Abstract
Objectives
To assess the efficacy and safety of canakinumab in Japanese patients with systemic juvenile idiopathic arthritis (sJIA).
Methods
This was an open-label, single-arm active treatment study. sJIA patients, aged ≥2 to <20 years, were administered canakinumab 4 mg/kg every 4 weeks for ≤48 weeks. The co-primary endpoints were the proportion of patients who achieved an adapted American College of Rheumatology pediatric (ACR pedi) 30 criteria at week 8, and the proportion of patients who successfully tapered corticosteroids at week 28. Herein, the efficacy and safety results up to 48 weeks are reported.
Results
Of the 19 patients enrolled, 15 (78.9%) had previously used tocilizumab. All patients achieved ACR pedi 30 at week 8 and 73.7% (14/19) successfully tapered corticosteroids at week 28. At week 48, ACR pedi 50/70/90/100 responses were achieved by 100.0%/100.0%/87.5%/68.8% of patients. The most common adverse events (AEs) were infections (271.6 patient-years), 42.1% (8/19) patients had serious AEs. Two potential cases of macrophage activation syndrome were identified. No deaths were reported.
Conclusion
Canakinumab was efficacious in Japanese patients with sJIA and was associated with substantial corticosteroid dose reduction in the majority of patients. The safety profile of canakinumab was consistent with that observed from previous studies.
ClinicalTrials.gov (identifier
NCT02396212).
Introduction
Systemic juvenile idiopathic arthritis (sJIA) is a distinct form of JIA, which accounts for ∼4.0–17.0% of JIA worldwide and 42.0–54.0% of JIA in Japan [Citation1–5]. In Japan, the estimated prevalence of sJIA is 11.3 per 100,000 children [Citation2,Citation3]. sJIA is associated with systemic symptoms such as remittent fever, evanescent erythematous rash, lymph node swelling, hepatosplenomegaly, serositis, elevated erythrocyte sedimentation rate, and acute-phase proteins (C-reactive protein (CRP) and serum amyloid A) [Citation1,Citation6,Citation7]. sJIA patients may also develop macrophage activation syndrome (MAS), a life-threatening complication, joint deformity, growth retardation, and osteoporosis [Citation8–12].
The pathogenesis of sJIA is not well understood. Potent pro-inflammatory cytokines, including interleukin (IL)-1, IL-6, and IL-18, have been reported to play an important role in the inflammatory process of sJIA [Citation2,Citation13–17]. Previous studies suggest that dysregulation of IL-1 plays a pivotal role in the pathogenesis of sJIA [Citation13–14].
Conventional treatments for sJIA include nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids. However, some sJIA patients inadequately respond to these drugs, specifically corticosteroids, or relapse on reducing corticosteroid dose. Moreover, long-term use of corticosteroids is known to be associated with certain toxicities [Citation18–21]. The advent of biologics, including IL-1 (anakinra, canakinumab) and IL-6 inhibitors (tocilizumab), provides an alternative and effective therapeutic option for sJIA [Citation18,Citation22,Citation23]. The management of sJIA via biologics is aimed to achieve and maintain clinical remission and withdrawing or tapering corticosteroids [Citation21,Citation24–27]. Consequently, it helps prevent the inhibition of growth caused by the long-term use of corticosteroids, and maintain the inactive disease state, resulting in enhanced physical function and quality of life (QoL) [Citation28,Citation29]. Tocilizumab is the first approved treatment for sJIA in Japan. However, some patients do not respond adequately or discontinue tocilizumab due to AEs (e.g. infusion reactions) [Citation23,Citation30,Citation31].
Canakinumab is a human anti-IL-1β monoclonal antibody that selectively binds to IL-1β, and prevents proinflammatory signaling [Citation32–34]. Previous studies have demonstrated the efficacy and safety of canakinumab (4 mg/kg subcutaneously (s.c.) every 4 weeks; q4w) in sJIA [Citation22,Citation35]. However, none of these studies included Japanese patients with sJIA. This phase III study was conducted to evaluate the efficacy and safety of canakinumab in Japanese patients with sJIA. Additionally, corticosteroid tapering with canakinumab over time was evaluated.
