Abstract
Objectives: Adverse drug reactions (ADRs) related to liver dysfunction are a common problem in patients with rheumatoid arthritis (RA) receiving iguratimod, but which patient subgroups go on to discontinue iguratimod treatment is unclear. A post-hoc analysis of a post-marketing surveillance study was performed to investigate factors influencing treatment continuation after the onset of liver dysfunction.
Methods: Types of ADR were compared between patients in whom iguratimod treatment was discontinued or continued in accordance with the judgment of the patient’s physician after the patient developed liver dysfunction as an ADR. Stepwise logistic regression analysis was also conducted to investigate factors associated with treatment discontinuation.
Results: The multivariate analysis found that concomitant use of methotrexate (MTX) at >8 mg/week (vs. no use) was associated with a significantly lower risk of discontinuation (OR: 0.136; 95%CI: 0.030–0.620), and previous treatment with MTX (vs. no use) was associated with a significantly higher discontinuation risk (OR: 4.045; 95%CI: 1.098–14.908).
Conclusion: Although concomitant use of MTX during iguratimod treatment does not appear to influence treatment discontinuation due to abnormal liver function, liver function tests are of importance to continued treatment in patients receiving iguratimod who have a history of MTX use.
Introduction
In the treatment of rheumatoid arthritis (RA), the use of disease-modifying antirheumatic drugs (DMARDs) is recommended as early as possible after diagnosis [Citation1]. However, DMARDs show a high incidence of adverse drug reactions (ADRs), including frequencies of 30–50% for gastrointestinal disorder, liver dysfunction and kidney dysfunction. The first-line DMARD is methotrexate (MTX), which is known to cause ADRs including bone marrow damage, interstitial pneumonia, infection, gastrointestinal disorders and liver dysfunction. Folic acid is concomitantly administered to prevent liver dysfunction and gastrointestinal symptoms as ADRs due to MTX, and its concomitant use is particularly recommended in patients with risk factors for ADR, such as the elderly and those with decreased renal function [Citation2]. Identifying which patients are susceptible to these ADRs and whether these ADRs necessitate treatment discontinuation is important, not only so that ADRs can be dealt with in a timely manner, but also to ensure continuation of RA treatment and achievement of treatment goals.
Iguratimod is a conventional synthetic DMARD (csDMARD) developed in Japan. Many patients in phase-III studies developed serious ADRs in the form of liver dysfunction due to increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and gastrointestinal disorders due to peptic ulcers [Citation3]. After regulatory approval in 2012, a post-marketing surveillance study of all patients using iguratimod was conducted. The all-case study found a 38.26% incidence of ADRs, the most common of which were gastrointestinal disorders (10.43%) and liver dysfunction (9.71%) [Citation4]. Among gastrointestinal disorders, peptic ulcer was an ADR requiring particular attention, and tended to be common among patients taking concomitant non-steroidal anti-inflammatory drugs (NSAIDs) and patients with a history of ulcer. Bleeding-related ADRs observed in the all-case study were suspected to be attributable to interaction with warfarin, and the details of this have been reported previously [Citation5]. Increases in ALT and AST were common in cases of liver dysfunction, but many patients showed transient changes and were able to continue treatment, whereas others had to discontinue treatment. Risk factors for liver dysfunction were investigated [Citation4], but the characteristics of patients who had to discontinue treatment were not studied. Whether to discontinue treatment is determined subjectively by physicians, with the decision being made based on a comprehensive evaluation of objective data including patient background information and laboratory test results. Therefore, investigation of factors influencing physician decisions to discontinue is important, as this will provide clinically important data for achieving the treatment goals of iguratimod. We therefore conducted a post-hoc analysis of the post-marketing surveillance study to investigate patient factors influencing the physician’s decision to discontinue treatment after the onset of liver dysfunction.
