Abstract
Real-world evidence, based on real-world data from routine clinical treatment, is becoming increasingly important for providing high-quality medical care. Large-scale cohort studies can provide useful access to some of this real-world evidence, as shown by the IORRA (Institute of Rheumatology, Rheumatoid Arthritis) cohort in Japan. This large cohort study of patients with rheumatoid arthritis (RA) has been surveying enrolled participants since its inception in 2000. In the last 19 years, it has served as a database for a wide range of research in areas including transitions in medical care at the clinical level, changes in therapeutic drugs, approaches to comorbidities, developments in pharmacoeconomics, and the effects of genomic information on treatment options. This research has resulted in the publication of 133 articles in English to date. IORRA monitors changes in the management of RA, and has quantified over time the daily experience of clinicians who provide routine medical care. Such observational databases, which reflect the reality of daily clinical practice, will become increasingly important and may provide a model for similar research in other disease areas.
Introduction
Rheumatoid arthritis (RA) is a progressive and debilitating chronic autoimmune disease of the joints that until recently had the almost certain outcome of joint deterioration, restrictions on daily activities, extraarticular morbidity, and diminished quality of life. The development of biologic disease-modifying anti-rheumatic drugs (bDMARDs) and targeted synthetic anti-rheumatic drugs (tsDMARDs) have revolutionized the treatment of this condition, but questions remain about how to integrate these new forms of treatment into ordinary clinical practice. Most evidence obtained in the course of new drug development is based on randomized controlled trials (RCTs) and the meta-analysis of those trials. However, although evidence-based medicine places great importance on RCTs and has used them to make clinical medicine more scientific, RCTs do not always accurately model the realities of routine clinical practice. RCTs inherently provide only a short-term evaluation of outcome measures for their subjects. Such results provide insufficient evidence for long-term outcomes in chronic RA patients who are under long-term treatment by rheumatologists [Citation1]. Today evidence-based clinical medicine is beginning to shift from depending solely on RCTs towards incorporating more real-world observational research. Cohort research, based on reliable documentation of routine clinical practice, provides access to real-world data (RWD) and real-world evidence (RWE).
The IORRA cohort: 19 years of data on RA in Japan
The Institute of Rheumatology at Tokyo Women’s Medical University is the largest specialized treatment facility for RA in Japan, treating approximately 6000 RA patients. There are an estimated 600,000 patients with RA in Japan, which means that the Institute treats approximately 1% of all Japanese RA patients. To access the information from this unique data pool and make that information readily available for research, we established the Institute of Rheumatology, Rheumatoid Arthritis (IORRA) cohort in 2000 modeled after the American Rheumatism Association Medical Information System (ARAMIS). Data have been collected twice annually for 19 years since October 2000; as of this writing (April 2019), the 38th data collection is underway. All RA patients who come to our institute and provide informed consent are registered in the cohort and given a 30-page form by their principal physician. They are asked to fill in the necessary sections at home and to return the form by mail.
The IORRA database consists of three domains: items self-reported by the patient (physical disability, a visual analog scale (VAS), symptoms, comorbidities such as fracture, malignancy, or infection, imaging procedures, surgeries, hospitalizations, drugs being taken, adverse drug reactions, etc.), assessments by the physician (joint-related findings such as swelling and pain, VAS, and extraarticular diseases), and laboratory test values (including assessment of disease activity and of adverse events). At each data collection, we receive forms from 5000 to 6000 participants; our response rate exceeds 98%. Physical disability of patients is monitored by the validated Japanese version of the Health Assessment Questionnaire (H-HAQ) throughout the studies [Citation2].
Analysis of this 19-year cumulative database of information from RA patients has led to the publication of 133 scientific papers in English. All research was approved by the Ethics Committee of Tokyo Women’s Medical University.
Findings from the IORRA cohort
Genomic information that contributes to the development and progression of RA
Both genetic and environmental factors contribute to the development of RA. With approval from the Genetic Ethics Committee and informed consent from each patient, the IORRA cohort has successfully collected and analyzed patient DNA for genomic research. Since the first genome-wide association study (GWS) in 2002, IORRA data have provided the basis for a number of significant results that demonstrate a complex genetic component of RA. In 2003, RIKEN confirmed that PAD14 (peptidyl arginine deaminase type IV) is an RA susceptibility gene [Citation3]; this finding was supported by data from IORRA [Citation4]. In 2014, IORRA and major RA research institutes inside and outside Japan cooperated in a GWAS meta-analysis of 100,000 patients, identifying 42 novel RA risk loci and bringing the total of recognized RA risk loci to 101 [Citation5]. Most of these gene clusters are involved in immune system functions. IORRA’s specific wealth of clinical information has also contributed to progress in heritability analysis, twin studies, and risk factor analysis for joint destruction and bone fracture in RA patients.
