Abstract
Objectives: To examine time trends in the characteristics of patients with rheumatoid arthritis (RA) undergoing primary total joint replacement (TJR).
Methods: Biologics were approved in Japan for use in patients with RA in July 2003. A total of 403 large joints in 282 patients who underwent TJR at our institute between 1 January 2004 and 31 December 2017 were retrospectively examined.
Results: A significant decreasing trend was observed in the number of TJRs performed from 2004 to 2017 (p = 0.013). No significant trend was observed in time from RA onset to TJR (p = 0.294). Age at RA onset (p = 0.034) showed a significant increasing trend, and serum C-reactive protein (CRP) levels showed a significant decreasing trend (p < 0.001). Negative CRP (defined as ≤0.3 mg/dl; partial regression coefficient (B) = 2.44, p = 0.016) was independently associated with time from RA onset to TJR as well as age at RA onset and juxta-articular osteophyte formation.
Conclusion: The number of TJRs decreased since the approval of biologics in Japan, and changes were observed in the characteristics of patients with RA undergoing TJR. Negative CRP was an independent factor associated with longer time from RA onset to TJR.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease associated with irreversible joint destruction and functional disability. Preventing these outcomes is a goal of RA treatment [Citation1]. Biologics were approved for use in Japan to treat RA in July 2003. Newer medications including biologics and more aggressive treatment strategies have enabled more patients with RA to achieve this goal. When severe large joint destruction causes functional disability, total joint replacement (TJR) is performed to improve function and pain in patients with RA, as well as those with osteoarthritis (OA) [Citation2]. The number of TJRs performed in patients with RA has been on the decline since newer medications became available in Japan [Citation3,Citation4], as well as in other countries [Citation5–7]; however, some patients with RA require TJR despite receiving aggressive treatment [Citation8,Citation9]. TJR thus remains an important treatment option for RA patients with joint destruction.
Several patient characteristics at the time of surgery have been reported to affect outcomes following TJR in patients with RA. Longer disease duration is associated with an increased risk of infection [Citation10], and both higher disease activity and higher serum C-reactive protein (CRP) levels are associated with worse function [Citation11]. Higher disease activity is also associated with an increased risk of death, in addition to older age [Citation12,Citation13]. These characteristics of patients undergoing TJR are likely to have changed due to recent advances in RA drug therapy.
Examination of time trends in the characteristics of patients with RA undergoing TJR may allow us to better understand temporal changes in the outcomes of TJR in this patient population. Several studies have examined time trends in the characteristics of patients at the time of TJR, but those studies mainly targeted patients with OA [Citation14–16]. Little has been reported on this aspect in patients with RA undergoing TJR. A previous cohort study in Japan showed an increasing trend in disease duration at the time of TJR since the approval of the first biologic agent in Japan [Citation17]. We hypothesized that recent, more aggressive treatment strategies to suppress disease activity might have led to delays in TJR in patients with RA.
This study aimed to examine time trends in the characteristics of patients with RA undergoing primary TJR, and the association between time from RA onset to TJR and levels of serum CRP, an objective biomarker of disease activity in RA.
Subjects and methods
Subjects
A total of 286 patients (407 joints) with RA underwent primary TJR of large joints (i.e. shoulder, elbow, hip, knee, and ankle) at our institute between 1 January 2004 and 31 December 2017. Of these, 403 joints in 282 patients were retrospectively examined, excluding four joints in four patients who had fractures. Of the 282 patients, 181 (64%) underwent one joint replacement, 84 (30%) underwent two joint replacements, 14 (5%) underwent three joint replacements, and three (1%) underwent four joint replacements during the study period. All patients met the 1987 American College of Rheumatology (ACR) classification criteria [Citation18] or the new ACR/European League Against Rheumatism (EULAR) diagnostic criteria [Citation19]. This study was approved by the Ethics Committee of Nagoya University Graduate School of Medicine (Protocol no. 2018-0260) and complied with the principles set forth in the Declaration of Helsinki. Informed consent was obtained by an opt-out procedure. Patient anonymity was maintained during data collection, and the security of personal information was strictly controlled.