Methods
Study design
This was an open-label, single-arm active treatment study of canakinumab, with a screening period of 28 days. Patients were administered canakinumab (Ilaris®, Novartis Pharma Stein AG, Stein, Switzerland) 4 mg/kg (maximum dose was 300 mg) q4w s.c., without any dose adjustments (). Patients who did not meet adapted American College of Rheumatology pediatric (ACR pedi) 30 criteria by week 12 were discontinued from the study. Two interim analyses (at weeks 28 and 48) were performed during the study to collect data after all patients completed each assessment. Herein, the study results up to 48 weeks are reported.
Figure 1. Study design. max: maximum; q4w: every 4 weeks; sJIA: systemic juvenile idiopathic arthritis.
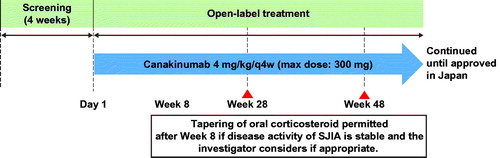
The study was conducted as per the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by an independent ethics committee in each hospital. All patients or parents or legal guardians of patients provided written informed consent.
Patients
Patients, aged ≥2 to <20 years, with a confirmed diagnosis of sJIA as per International League Against Rheumatism criteria at least 3 months prior to enrollment, including active systemic features, arthritis, and CRP >30 mg/L were included in this study [Citation36]. Patients were allowed to continue prior therapy, with stable doses of methotrexate (maximum of 20 mg/m2/week) for ≥4 weeks prior to baseline, NSAIDs for ≥1 week prior to baseline and/or systemic oral corticosteroid (≤1.0 mg/kg/day; prednisolone conversion, maximum 60 mg/day) for ≥3 days prior to baseline. Patients should have a negative test result for tuberculosis at screening or 4 weeks prior to screening. Major exclusion criteria were concomitant treatment with another biologic agent or disease-modifying drug (washout of 30 days or ≥5 half-lives), history of active MAS within 6 months before enrollment, hypersensitivity to study drug or biologics, and live-virus vaccination within 3 months before enrollment.
Assessments
Efficacy assessments
The co-primary endpoints were the proportion of patients who achieved ACR pedi 30 criteria at week 8, and the proportion of patients with canakinumab treatment who were able to taper corticosteroids successfully at week 28. Secondary endpoints included the proportion of patients who met ACR pedi 30/50/70/90/100 criteria, inactive disease status, flare, change in components of ACR pedi criteria, change in CRP levels, corticosteroid tapering over time from week 8, change in plasma concentrations of IL-6 and IL-18 after treatment with canakinumab, and safety and tolerability of canakinumab.
The ACR pedi 30/50/70/90/100 criteria was defined as improvements of ≥30%/≥50%/≥70%/≥90%/100% from baseline in ≥3 of the six variables in JIA core set and no intermittent fever (body temperature ≤38 °C) in the preceding week, with no more than one of the six variables worsening by >30%. The six JIA components were the number of joints with active arthritis, the number of joints with a limited range of motion, physician’s global assessment (PGA), and patients’/parents’ global assessment (PPGA) of disease activity on a 100 mm visual analog scale (VAS), standardized CRP level (normal range: 0–10 mg/L), and functional ability (using the Disability Index of the Childhood Health Assessment Questionnaire; Japanese version; CHAQ-DI [Citation37], on a scale of 0–3) [Citation22,Citation35,Citation38–40]. Flare was defined as the recurrence of fever due to sJIA lasting for ≥2 consecutive days; a worsening of ≥30% in ≥3 of the six response variables, with no more than one variable improving by ≥30% (worsening in ≥2 joints, worsening of ≥20 mm in PGA or PPGA, CRP >30 mg/L). Inactive disease was defined as the absence of active arthritis, fever, rash, serositis, splenomegaly, hepatomegaly, or generalized lymphadenopathy attributable to sJIA, a normal CRP level, a PGA score ≤10 mm, and duration of morning stiffness ≤15 minutes [Citation41].
