMIC Co., Ltd. a contract research organization, for conducting all of the analyses.
References
- Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–77.
- Japan College of Rheumatology. editors. Guidelines for the Management of Rheumatoid Arthritis. Tokyo: Medical Review Co. Ltd; 2014
- Ministry of Health, Labour and Welfare Administrative Notice No. 0217-1 in 2006. Guidelines for the Clinical Evaluation Method of Anti-rheumatoid Arthritis Drugs. Ministry of Health Labour and Welfare; 2006. Available from: https://www.pmda.go.jp/files/000206217.pdf
- Balsa A, de Miguel E, Castillo C, Peiteado D, Martín-Mola E. Superiority of SDAI over DAS-28 in assessment of remission in rheumatoid arthritis patients using power Doppler ultrasonography as a gold standard. Rheumatology (Oxford). 2010;49(4):683–90.
- van Gestel AM, Prevoo ML, van't Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis: comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39(1):34–40.
- Saevarsdottir S, Wallin H, Seddighzadeh M, Ernestam S, Geborek P, Petersson IF, et al. Predictors of response to methotrexate in early DMARD naive rheumatoid arthritis: results from the initial open-label phase of the SWEFOT trial. Ann Rheum Dis. 2011;70(3):469–75.
- Mimori T, Harigai M, Atsumi T, Fujii T, Kuwana M, Matsuno H, et al. Safety and effecriveness of 24-week treatment with iguratimod, a new oral disease-modifying antirheumatic drug, for patients with rheumatoid arthritis: interim analysis of post-marketing surveillance study of 2679 patients in Japan. Mod Rheumatol. 2017;27(5):755–65.
- Mimori T, Harigai M, Atsumi T, Fujii T, Kuwana M, Matsuno H, et al. Safety and effectiveness of iguratimod in patients with rheumatoid arthritis: final report of a 52-week, multicenter postmarketing surveillance study. Mod Rheumatol. 2019;29(2):314–23.
- Ishiguro N. W57-1: Final report of Iguratimod post-marketing surveillance in patients with rheumatoid arthiritis (52 weeks) [abstract]. Mod Rheumatol. 2016;(Suppl):S158.
- Kato E, Sawada T, Tahara K, Hayashi H, Tago M, Mori H, et al. The age at onset of rheumatoid arthritis is increasing in Japan: a nationwide database study. Int J Rheum Dis. 2017;20(7):839–45.
- Aikawa Y, Yamamoto M, Yamamoto T, Morimoto K, Tanaka K. An anti-rheumatic agent T-614 inhibits NF-kappaB activation in LPS- and TNF-alpha-stimulated THP-1 cells without interfering with IkappaBalpha degradation. Inflamm Res. 2002;51(4):188–94.
- Kawakami A, Tsuboi M, Urayama S, Matsuoka N, Yamasaki S, Hida A, et al. Inhibitory effect of a new anti-rheumatic drug T-614 on costimulatory molecule expression, cytokine production, and antigen presentation by synovial cells. J Lab Clin Med. 1999;133(6):566–74.
- Kohno M, Aikawa Y, Tsubouchi Y, Hashiramoto A, Yamada R, Kawahito Y, et al. Inhibitory effect of T-614 on tumor necrosis factor-alpha induced cytokine production and nuclear factor-kappaB activation in cultured human synovial cells. J Rheumatol. 2001;28(12):2591–6.
- Tanaka K, Yamamoto T, Aikawa Y, Kizawa K, Muramoto K, Matsuno H, et al. Inhibitory effects of an anti-rheumatic agent T-614 on immunoglobulin production by cultured B cells and rheumatoid synovial tissues engrafted into SCID mice. Rheumatology. 2003;42(11):1365–71.
- Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322–8.
- Rosenthal GJ, Germolec DR, Lamm KR, Ackermann MF, Luster MI. Comparative effects on the immune system of methotrexate and trimetrexate. Int J Immunopharmacol. 1987;9(7):793–801.
- Hirata S, Matsubara T, Saura R, Tateishi H, Hirohata K. Inhibition of in vitro vascular endothelial cell proliferation and in vivo neovascularization by low-dose methotrexate. Arthritis Care Res. 1989;32(9):1065–73.
- Hara M, Ishiguro N, Katayama K, Kondo M, Sumida T, Mimori T, et al. Safety and efficacy of combination therapy of iguratimod with methotrexate for patients with active rheumatoid arthritis with an inadequate response to methotrexate: an open-label extension of a randomized, double-blind, placebo-controlled trial. Mod Rheumatol. 2014;24(3):410–8.
- Ishiguro N, Yamamoto K, Katayama K, Kondo M, Sumida T, Mimori T, et al. Concomitant iguratimod therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate: a randomized, double-blind, placebo-controlled trial. Mod Rheumatol. 2013;23(3):430–9.
- Li R, Zhao JX, Su Y, He J, Chen LN, Gu F, et al. High remission and low relapse with prolonged intensive DMARD therapy in rheumatoid arthritis (PRINT): a multicenter randomized clinical trial. Medicine (Baltimore). 2016;95(28):e3968.
- Suto T, Yonemoto Y, Okamura K, Sakane H, Takeuchi K, Tamura Y, et al. The three-year efficacy of iguratimod in clinical daily practice in patients with rheumatoid arthritis. Mod Rheumatol. 2018;10:1–20.