Data collection
Demographic and clinical data of patients at the time of surgery were retrospectively collected from clinical records, and included the following: sex, age at surgery, age at RA onset, surgery site, use of methotrexate (MTX), biologics, and/or glucocorticoids, and serum CRP levels. Radiographic assessment was performed using preoperative plain radiographs in two projections including the anteroposterior projection. Two observers evaluated osteophyte formation, i.e. a hallmark of OA [Citation20], by comparisons with normal joint radiographs. Osteophytes were defined as bony protrusions emerging from the juxta-articular cortical shell. In cases of disagreement, a consensus was reached by discussion between the two observers.
Statistical analysis
All data were analyzed using the joint as the statistical unit of analysis. Time trends over the study period were assessed using the Jonkheere–Terpstra trend test for continuous variables and the Cochran–Armitage trend test for categorical variables. Patients were divided into two groups according to year of surgery (2004–2010 and 2011–2017), and according to serum CRP levels based on normal values at our institution (≤0.3 mg/dl [CRP(–) group] and >0.3 mg/dl [CRP(+) group]). Data at the time of surgery were compared between the two groups using the Mann–Whitney U test for continuous variables and the chi-squared test for categorical variables. Time from RA onset to TJR was compared according to the following baseline categorical variables using the Mann–Whitney U test: sex, age at RA onset [elderly-onset RA (EORA) vs. younger-onset RA (YORA)], surgery site, use of MTX, biologics, and/or glucocorticoids, CRP negativity, and osteophyte formation. EORA was defined as RA onset at ≥60 years of age and YORA as onset at <60 years of age [Citation21]. TJR of knee and hip joints are generally more attributable to OA than RA, whereas TJRs of elbow, shoulder, and ankle joints are not. Accordingly, with respect to surgery site, patients were divided into the following two groups: knee/hip and others. In addition, factors associated with time from RA onset to TJR were assessed by multiple regression analysis with variables found to be significant (p < 0.05) in the above univariate analyses.
The Cochran–Armitage trend test was performed using EZR version 1.36 (Saitama Medical Centre, Jichi Medical University, Saitama, Japan) [Citation22]. All other statistical analyses were performed using SPSS version 24.0 software (IBM, Armonk, NY, USA). p < 0.05 was considered statistically significant.
Results
Subject characteristics at the time of surgery
shows the demographic, clinical, and radiographic characteristics of all patients included in this study at the time of surgery. Median [interquartile range (IQR)] age at surgery and at RA onset were 65 (58, 71) and 49 (39, 59) years, respectively, and time from RA onset to TJR was 14 (8, 22) years. Among all patients, 93 (23%) had EORA and 149 (37%) had negative CRP (i.e. ≤0.3 mg/dl). During the study period, total knee replacement was the most frequently performed TJR (66%), followed by total hip replacement (22%).
Table 1. Patient characteristics at the time of surgery.
Time trends
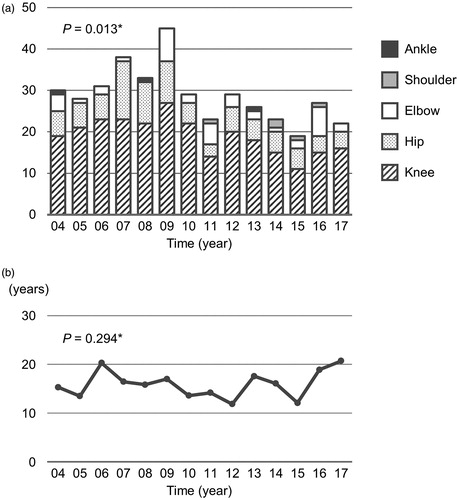
The number of TJRs performed per year showed a significant decreasing trend from 2004 to 2017 (p = 0.013) (. When stratified by surgical site, significant decreasing trends were observed in the numbers of knee (p = 0.028) and hip (p = 0.011) TJRs. No significant trends were observed in the numbers of elbow (P = 0.651), shoulder (p = 0.059), and ankle (p = 0.312) TJRs. Time from RA onset to TJR showed no significant trend (p = 0.294) (.
Figure 1. (a) Time trends in numbers of total joint replacements and (b) time from rheumatoid arthritis onset to total joint replacement. *The Jonkheere–Terpstra trend test.