Corticosteroid tapering
Patients using concomitant corticosteroids at baseline were allowed to taper their dose according to the study protocol from week 8 until the end of study, at the discretion of investigators. If patient’s disease activity worsened during corticosteroid tapering, then the patient could be returned to the immediate prior or higher dose, if deemed necessary by the investigator, but tapering could not be resumed for ≥2 weeks. Successful corticosteroid tapering was defined as a reduction in prednisolone equivalent dose from baseline dose of >0.8 mg/kg/day to ≤0.5 mg/kg/day, or from ≥0.5 mg/kg/day, and ≤0.8 mg/kg/day by ≥0.3 mg/kg/day, or any initial dose to ≤0.2 mg/kg/day, or any reduction from an initial dose of ≤0.2 mg/kg/day, while maintaining ACR pedi 30 criteria.
IL-6 and IL-18 concentrations
Plasma concentrations of IL-6 and IL-18 were measured at baseline, weeks 2, 4, 12, 24, and 48 by chemiluminescence enzyme immunoassay using the human IL-6 QuantiGlo ELISA kit (R&D Systems, Minneapolis, MN) and by enzyme immunoassay using the human IL-18 ELISA kit (MBL International, Woburn, MA), respectively. IL-6 and IL-18 were analyzed with a lower limit of quantification (LLOQ) of 0.300 pg/mL and 25.6 pg/mL, respectively. The upper limit of quantification (ULOQ) of IL-18 was 5000 pg/mL. IL-6 and IL-18 values below the LLOQ were replaced by LLOQ/2 and IL-18 values above the ULOQ were replaced by the ULOQ.
Safety assessments
Safety and tolerability of canakinumab was assessed in terms of adverse events (AEs) and serious AEs (SAEs). An independent MAS adjudication committee (MASAC) reviewed potential cases of MAS. Potential cases were identified through systematic database search of specified AE terms and/or (abnormal) laboratory criteria [Citation42] specified by the MASAC. The committee reviewed the identified cases and provided the report of adjudication outcome to the sponsor. The outcome of the adjudication was reported on the MAS Adjudication case report form after the completion of the study.
Exploratory analyses
The primary analysis for the co-primary endpoints was repeated for a subgroup defined by prior use of tocilizumab (yes/no). In addition, treatment-emergent AEs were also summarized for the subgroup.
Statistical methods
The full analysis and safety set included all patients who received ≥1 dose of canakinumab during the study. For ACR pedi 30/50/70/90/100 criteria at week 8, a missing response was imputed as non-responder regardless of the reason for missing data (non-responder imputation, NRI). After week 8, no imputation was applied. For corticosteroid tapering at week 28, a missing value was imputed using last observation carried forward from weeks 8 to 28. Patients who discontinued before week 8 were regarded as treatment failures. Missing values were not imputed for the other efficacy analyses. AE coding was done using the Medical Dictionary for Regulatory Activities (MedDRA version 20.0).
Results
Patient disposition and baseline characteristics
Of the 19 enrolled patients, 16 (84.2%) completed 48 weeks of treatment. Three patients discontinued prior to week 28 due to an AE (n = 1) or lack of efficacy (n = 2). summarizes the demographic and baseline disease characteristics of all patients. The median age of patients was 9.0 years and 68.4% (13/19) of patients were female. The median time from sJIA diagnosis to study entry was 5.9 (0.4–17.3) years. All patients were receiving an oral corticosteroid at baseline and the median (min–max) oral prednisolone equivalent dose was 0.2 (0.08–0.94) mg/kg/day, with 73.7% (14/19) of patients receiving >0 to ≤0.4 mg/kg/day. Methotrexate was also used in 47.4% (9/19) of patients as concomitant medication. The most commonly used prior sJIA medication was tocilizumab (78.9%; 15/19).
Table 1. Baseline demographic and disease characteristics.
Efficacy
ACR pedi 30 at week 8 and corticosteroid tapering at week 28 (NRI)
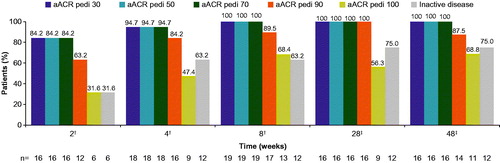
All 19 patients, including four who received an oral corticosteroid at increased dosage and/or intravenous (i.v.) corticosteroid until week 8 for the treatment of AEs (sJIA flare) and one patient who discontinued the study before week 8 due to lack of efficacy, achieved ACR pedi 30 by week 8, with 84.2% (16/19) and 94.7% (18/19) of patients achieving ACR pedi 30 at weeks 2 and 4, respectively (). The last visit of the discontinued patient was on day 41, which was within the visit window of week 8 assessment defined in the study protocol (days 32–60), therefore the data on that day were included in the week 8 analysis.