Methods
Patients
The all-case post-marketing surveillance study covered 2608 institutions throughout Japan from September 2012 to November 2015. All-cases using iguratimod between September 2012 and April 2013 were registered, and safety and efficacy under conditions of actual use were evaluated up to 52 weeks after iguratimod administration. For the safety evaluation, blood disorders, liver dysfunction, kidney dysfunction, gastrointestinal disorders (peptic ulcer), interstitial pneumonia and infection were focused on as special interest of study [Citation5]. To investigate factors impacting the decision process of physicians, as to whether or not to continue treatment after the onset of liver dysfunction, the post-hoc analysis included those patients in the safety analysis set who developed liver dysfunction as an ADR after iguratimod administration. The all-case study was conducted in accordance with the Good Post-marketing Study Practice (GPSP) Ordinance of the Japanese Ministry of Health, Labour and Welfare, and was registered in advance in JAPIC (JapicCTI-152782 and JapicCTI-132051) and ClinicalTrials.gov (NCT01850966). The all-case study was conducted by Eisai Co., Ltd and Toyama Chemical Co., Ltd.
Safety assessment
Adverse events were collected from reports by participating physicians and coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 19.1. AEs and ADRs were defined and classified in accordance with ICH E2A [Citation6] and E2D [Citation7] guidelines. AEs were classified by Preferred Term (PT) and System Organ Class (SOC).
Classification of liver dysfunction
The definitions used in the previously reported all-case study results were used [Citation5]. Among AEs collected in the study, events related to liver function under MedDRA SOC and PT were defined as liver dysfunction. Alcohol-related, pregnancy-related and liver neoplasm-related liver disorders were excluded based on a Standardised MedDRA Query (SMQ) for liver disorder. Events related to interactions with warfarin were also excluded.
Definition of transient liver dysfunction based on laboratory values
To investigate whether elevated laboratory values due to liver dysfunction ADRs were transient or protracted, transient liver dysfunction was defined as a transient elevation in laboratory values. AST and ALT were selected from the study data, as these are generally used in liver function tests. Common reference ranges as defined by the Japanese Committee for Clinical Laboratory Standards were selected as the reference laboratory values for Japanese individuals (AST: <30 IU/L, ALT: male <42 IU/L, female <23 IU/L) [Citation8]. The normal range in this study was set at ≤2 × reference value, since the Japan College of Rheumatology MTX Clinical Guideline [Citation2] defines AST and ALT values 2 × reference value as the condition of liver disorder requiring cautious administration. In clinical studies, treatment discontinuation for elevated AST and ALT was not necessary if these values normalized within around 4 weeks [Citation9]. The package insert also recommends performing liver function tests at least once a month, and many patients with elevated laboratory test values show a return to the normal range within 4 weeks. In view of the above considerations, transient liver dysfunction was defined as both AST and ALT returning to the normal range (AST: <60 IU/L, ALT: male <84 IU/L female <46 IU/L) within 4 weeks after a liver dysfunction ADR.
Protracted liver dysfunction, by definition, did not meet the above criteria.
Analysis
SAS System version 9.3 (SAS Institute, Cary, NC, USA) was used for all analyses. The statistical significance level was set at two-tailed 5%.
Logistic regression analysis
Stepwise logistic regression analysis was also conducted to investigate factors associated with discontinuation as decided by the patient's physician after the onset of liver dysfunction. The significance level was set at 20% for the Wald test, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using the extracted factors for the final model.
In and , the following covariates were used: age, body weight, duration of RA, Steinbrocker stage classification, Steinbrocker functional classification, liver dysfunction at start of administration, kidney dysfunction at start of administration, comorbidities, allergy history, concomitant MTX dose, concomitant use of biological preparation, concomitant use of steroid, prior treatment with MTX, concomitant use of NSAIDs and concomitant use of folic acid.
Figure 1. Factors associated with post-ADR discontinuation. Results of a multivariate analysis of patient background factors. Five factors were left for the final model. Concomitant use of MTX >8 mg (vs. none) was the factor associated with a high likelihood of continuation and previous MTX treatment (vs. none) was the factor associated with a high likelihood of discontinuation.
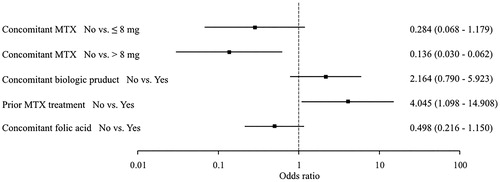
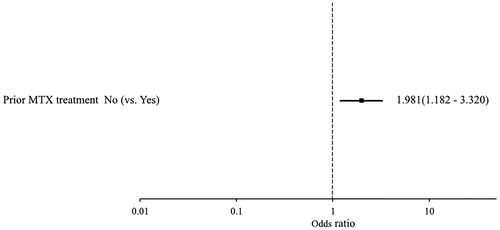
Figure 2. Factors associated with protraction of abnormal laboratory values. Patient background factors associated with protracted liver dysfunction were investigated based on laboratory test values. Multivariate analysis only selected previous MTX treatment for the final model, and showed previous MTX treatment (vs. no) as a factor significantly associated with protraction of abnormal laboratory values.