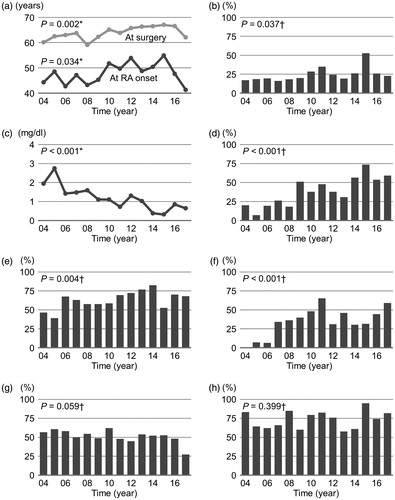
Both age at surgery and age at RA onset showed a significant increasing trend (p = 0.002 and 0.034, respectively) (), and the proportion of subjects with EORA showed a significant increasing trend (p = 0.037) (. Serum CRP levels showed a significant decreasing trend (p < 0.001) (), and the proportion of subjects with negative CRP showed a significant increasing trend (p < 0.001) (. Relative to the 2004–2010 group (n = 234), the 2011–2017 group (n = 169) was more likely to be older at surgery [median (IQR), 66 (60–73) vs. 63 (57-69) years, p = 0.005], be older at RA onset [51 (41–61) vs. 48 (37–57) years, p = 0.013], have a higher rate of EORA (28% vs. 20%, p = 0.042), have lower serum CRP levels [0.30 (0.07–0.95) vs. 0.88 (0.29–2.22) mg/dl, p < 0.001], and have a higher rate of negative CRP (50% vs. 27%, p < 0.001) (). With respect to RA treatment, a significant increasing trend was observed in the proportion of subjects receiving MTX (p = 0.004) and biologics (p < 0.001) ()).
Figure 2. Time trends in (a) age, (b) proportion of patients with elderly-onset rheumatoid arthritis (EORA), (c) serum C-reactive protein (CRP) levels, (d) proportion of patients with CRP ≤0.3 mg/dl, (e) methotrexate use, (f) biologic use, (g) glucocorticoid use, and (h) osteophytes on radiograph. Data are presented as mean values or percentage. *The Jonkheere–Terpstra trend test; †The Cochran–Armitage trend test.
Factors associated with time from RA onset to TJR
shows subject characteristics stratified by serum CRP levels. Relative to the CRP(+) group (n = 254), the CRP(–) group (n = 149) was more likely to have a higher rate of biologic use (43% vs. 28%, p = 0.003) and osteophyte formation (82% vs. 66%, p = 0.001).
Table 2. Patient characteristics stratified by serum C-reactive protein (CRP) levels.
A significantly longer time from RA onset to TJR was observed in the CRP(–) group than in the CRP(+) group [median (IQR), 16 (8, 24) vs. 12 (8, 20), p = 0.022] (), and in subjects with osteophytes than in those without osteophytes [median (IQR), 14 (8, 23) vs. 12 (7, 18), p = 0.019] (. Time from RA onset to TJR in patients with EORA was significantly shorter compared to that in patients with YORA [median (IQR), 6 (3, 10) vs. 17 (11, 24), p < 0.001] (. There was no significant difference in time from RA onset to TJR stratified by sex and use of MTX, biologics, and/or glucocorticoids. We next performed multiple regression analysis with CRP, age at RA onset, surgery site, and osteophyte formation as categorical variables. Negative CRP was associated with time from RA onset to TJR [partial regression coefficient (B) =2.44, p = 0.016] independently of age at RA onset, surgery site, and osteophyte formation ().
Figure 3. Time from rheumatoid arthritis (RA) onset to total joint replacement (TJR) stratified by (a) serum C-reactive protein (CRP) levels, (b) osteophytes on radiograph, (c) age at RA onset, and (d) surgery site. The box plot shows the median value (central line) and 25th and 75th percentiles (horizontal lines); whiskers indicate minimum and maximum values; and circles represent outliers. Statistical comparison was performed using the Mann–Whitney U test. EORA: elderly-onset RA; YORA: younger-onset RA.