Figure 2. ACR pediatric responses and inactive disease (with duration of morning stiffness) status. For the analyses of ACR pedi 30/50/70/90/100 criteria at week 8, missing response was imputed with non-responder regardless of the reason for missing data (NRI). No imputation was applied after week 8. †m = 19; ‡m = 16. Inactive disease was defined as no joints with active arthritis; no fever (body temperature ≤38 °C); no rheumatoid rash, serositis, splenomegaly, hepatomegaly, or generalized lymphadenopathy attributable to JIA; normal CRP; PGA of disease activity indicating no disease activity ≤10 mm; duration of morning stiffness ≤15 minutes. ACR pedi: adapted American college of rheumatology-pediatric; CRP: C-reactive protein; JIA: juvenile idiopathic arthritis; m: the total number of evaluable patients; n: number of patients with response; NRI: non-responder imputation; PGA: physician’s global assessment.
The corticosteroid tapering at week 28 is shown in . Of the 19 patients, 14 (73.7%) successfully tapered their corticosteroid dose, with two (10.5%) patients being corticosteroid-free at week 28. Of note, two of the 14 (14.3%) patients who successfully tapered their corticosteroid dose at week 28 received i.v. corticosteroid after week 8 due to AEs (MAS suspected and sJIA flare). These two patients achieved ACR pedi 90/100 response by week 2 and their oral corticosteroid doses were tapered after week 8. Overall, five (26.3%) patients were not able to taper corticosteroids, including one (5.3%) patient who discontinued the study before week 8.
Table 2. Corticosteroid tapering at week 28, by prior use of tocilizumab.
Response to treatment over time and inactive disease
More than 80.0% of patients achieved ACR pedi 30/70 at week 2 and ACR pedi 90 by week 4. By week 28, 100.0% (16/16), 100.0% (16/16), 100.0% (16/16), and 56.0% (9/16) of patients achieved ACR pedi 30/70/90/100, respectively. These responses were maintained or further increased up to week 48 ().
Inactive disease was achieved in 31.6% (6/19) and 63.2% (12/19) patients at weeks 2 and 4, respectively, which further increased to 75.0% (12/16) at week 48 ().
Flare
The flare criteria were met for three patients during the 48 weeks of treatment. Two patients had flares before week 8, and one patient experienced flare after the initiation of corticosteroid tapering. No patients had flare after week 28 to week 48.
Components of the ACR pedi criteria
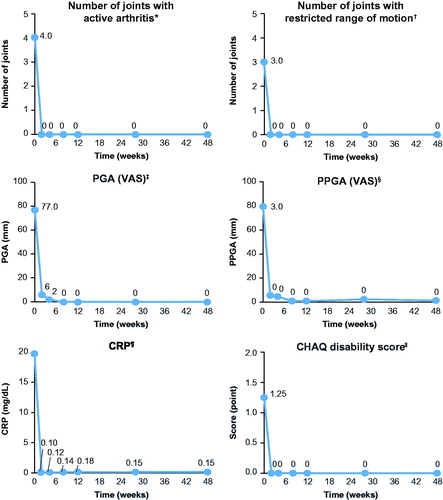
All components of the ACR pedi criteria showed improvement from week 2 to 28, and these improvements were sustained or further improved to week 48 (). The number of joints with active arthritis and the number of joints with limitation of motion showed a decline from baseline, with a median change of −4.0 and −2.5, respectively, at week 48. The PGA of disease activity showed a decline from baseline, with a mean change of −63.9 mm (–95.9%) at week 48. The PPGA of patient’s overall well-being showed an improvement, with a mean change of −68.6 mm (–88.4%) at week 48. Standardized CRP showed a rapid decline as early as day 3, with a median percent change from baseline of −99.4% (–188.7 mg/L) at week 48. The CHAQ-DI showed improvement starting at week 2 from baseline and the observed effect was sustained over time; the median score at baseline was 1.3, −0.6 at week 2, and −1.1 at week 48. The majority of patients (75.0%) showed a minimal clinical important difference (MCID) improvement at week 48.