Effects of changes in treatment strategies on disease activity in RA patients
The introduction of bDMARDs to the public marketplace in 2003 marked the start of dramatic advances in the treatment of RA, leading to improved clinical outcomes. The quality of life for patients registered with IORRA has improved with the effectiveness of today’s new therapeutic drugs, with regard to both disease activity and physical disability () [Citation6].
Figure 1. Changes of drug use and disease activity of RA patients in the IORRA cohort from 2000 to 2018. Disease activity was categorized using the DAS28 score and standard methods. This figure is the updated version of previously published in Yamanaka et al. [Citation6].
![Figure 1. Changes of drug use and disease activity of RA patients in the IORRA cohort from 2000 to 2018. Disease activity was categorized using the DAS28 score and standard methods. This figure is the updated version of previously published in Yamanaka et al. [Citation6].](/cms/asset/9d0f8af8-f851-49ca-9c98-b5f0053689a9/imor_a_1660028_f0001_c.jpg)
A treat-to-target strategy for RA has recently become the standard of care for RA, with the target of complete remission. Findings at IORRA support this strategy; our data show that long-term remission correlates inversely with disease progression to permanent disability. Specifically, we found a correlation between the number of remission criteria met by the patient and the level of physical function that was retained. shows the relationship between the achievement of each remission criterion and the progression of functional disability of patients across six consecutive data points during a 2.5-year period from 2008 through 2010, based on twice-annual monitoring of patient information in the IORRA cohort as noted above.
Table 1. J-HAQ score progression, odds ratio by logistic regression model, applied to patients meeting a variety of remission criteria over a period of 2.5 years.
We can restate these findings as ‘The more times a patient achieves remission, the less deterioration in physical function.’ In fact, disability (J-HAQ score) worsened in only 7% of patients who satisfied the Boolean Trial criteria and also in only 7% of patients who satisfied the Boolean Practice criteria [Citation7] for remission in all 6 IORRA surveys during that time () [Citation8]. Furthermore, IORRA data clearly demonstrated that tapering of corticosteroid and/or methotrexate is common after achieving remission through bDMARDs; this information could not have been obtained from ordinary controlled studies such as RCTs [Citation9].
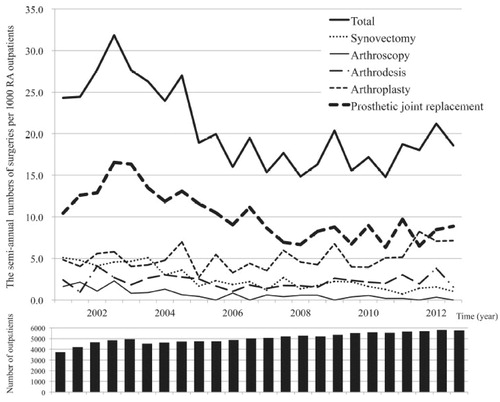
One of our studies examined the incidence of orthopedic surgery; our data show that the number of therapeutic knee and hip replacements has decreased () [Citation10]. Findings since the introduction of bDMARDs (2003) and the implementation of the treat-to-target strategy (2004) clearly demonstrate the increasing effectiveness of RA therapies after 2000.
Figure 2. The semiannual number of surgeries per 1000 outpatients with rheumatoid arthritis (RA) in a single institute-based large observational cohort (IORRA) study.
IORRA accumulated data on the incidence of extraarticular symptoms and comorbidities that commonly occur in RA patients, including interstitial pneumonia, lymphoproliferative disease, malignancy, cardiovascular disorders, infections, and depression. Data illustrated how comorbidities interfered with patient treatment options, resulting in suboptimal patient outcomes, increased disease activity, and disability [Citation11]. These findings emphasize the challenge of determining the best treatment for patients with comorbidities.