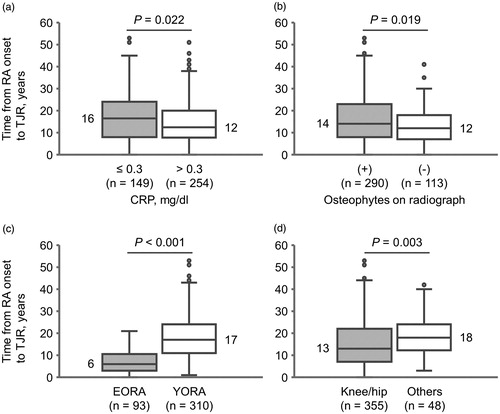
Table 3. Factors associated with time from rheumatoid arthritis (RA) onset to total joint replacement.
Discussion
In this study, we found that the number of TJRs performed in patients with RA decreased from 2004 to 2017. Over this period, the demographic and clinical characteristics of patients with RA at the time of TJR also showed changes. Specifically, there were increases in both age at surgery and age at RA onset, as well as in the proportion of patients receiving MTX and/or biologics, and a decrease in serum CRP levels. Contrary to a previous report [Citation17], no significant change was observed in time from RA onset to TJR. Multivariate analysis revealed that negative CRP (i.e. ≤0.3 mg/dl) was independently associated with time from RA onset to TJR. To our knowledge, this is the first study to report time trends in the characteristics of patients at the time of TJR, specifically with a focus on patients with RA.
This study found a decrease in the number of TJRs performed in patients with RA since 2004, along with an increase in the proportion of patients with negative CRP and those receiving MTX and/or biologics, consistent with findings from previous studies in Japan [Citation3,Citation4]. Moreover, according to our hypothesis that suppression of disease activity may lead to delays in TJR, negative CRP was correlated with longer times from RA onset to TJR. Notably, the use of MTX and/or biologics was not associated with time from RA onset to TJR. This may be due to subjects being limited to patients undergoing TJR and selection bias for treatment. Tight control of disease activity is important in patients with RA from the perspective of avoiding TJR [Citation23]. Our previous longitudinal studies revealed that a significantly lower number of TJRs were performed in patients receiving combination therapy with biologics and MTX than in patients receiving biologic monotherapy [Citation8,Citation9]. A cohort study showed that greater duration of exposure to MTX soon after an RA diagnosis was associated with delays in TJR [Citation24]. Although causality cannot be proven due to the cross-sectional design, our findings suggest that suppression of disease activity with newer medications might have decreased and delayed the need for TJR in patients with RA.
Recent studies have shown that levels of serum CRP and disease activity were reduced in patients with RA, with an accompanying increase in the proportion of patients receiving DMARDs such as MTX and biologics [Citation25,Citation26]. In this study, serum CRP levels dramatically decreased over the course of the study period, and more than 50% of patients had negative CRP between 2014 and 2017. Relative to the CRP(+) group, the CRP(–) group was more likely to have a higher rate of biologic use. These findings suggest that newer medications including biologics have reduced disease activity in patients with RA undergoing TJR, as well as in the general population of patients with RA. Preoperative disease activity and serum CRP levels have been reported to be negatively correlated with postoperative function in patients undergoing total hip replacement [Citation11]. Better control of preoperative disease activity, which is now achievable, may further improve outcomes in patients with RA who undergo TJR in the future.
A higher rate of osteophyte formation was observed in the CRP(–) group compared to the CRP(+) group. Moreover, osteophyte formation was correlated with longer times from RA onset to TJR. Osteophyte formation is included in the definition of structural remodeling of large joints according to the ‘assessment of rheumatoid arthritis by scoring of large joint destruction and healing in radiographic imaging’ (ARASHI) score [Citation27]. The ARASHI score suggests that osteophyte formation may contribute to the stability of large joints. Accordingly, osteophytes formed as a result of suppressed inflammation could delay TJR via stabilization of large joints. In other words, our findings suggest that an earlier TJR is required for damaged joints without osteophytes due to residual inflammation.
According to a nationwide database study in Japan, age at RA onset has increased significantly over the last decade, likely due to the country’s rapidly aging population [Citation28]. In this study, age at RA onset increased in patients undergoing TJR over the past 14 years. These changes can be attributed to an increase in age at RA onset in patients with RA as a whole. Given the bias of preferentially avoiding TJR in younger patients, patients with EORA naturally had a shorter time to TJR. Large joints are more often involved in patients with EORA [Citation29], and disease activity is higher at onset [Citation30] compared to patients with YORA. These clinical features of EORA also may have led to an earlier TJR in older patients.