Furthermore, among the possible covariates, variance inflation factors (VIFs) were calculated as variables to be used in the multivariate analysis. For qualitative variables, however, VIFs were obtained after these variables had been converted to integers as shown in the examples below: duration of RA (>5 years, 0; ≥5 years and <10 years, 1; ≥10 years, 2) and Steinbrocker stage classification (Stages I + II, 0; Stages III + IV, 1).
Results
Type of ADR and patient background
Liver dysfunction occurred in 9.71% (259/2666) of the 2666 patients in the safety analysis set of this study. Among these 259 patients, 42.47% (110/259) continued iguratimod treatment after the ADR, and 57.53% (149/259) discontinued iguratimod treatment after the ADR, either because of the ADR or for some other reason.
In order to investigate whether or not the ADR types influenced the decisions of physicians, we investigated the post-ADR status of iguratimod administration by the types of ADRs (). In this study, all 259 patients were included in the further statistical analysis. Although ADRs of liver dysfunction, increased ALT, and increased AST each differed by ≥5%, these events did not appear to strongly influence whether iguratimod treatment was continued or discontinued. A patient with an ADR who showed ‘increased HBV DNA levels’ had concomitantly used Entecavir after consulting with a liver specialist, and fully recovered. Post-ADR treatment status was also investigated by patient background factor, since these factors could have influenced whether treatment was continued after the ADR (). Age, concomitant use/nonuse of MTX, and concomitant use/nonuse of folic acid were categories with a ≥ 10% difference in proportion of patients. Among the 130 patients treated with MTX prior to iguratimod administration, 116 patients continued to use MTX after starting iguratimod administration. On the other hand, among the 129 patients not previously treated with MTX, 122 patients did not use MTX after starting iguratimod administration (Supplemental Table 1).
Table 1. Post-ADR Status of iguratimod administration by type of ADR (liver dysfunction).
Table 2. Background characteristics of patients with liver dysfunction ADRs.
Factors leading to discontinuation of iguratimod treatment after the onset of an ADR
Multivariate analysis was performed to further investigate patient background factors influencing post-ADR iguratimod treatment continuation as decided by the patient's physician after the ADR. The same patient background factors listed in were used. The multivariate analysis revealed the VIFs for each explanatory variable to be 4 or less, showing no collinearity. Thus, we employed stepwise analysis to determine the final model (Supplemental Table 2). Concomitant use of MTX >8 mg/week (vs. no use) was associated with a significantly low risk of discontinuation (OR: 0.136; 95%CI: 0.030–0.620), and previous treatment with MTX (vs. no use) was associated with a significantly high risk of discontinuation (OR: 4.045; 95%CI: 1.098–14.908) (). The analysis also found that concomitant use of MTX ≤8 mg/week (vs. no use) was associated with a not significantly but low trend toward a greater risk of discontinuation (OR: 0.284; 95%CI: 0.068–1.179).
Risk of ADR prolongation based on laboratory test values
Many liver dysfunction ADRs were due to elevated laboratory test values. No patients had treatment discontinued because of elevated ALT and AST in phase-III studies which involved coadministration with an MTX [Citation10], but discontinuations were observed in the all-case study. Although elevated laboratory test values were transient in many cases, treatment may have been discontinued in some cases when an elevated value remained abnormally high for a protracted period. Patients with liver dysfunction ADRs in whom concentrations of ALT and AST had been measured were therefore divided according to whether elevated laboratory values were transient or protracted, and factors were investigated in patients with protracted elevation. In this study, transient liver dysfunction was defined as both AST and ALT returning to their normal ranges (AST: <60 IU/L, ALT: male <84 IU/L female <46 IU/L) within 4 weeks after a liver dysfunction ADR, while the remainder were defined as protracted liver dysfunctions (Refer to the Methods section). Multivariate analysis identified prior MTX treatment (vs. no use) as a factor associated with protraction of abnormal laboratory values (OR 1.981; 95%CI: 1.182–3.320) (). This could explain why treatment discontinuation was common in patients who had previously used MTX.