IORRA also clearly elucidated morbidity and mortality in RA patients [Citation12]. Quality of life and life expectancy are generally considered to be worse for these patients than for the general population, and this relationship remained consistent when comparing findings from European, North American, and Japanese cohort studies. However, the causes of death differed among these populations. Among Japanese RA patients under treatment with bDMARDs, the two biggest causes of death were pulmonary involvement including pneumonia and interstitial pneumonia and malignancy, while cardiovascular disease was the most common cause of death in RA patients. When tumor necrosis factor inhibitors (TNF-I) are introduced, the incidence of infections increases in the first three to six months, which led to some concerns about worsening morbidity and mortality in Japanese RA patients. However, a study of the use of bDMARDs, based on patient data from six medical institutions specializing in RA and the IORRA cohort data, showed mortality comparable to that of the general population [Citation13]. This reassured clinicians about the safety of TNF-I.
Osteoporosis in patients with RA
The IORRA cohort provided valuable data on fractures and osteoporosis in RA. One data analysis showed that fractures were reported in 14% of registered RA patients during a 5-year period, and that women experienced multiple fractures at a significantly higher rate than men. The incidence of non-vertebral fractures over 10 years in the IORRA cohort, showed no change in the fracture rate during that time, but the non-vertebral fracture rate increased sharply with age in women. Non-vertebral fractures occurred about three times as often as vertebral fractures, and age was strongly associated with increasing frequency of fractures in the vertebrae as well as at the proximal end of the femur. These findings led to a prospective investigation of fracture risk factors stratified by fracture site. Results showed that the risk of all fractures increased with age, that women were at risk for fractures of the vertebrae and the distal radius, that body mass index (BMI) predicted fracture of the proximal femur and the distal radius, that knee replacement surgery was a predictor of proximal femoral fracture, that DAS28 (disease activity score) was a predictor of vertebral fracture, that the JHAQ-DI (Japanese health assessment questionnaire and disability index) was a predictor of vertebral and proximal femoral fractures, and that the use of oral prednisolone significantly increased the risk of fracture at these three sites [Citation14].
The relationship between Vitamin D deficiency and osteoporosis in RA patients has also been analyzed. IORRA data showed vitamin D deficiency [serum 25-hydroxyvitamin D level <20 ng/mL] in 75% of women and 56% of men, and indicated a significant correlation between osteoporosis and female sex, younger age, physical disability, and use of oral prednisolone [Citation15].
Pharmacoeconomics of RA therapy
Although researchers have made great strides in the treatment of RA, the advent of expensive drug therapy has brought the cost of therapy into question and raised important social issues. Health insurance system is different in each country, and copayment of patients in Japan is 30%. Although there are several systems to support patients financially, pharmacoeconomic investigation, especially in patients receiving bDMARD, is quite important in Japan. Investigations have thus been initiated regarding the relationships between the rising cost of medical treatment, disease activity, and physical disability. All illness and medical treatment, including RA, involves direct costs for medical treatment and non-medical care, and indirect costs, such as the patient’s productivity loss and burden on their families and society. The physical disability associated with RA has always meant high indirect costs, and the recent advent of bDMARD has sharply increased the direct costs of RA treatment. A survey of direct costs affecting the IORRA cohort showed that increased direct treatment costs were associated with worsening disease activity as assessed by the DAS28, increased physical disability as assessed by the J-HAQ-DI, and reduced quality of life as assessed by the EQ-5D (a standardized test for measuring health-related quality of life) [Citation16]. These findings suggest that inadequate RA treatment tends to be associated with higher treatment costs.
At present, bDMARDs are much more expensive to administer and monitor than traditional antirheumatic medications. Although these new agents significantly inhibit disease activity and slow the progression of joint destruction and the deterioration of physical ability, their cost-effectiveness must be thoroughly investigated. Researchers have been able to apply simulation analysis to the IORRA cohort, accessing routine treatment data from Japanese patients with RA. With this approach, we showed it is economically feasible to use bDMARDs for treating patients with RA who have high disease activity [Citation17].
Comparison with RA cohorts in other parts of the world
Joint research has been conducted with representative cohort research consortia that are conducting RA research around the world, including the IORRA cohort in Japan, the Consortium of Rheumatology Research of North America (CORRONA) in the US, the Norfolk Arthritis Register (NOAR) in the United Kingdom, and the Swedish Rheumatology Registry for Early RA (SRR) [Citation18]. This joint research focused particularly on comparing the incidence of infection, malignant neoplasm, and cardiovascular disease in patients treated with fostamatinib in a clinical trial program. No significant differences were found in the outcomes of these cohorts after adjusting for age and sex. The results provide a meaningful rationale for conducting a world-wide epidemiological study of RA [Citation19].