According to a previous study in Japan, the disease duration of patients undergoing total knee arthroplasty gradually increased from 2003 to 2009 [Citation17]. In contrast, time from RA onset to TJR showed no increase during our study period, despite the significant decrease in serum CRP levels. This may be due to the significant increase in age at RA onset over time, which was negatively correlated with time from RA onset to TJR. The discrepancy between the previous study and this study is likely due to the longer and more recent study period of our study. Indeed, our findings are supported by a more recent study [Citation4], which found no significant difference in the disease duration of patients who underwent orthopedic surgery between 2004 and 2014. The increase in age at surgery might have resulted from the increase in age at RA onset and no significant changes in time from RA onset to TJR during our study period.
This study has some limitations worth noting. First, this study did not include information regarding the total number of patients with RA in our institute each year. Therefore, it is impossible to determine the annual proportion of patients with RA undergoing TJR. However, we presume that the annual incidence of TJR has decreased over time, as it is unlikely that the total number of patients with RA decreased in our institute during the study period. Second, we could not obtain sufficient data concerning measures of disease activity (e.g. Disease Activity Score in 28 joints) and physical function in order to consider these factors. Finally, since we adopted a cross-sectional design, our findings cannot demonstrate causality (i.e. that suppression of disease activity and age at RA onset influence time to TJR). Further longitudinal studies will be needed to clarify this aspect.
In conclusion, the number of TJRs performed in patients with RA has decreased since biologics were approved in Japan. Moreover, age at surgery, age at RA onset, and the proportion of patients receiving MTX and/or biologics increased, while disease activity decreased, in patients with RA undergoing TJR. Negative CRP, defined as ≤0.3 mg/dl, was independently associated with longer times from RA onset to TJR. Newer medications and more aggressive treatment strategies to suppress disease activity can reduce the number of and delay TJR in patients with RA.
Conflict of interest
None.
References
- Breedveld FC, Kalden JR. Appropriate and effective management of rheumatoid arthritis. Ann Rheum Dis. 2004;63(6):627–33.
- Goodman SM, Johnson B, Zhang M, Huang WT, Zhu R, Figgie M, et al. Patients with rheumatoid arthritis have similar excellent outcomes after total knee replacement compared with patients with osteoarthritis. J Rheumatol. 2016;43(1):46–53.
- Momohara S, Inoue E, Ikari K, Kawamura K, Tsukahara S, Iwamoto T, et al. Decrease in orthopaedic operations, including total joint replacements, in patients with rheumatoid arthritis between 2001 and 2007: data from Japanese outpatients in a single institute-based large observational cohort (IORRA). Ann Rheum Dis. 2010;69(01):312–3.
- Matsumoto T, Nishino J, Izawa N, Naito M, Hirose J, Tanaka S, et al. Trends in treatment, outcomes, and incidence of orthopedic surgery in patients with rheumatoid arthritis: an observational cohort study using the Japanese National Database of Rheumatic Diseases. J Rheumatol. 2017;44(11):1575–82.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331–7.
- Leon L, Abasolo L, Carmona L, Rodriguez-Rodriguez L, Lamas JR, Hernandez-Garcia C, et al. Orthopedic surgery in rheumatoid arthritis in the era of biologic therapy. J Rheumatol. 2013;40(11):1850–5.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983–2007. Ann Rheum Dis. 2010;69(5):868–71.
- Asai S, Kojima T, Oguchi T, Kaneko A, Hirano Y, Yabe Y, et al. Effects of concomitant methotrexate on large joint replacement in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a multicenter retrospective cohort study in Japan. Arthritis Care Res (Hoboken). 2015;67(10):1363–70.
- Asai S, Takahashi N, Funahashi K, Yoshioka Y, Takemoto T, Terabe K, et al. Concomitant methotrexate protects against total knee arthroplasty in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors. J Rheumatol. 2015;42(12):2255–60.
- Momohara S, Kawakami K, Iwamoto T, Yano K, Sakuma Y, Hiroshima R, et al. Prosthetic joint infection after total hip or knee arthroplasty in rheumatoid arthritis patients treated with nonbiologic and biologic disease-modifying antirheumatic drugs. Mod Rheumatol. 2011;21(5):469–75.