Discussion
This is the first research paper to investigate treatment continuation after the onset of liver dysfunction ADRs, which are often seen in patients treated with iguratimod. The decision made by the patient’s physician regarding treatment continuation after the onset of liver dysfunction ADRs was unrelated to the type of ADR. As to the factors affecting the physician’s decision to discontinue treatment after ADR onset, concomitant use of MTX >8 mg/week was associated with a low risk compared to no use, and prior MTX treatment was associated with a high risk. Analysis using laboratory test values also clarified that prior MTX treatment was a factor associated with protraction of elevated laboratory values.
The incidence of liver dysfunction in the all-case study was 9.71%, with increased ALT and/or increased AST accounting for much of this percentage. In a phase-III comparison study with salazosulfapyridine, the incidence of liver dysfunction in the iguratimod group was 21.5% [Citation3], and in a phase-III coadministration study with MTX, the incidence of increased AST was 9.8% and increased ALT was 5.5% [Citation10]. The population included in our research does not appear to be a special population, since the incidence of liver dysfunction, including abnormal laboratory values, was broadly similar in the clinical studies.
MTX pneumonia and bone-marrow disorder ADRs are known to lead to discontinuation of MTX treatment [Citation11], and the same is true for headache, dizziness and leukopenia in salazosulfapyridine treatment [Citation12]. Elevated laboratory values were often seen in the all-case study, and although no patients developed serious liver dysfunction [Citation4], it seemed possible that specific ADRs were resulting in discontinuation. The presence of bias in ADRs was therefore investigated. Among MedDRA PTs, abnormal liver function was common in discontinuations, while increased AST and increased ALT tended to be common in patients who continued treatment. However, despite having different MedDRA codes, ADRs were due to increases in laboratory values (AST and ALT). These differences across continuations and discontinuations were therefore suspected to arise from differences in the ADR terms reported by clinicians. A phase-III coadministration study with MTX found that the iguratimod + MXT group and placebo + MTX group showed increased AST at incidence of 9.8% and 5.7%, respectively, and increased ALT of 5.5% and 8.0%, respectively, and the incidence of AST or ALT >100 IU was 1.8% [Citation10]. These findings suggest that the type of liver dysfunction ADR arising from iguratimod treatment did not influence the patient’s physician when deciding whether or not to discontinue treatment. The incidence of these ADRs was also similar in the all-case study and in the phase-III MTX coadministration study.
Among the factors affecting the decision-making process of the patient’s physician regarding iguratimod discontinuation after ADR onset, concomitant use of MTX >8 mg/week was associated with a significantly lower risk of discontinuation compared to nonuse. Concomitant use of MTX ≤8 mg/week also tended to carry a lower risk of discontinuation compared to nonuse. This indicates that the dose of concomitant MTX does not influence the risk of post-ADR discontinuation. Dose-dependent increases in the occurrence of ADRs due to MTX have been reported [Citation13]. A retrospective Spanish study found that coadministration with MTX or hydroxychloroquine was a risk factor for suspension of leflunomide treatment [Citation14]. Differences were therefore expected in this study depending on concomitant use and dose of MTX, but our results did not bear this out. When using MTX to treat RA, the dose is increased to a sufficient amount if the initial treatment is ineffective. Although there is evidence that the increase in incidence of ADRs is limited when increasing MTX to a sufficient dose [Citation11], other drugs are sometimes used additionally in consideration of ADR risk factors. Japanese guidelines similarly recommend a normal starting MTX dosage of 6–8 mg/week, which is increased depending on tolerability and the presence of risk factors for MTX-related ADRs [Citation2]. This means that patients concomitantly using MTX >8 mg/week probably are at less risk for developing ADRs that would necessitate MTX treatment discontinuation. Namely, these patients may have a higher tolerance to drugs including iguratimod, and thus would be less prone to develop ADRs leading to treatment discontinuation. Many patients in this study who had previously used MTX were administered iguratimod while continuing to receive MTX. It is therefore possible that patients previously treated with MTX included patients in whom the MTX dose was unable to be increased. However, since the all-case study did not collect detailed information as reasons for changing concomitant drug dose, factors such as MTX tolerance could not be investigated in detail. Furthermore, concomitant use of several DMARDs had no influence on the post-ADR continuation rate compared to drug monotherapy [Citation15], and concomitant use of csDMARD and MTX is not currently thought to cause an increase in ADRs compared to MTX monotherapy [Citation16]. The all-case study also found that concomitant MTX was not a risk factor for ADRs [Citation9], indicating that the results of this study were in line with the conclusions of previous reports.