Limitations
The IORRA cohort has become well-established, continues to provide ongoing information on daily clinical practice in Japanese RA patients, and has been the source of data for many published articles. However, there are several limitations in the IORRA cohort itself and in publications using the IORRA database. First, IORRA is a single institute-based cohort study, and although it covers approximately 1% of all RA patients in Japan, it is not representative of the entire population of Japanese RA patients. In particular, IORRA data is collected from patients at the Institute of Rheumatology, Tokyo Women’s Medical University, a typical urban medical institution located in the middle of Tokyo. Second, IORRA data is essentially collected when patients visit our Institute, and thus does not include much information from patients after they discontinue their visits. We tried to collect such information by mail, but approximately half of those patients did not respond to our request. Third, most of the IORRA database is constructed from individual patient questionnaires. We consider this approach to be quite intriguing, since patient-reported outcome has become increasingly important recently. However, information from such questionnaires is prone to ‘recall bias,’ with underestimation by some patients and overestimation by others. We tried to use medical charts to validate the accuracy of questionnaire-based patient information, but there are limits to what can be accomplished with this methodology. In addition to these limitations, each study includes inherent limitations. We have tried to minimize the influence of these limitations to the interpretation of IORRA publications, and we will continue to make every effort to do so. To minimize potential bias, we must maintain the commitment of all IORRA project members, particularly by motivating patients, rheumatologists, statisticians, and subscribers. This may be the most important lesson learned from this cohort study.
Summary
Our IORRA cohort has provided data for published papers responding to a variety of clinical questions on routine clinical practice. Real-world evidence, derived from the real-world data obtained from IORRA, can thus quantify clinician and patient experience in routine RA treatment over time. We were the first Japanese institution to do this kind of RA data collection to a global standard, and as pioneers, we helped to open the way for the many organizations that are doing observational research in Japan today. This kind of large-scale cohort, observed and recorded over an extended period, can be used to investigate a wide range of diseases, and can positively affect the decision-making of clinicians in Japan and around the world. We hope that this will, in time, result in improved prognosis, not only for RA but also for patients with other chronic diseases around the world.
Conflict of interest
HY has received speaker bureaus from Bristol-Meyers-Squibb, Pfizer, Teijin Pharma, and YLbio and research grant from AbbVie, Eisai, Bristol-Meyers, Novartis, Behringer, Astellas, Kaken, Nippon-Shinyaku, Pfizer, UCB, Ayumi, Ono, Daiichi-Sankyo, Taisyo-Toyama, Takeda, Tanabe-Mitsubishi, Chugai, Teijin Pharma, Torii, and YLbio.
ET has received lecture fees or consulting fees from Abbvie, Asahi Kasei, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Janssen, Nippon Kayaku, Pfizer, Takeda, Taisho Toyama, and UCB.
AN has received research grants from Asahi Kasei, Chugai, Daiichi Sankyo, Kissei, Mitsubishi Tanabe and Pfizer, and speaker fee from Abbvie GK, Actelion, Asahi Kasei, Astellas, Ayumi, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Janssen, Kissei, Mitsubishi Tanabe, Ono, Sanofi, Taisho, and Takeda, and consulting fees from Nippon Kayaku.
TF has served on speaker bureaus for the Asahi Kasei, Bristol-Myers Squibb, Chugai, Eisai, Ono, Pfizer, Takeda, and UCB.
KI has received speakers’ bureau fees from AbbVie, Astellas, Ayumi, Bristol- Meyers, Chugai, Daiichi Sankyo, Janssen Pharmaceutical, Lilly, Pfizer, Takeda, Tanabe-Mitsubishi and UCB.
AT has received speakers’ bureau fees from Teijin Pharma.
EI has declared no COI.
MH has received unrestricted research grants from Abbvie Japan, Ayumi, Bristol Myers Squibb, Eisai, Nippon Kayaku, Mitsubishi Tanabe, and Teijin Pharma, and has received speaker’s fees from Kissei, Ltd, Eli Lilly, and Chugai.
Acknowledgments
The authors would like to express our sincere thanks to all people involved with the IORRA cohort project.
References
- Yamanaka H, Tohma S. Potential impact of observational cohort studies in Japan on rheumatoid arthritis research and practice. Mod Rheumatol. 2006;16(2):75–6.