- Imagama T, Tokushige A, Seki K, Seki T, Ogasa H, Taguchi T. Risk factors associated with short-term clinical results after total hip arthroplasty for patients with rheumatoid arthritis. Orthopedics 2018;41(6):e772
- Cordtz RL, Zobbe K, Højgaard P, Kristensen LE, Overgaard S, Odgaard A, et al. Predictors of revision, prosthetic joint infection and mortality following total hip or total knee arthroplasty in patients with rheumatoid arthritis: a nationwide cohort study using Danish healthcare registers. Ann Rheum Dis. 2018;77(2):281–8.
- Haraguchi A, Nakashima Y, Miyahara H, Esaki Y, Okazaki K, Fukushi JI, et al. Minimum 10-year results of cementless total hip arthroplasty in patients with rheumatoid arthritis. Mod Rheumatol. 2017;27(4):598–604.
- Kirksey M, Chiu YL, Ma Y, Della Valle AG, Poultsides L, Gerner P, et al. Trends in in-hospital major morbidity and mortality after total joint arthroplasty: United States 1998–2008. Anesth Analg. 2012;115(2):321–7.
- Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637–47.
- Singh JA, Lewallen DG. Time trends in the characteristics of patients undergoing primary total knee arthroplasty. Arthritis Care Res (Hoboken). 2014;66(6):897–906.
- Momohara S, Ikari K, Kawakami K, Iwamoto T, Inoue E, Yano K, et al. The increasing disease duration of patients at the time of orthopaedic surgery for rheumatoid arthritis. Rheumatol Int. 2012;32(10):3323–4.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81.
- Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthr Cartilage. 2007;15(Suppl A):A1–56.
- Terkeltaub R, Esdaile J, Décary F, Tannenbaum H. A clinical study of older age rheumatoid arthritis with comparison to a younger onset group. J Rheumatol. 1983;10:418–24.
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR' for medical statistics. Bone Marrow Transpl. 2013;48(3):452–8.
- Sano H, Arai K, Murai T, Fujisawa J, Kondo N, Netsu T, et al. Tight control is important in patients with rheumatoid arthritis treated with an anti-tumor necrosis factor biological agent: prospective study of 91 cases who used a biological agent for more than 1 year. Mod Rheumatol. 2009;19(4):390.
- Widdifield J, Moura CS, Wang Y, Abrahamowicz M, Paterson JM, Huang A, et al. The longterm effect of early intensive treatment of seniors with rheumatoid arthritis: a comparison of 2 population-based cohort studies on time to joint replacement surgery. J Rheumatol. 2016;43(5):861–8.
- Yamanaka H, Seto Y, Tanaka E, Furuya T, Nakajima A, Ikari K, et al. Management of rheumatoid arthritis: the 2012 perspective. Mod Rheumatol. 2013;23(1):1–7.
- Pedersen AB, Mor A, Mehnert F, Thomsen RW, Johnsen SP, Nørgaard M. Rheumatoid arthritis: trends in antirheumatic drug use, C-reactive protein levels, and surgical burden. J Rheumatol. 2015;42(12):2247–54.
- Kaneko A, Matsushita I, Kanbe K, Arai K, Kuga Y, Abe A, et al. Development and validation of a new radiographic scoring system to evaluate bone and cartilage destruction and healing of large joints with rheumatoid arthritis: ARASHI (Assessment of rheumatoid arthritis by scoring of large joint destruction and healing in radiographic imaging) study. Mod Rheumatol. 2013;23:1053–62.
- Kato E, Sawada T, Tahara K, Hayashi H, Tago M, Mori H, et al. The age at onset of rheumatoid arthritis is increasing in Japan: a nationwide database study. Int J Rheum Dis. 2017;20(7):839–45.
- Inoue K, Shichikawa K, Nishioka J, Hirota S. Older age onset rheumatoid arthritis with or without osteoarthritis. Ann Rheum Dis. 1987;46(12):908–11.
- van der Heijde DM, van Riel PL, van Leeuwen MA, van 't Hof MA, van Rijswijk MH, van de Putte LB. Older versus younger onset rheumatoid arthritis: results at onset and after 2 years of a prospective followup study of early rheumatoid arthritis. J Rheumatol. 1991;18:1285–9.