On the other hand, ‘Prior MTX treatment’ was associated with a significantly higher probability of a physician deciding to discontinue treatment Post-ADR iguratimod discontinuation was more strongly influenced by patient background factors before iguratimod administration than by the condition of the patient during administration. Registry research into reasons for discontinuation of treatment with biologic DMARDs has reported that the factors most likely to lead to treatment discontinuation are patient background factors such as number of DMARDs used in prior treatment [Citation17]. The factors affecting the decision of the patient’s physician to discontinue treatment after the onset of liver dysfunction in this study was therefore also likely to be a background factor existing before iguratimod administration. In routine clinical practice, certain liver dysfunctions are commonly seen in patients receiving long-term MTX administration, although the treatment is continued in many cases [Citation18]. Liver dysfunction is often observed during MTX treatment and is regarded as an ADR of special interest [Citation2]. Some of the included patients previously treated with MTX may have developed liver dysfunction or at least shown its signs before receiving iguratimod. When these patients again develop drug-induced liver dysfunction, their physicians are likely to discontinue iguratimod treatment for fear of exacerbating the condition. Thus, it is reasonable to suppose that the patient's physician chose to discontinue iguratimod if there was protracted elevation of liver laboratory values, after considering the patient's background factors. Notably, serious ADRs were probably prevented as a result of this decision.
As a post-hoc analysis of an all-case study, this study had the following limitations. All-case study collects information within the scope of routine practice and therefore cannot collect information on a large number of background factors. This was not a specialized study designed to investigate liver dysfunction ADRs. The presence of unmeasured confounding factors thus cannot be ruled out. In addition, registered patients had only been started administration of iguratimod for about 7 months after the start of marketing, and the sample size in this study was small compared to the overall all-case study. Further research is therefore needed when greater experience of use has been accumulated.
In conclusion, although concomitant use of MTX at any dose during iguratimod treatment does not appear to influence treatment discontinuation due to abnormal liver function, liver function tests are of particular importance for continuing treatment in patients receiving iguratimod who have a history of MTX use. Iguratimod discontinuation may also prevent significant ADRs in patients with protracted abnormal laboratory values.
Author contributions
NI contributed to acquisition and interpretation of the data, as well as both writing and critically reviewing the manuscript. KS, AY, SI, and M I contributed to the design, conduct, and analysis of the study and either writing or critically reviewing the manuscript. All authors approved the final version of this manuscript for submission.
Conflict of interest
N. Ishiguro received research grants or speaker’s fees from Astellas Pharma Inc., AbbVie GK, Asahi Kasei Corp., Bristol-Myers Squibb Company, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., Kaken Pharmaceutical Co., Ltd., Medical Corporation SANJINKAI, Medical Corporation TOUKOUKAI, Mitsubishi Tanabe Pharma Corp., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Taisho Toyama Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Zimmer Biomet G.K. and received consulting fees from Ono Pharmaceutical Co., Ltd. K. Shibata, A. Yoshimura, S. Ikeuchi and M. Ishii are employees of Eisai Co., Ltd.
Supplemental Material
Download MS Word (43.6 KB)Acknowledgements
We are grateful to the physicians and staff at the 2608 institutions that took part in the all-case post-marketing surveillance study, and also wish to thank the Japan College of Rheumatology PMS (post-marketing surveillance) Committee for safety advice during the study and Professor Kazuhiko Yamamoto for medical advice. We thank CMIC Co., Ltd. a contract research organization, for conducting all of the analyses.
References
- Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–77.
- Subcommittee on Development of Guidelines for the Use of Methotrexate in the Treatment of Rheumatoid Arthritis., editors. Japan College of Rheumatology Guideline for the Use of Methotrexate in Patients with Rheumatoid Arthritis. Tokyo: Yodosha; 2016.