- Matsuda Y, Singh G, Yamanaka H, Tanaka E, Urano W, Taniguchi A, et al. Validation of a Japanese version of the Stanford Health Assessment Questionnaire in 3763 patients with rheumatoid arthritis. Arthritis Rheum. 2003;49(6):784–8.
- Suzuki A, Yamada R, Chang X, Tokuhiro S, Sawada T, Suzuki M, et al. Functional haplotypes of PAD14, citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet. 2003;34(4):395–402.
- Ikari K, Kuwahara M, Nakamura T, Momohara S, Hara M, Yamanaka H, et al. Association between PADI4 and rheumatoid arthritis: a replication study. Arthritis Rheum. 2005;52(10):3054–7.
- Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 2014;506(7488):376–81.
- Yamanaka H, Seto Y, Tanaka E, Furuya T, Nakajima A, Ikari K, et al. Management of rheumatoid arthritis: the 2012 perspective. Mod Rheumatol. 2013;23(1):1–7.
- Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63(3):573–86.
- Shidara K, Nakajima A, Inoue E, Hoshi D, Sugimoto N, Seto Y, et al. Continual maintenance of remission defined by the ACR/EULAR criteria in daily practice leads to better functional outcomes in patients with rheumatoid arthritis. J Rheumatol. 2017;44(2):147–53.
- Shimizu Y, Tanaka E, Inoue E, Shidara K, Sugimoto N, Seto Y, et al. Reduction of methotrexate and glucocorticoids use after the introduction of biological disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis in daily practice based on the IORRA cohort. Mod Rheumatol.2018;28(3):461–7.
- Momohara S, Inoue E, Ikari K, Ochi K, Ishida O, Yano K, et al. Recent trends in orthopedic surgery aiming to improve quality of life for those with rheumatoid arthritis: data from a large observational cohort. J Rheumatol. 2014;41(5):862–6.
- Nakajima A, Inoue E, Shimizu Y, Kobayashi A, Shidara K, Sugimoto N, et al. Presence of comorbidity affects both treatment strategies and outcomes in disease activity, physical function, and quality of life in patients with rheumatoid arthritis. Clin Rheumatol. 2015;34(3):441–9.
- Nakajima A, Inoue E, Tanaka E, Singh G, Sato E, Hoshi D, et al. Mortality and cause of death in Japanese patients with rheumatoid arthritis based on a large observational cohort, IORRA. Scand J Rheumatol. 2010;39(5):360–7.
- Nakajima A, Saito K, Kojima T, Amano K, Yoshio T, Fukuda W, et al. No increased mortality in patients with rheumatoid arthritis treated with biologics: results from the biologics register of six rheumatology institutes in Japan. Mod Rheumatol. 2013;23(5):945–52.
- Furuya T, Inoue E, Hosoi T, Taniguchi A, Momohara D, Yamanaka H. Risk factors associated with the occurrence of hip fracture in Japanese patients with rheumatoid arthritis: a prospective observational cohort study. Osteoporos Int. 2013;24(4):1257–65.
- Furuya T, Hosoi T, Tanaka E, Nakajima A, Taniguchi A, Momohara S, et al. Prevalence of and factors associated with vitamin D deficiency in 4,793 Japanese patients with rheumatoid arthritis. Clin Rheumatol. 2013;32(7):1081–7.
- Tanaka E, Hoshi D, Igarashi A, Inoue E, Shidara K, Sugimoto N, et al. Analysis of direct medical and nonmedical costs for care of rheumatoid arthritis patients using the large cohort database, IORRA. Mod Rheumatol.2013;23(4):742–51.
- Tanaka E, Inoue E, Yamaguchi R, Shimizu Y, Kobayashi A, Sugimoto N, et al. Pharmacoeconomic analysis of biological disease modifying antirheumatic drugs in patients with rheumatoid arthritis based on real-world data from the IORRA observational cohort study in Japan. Mod Rheumatol 2016;29:1–10.
- Nyberg F, Askling J, Berglind N, Franzén S, Ho M, Holmqvist M, et al. Using epidemiological registry data to provide background rates as context for adverse events in a rheumatoid arthritis drug development program: a coordinated approach. Pharmacoepidemiol Drug Saf. 2015;24(11):1121–32.
- Yamanaka H, Askling J, Berglind N, Franzen S, Frisell T, Garwood C, et al. Infection rates in patients from five rheumatoid arthritis (RA) registries: contextualising an RA clinical trial programme. RMD Open. 2017;3(2):e000498.