- Hara M, Abe T, Sugawara S, Mizushima Y, Hoshi K, Irimajiri S, et al. Efficacy and safety of iguratimod compared with placebo and salazosulfapyridine in active rheumatoid arthritis: a controlled, multicenter, double-blind, parallel-group study. Mod Rheumatol. 2007;17(1):1–9.
- Hara M, Abe T, Sugawara S, Mizushima Y, Hoshi K, Irimajiri S, et al. Long-term safety study of iguratimod in patients with rheumatoid arthritis. Mod Rheumatol. 2007;17(1):10–6.
- Mimori T, Harigai M, Atsumi T, Fujii T, kuwana M, Matsuno H, et al. Safety and effectiveness of iguratimod in patients with rheumatoid arthritis: Final report of a 52-week, multicenter postmarketing surveillance study. Mod Rheumatol. 2018;27:1–10.
- Mimori T, Harigai M, Atsumi T, Fujii T, Kuwana M, Matsuno H, et al. Safety and effectiveness of 24-week treatment with iguratimod, a new oral disease-modifying antirheumatic drug, for patients with rheumatoid arthritis: interim analysis of post-marketing surveillance study of 2679 patients in Japan. Mod Rheumatol. 2017;27(5):755–65.
- ICH Harmonized Tripartite Guideline. Clinical safety data management: definitions and standards for expedited reporting E2A step 4. [dated 1994 Oct 27]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf [last accessed 30 Jul 2019]
- ICH Harmonized Tripartite Guideline. Post-approval safety data management: definitions and standards for expedited reporting E2D step 4. [dated 2003 Nov 12]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2D/Step4/E2D_Guideline.pdf [last accessed 30 Jul 2019]
- Japanese Committee for clinical laboratory standards. Common reference intervals for standardized clinical laboratory test in Japan. [updated 2014 Mar 31]. Available from: http://www.jccls.org/techreport/public_comment_201405_p.pdf [last accessed 30 Jul 2019]
- Ishiguro N, Yamamoto K, Katayama K, Kondo M, Sumida T, Mimori T, et al. Concomitant iguratimod therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate: a randomized, double-blind, placebo-controlled trial. Mod Rheumatol. 2013;23(3):430–9.
- Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009;68(7):1100–4.
- Amos RS, Pullar T, Bax DE, Situnayake D, Capell HA, McConkey B. Sulphasalazine for rheumatoid arthritis: toxicity in 774 patients monitored for one to 11 years. Br Med J (Clin Res Ed). 1986;293(6544):420–3.
- Yamanaka H, Inoue E, Tanaka E, Nakajima A, Taniguchi A, Terai C, et al. Influence of methotrexate dose on its efficacy and safety in rheumatoid arthritis patients: evidence based on the variety of prescribing approaches among practicing Japanese rheumatologists in a single institute-based large observational cohort (IORRA). Mod Rheumatol. 2007;17(2):98–105.
- Rodriguez-Rodriguez L, Jover-Jover JA, Fontsere O, Peña-Blanco RC, León L, Fernández-Gutierrez B, Abásolo L. Leflunomide discontinuation in rheumatoid arthritis and influence of associated disease-modifying anti-rheumatic drugs: a survival analysis. Scand J Rheumatol. 2013;42(6):433–6.
- Abasolo L, Leon L, Rodriguez-Rodriguez L, Tobias A, Rosales Z, Maria Leal J, et al. Safety of disease-modifying antirheumatic drugs and biologic agents for rheumatoid arthritis patients in real-life conditions. Semin Arthritis Rheum. 2015;44(5):506–13.
- Katchamart W, Trudeau J, Phumethum V, Bombardier C. Efficacy and toxicity of methotrexate (MTX) monotherapy versus MTX combination therapy with non-biological disease-modifying antirheumatic drugs in rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2009;68(7):1105–12.
- Strand V, Miller P, Williams SA, Saunders K, Grant S, Kremer J. Discontinuation of Biologic Therapy in Rheumatoid Arthritis: Analysis from the Corrona RA Registry. Rheumatol Ther. 2017;4(2):489–502.
- Yazici Y, Sokka T, Kautiainen H, Swearingen C, Kulman I, Pincus T. Long term safety of methotrexate in routine clinical care: discontinuation is unusual and rarely the result of laboratory abnormalities. Ann Rheum Dis. 2005; 64(2):207–11.