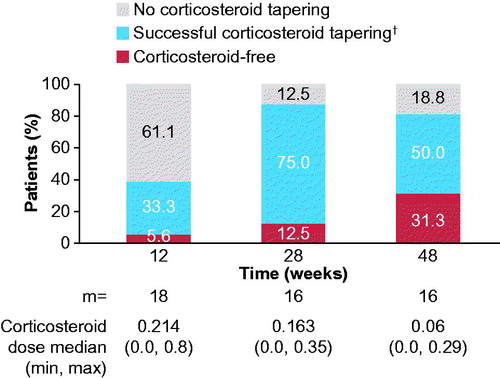
Figure 3. Corticosteroid tapering over time from week 8. Analyses based on observed data. †Corticosteroid dose reduced from >0.8 mg/kg/day to ≤0.5 mg/kg/day, or from ≥0.5 mg/kg/day and ≤0.8 mg/kg/day by ≥0.3 mg/kg/day, or from any initial dose to ≤0.2 mg/kg/day, or any reduction from an initial dose of ≤0.2 mg/kg/day, while maintaining ACR pedi 30 criterion. ACR pedi: adapted American college of rheumatology pediatric; m: the total number of evaluable patients.
Corticosteroid tapering over time from week 8
The proportion of patients who successfully tapered corticosteroids increased from week 12 (38.9%; 7/18) to 28 (87.5%; 14/16), and remained stable to week 48 (81.3%; 13/16). The proportion of corticosteroid-free patients increased from week 28 (12.5%; 2/16) to week 48 (31.3%; 5/16; ). At week 28, 62.5% (10/16) of patients were using ≤0.2 mg/kg/day of corticosteroid, which remained stable to week 48 (50.0%; 8/16).
Figure 4. Change over time in the components of the ACR pediatric criteria. Analyses based on observed data. *The range of possible values for number of joints with active arthritis was 0–73. †The range of possible values for number of joints with limited range of motion was 0–69. ‡The PGA of disease activity was based on a 100-mm VAS, with higher scores indicating more active disease. §The parent’s global assessment of the patient’s overall well-being was based on a 100-mm VAS, with higher scores indicating more active disease. ![]() The level of CRP was standardized to a normal range of 0–10 mg/L. ǁPhysical function was assessed by means of the cross-culturally adapted and validated version of the CHAQ-DI, with scores ranging from 0 to 3 and higher scores indicating greater disability. ACR pedi: adapted American college of rheumatology pediatric; CHAQ-DI: childhood health assessment questionnaire – disability index; CRP: C-reactive protein; PGA: physician’s global assessment; PPGA: patients’/parents’ global assessment; sJIA: systemic juvenile idiopathic arthritis; VAS: visual analog scale.
The level of CRP was standardized to a normal range of 0–10 mg/L. ǁPhysical function was assessed by means of the cross-culturally adapted and validated version of the CHAQ-DI, with scores ranging from 0 to 3 and higher scores indicating greater disability. ACR pedi: adapted American college of rheumatology pediatric; CHAQ-DI: childhood health assessment questionnaire – disability index; CRP: C-reactive protein; PGA: physician’s global assessment; PPGA: patients’/parents’ global assessment; sJIA: systemic juvenile idiopathic arthritis; VAS: visual analog scale.
Exploratory analysis of co-primary endpoints
All patients (100.0%) with/without prior use of tocilizumab, achieved ACR pedi 30 at week 8. At week 28, 75.0% (3/4) tocilizumab-naïve patients and 73.3% (11/15) of patients with prior exposure to tocilizumab successfully tapered corticosteroids ().
IL-6 and IL-18 concentrations after canakinumab treatment
The median level of IL-6 at baseline in all patients was 84.2 (2.07–1230.0) pg/mL. A 99.1% reduction in IL-6 levels was observed as early as week 2 (0.74 (0.15–61.4, n = 19) pg/mL). Upon treatment with canakinumab, plasma IL-6 remained low throughout the study (0.55 (0.15–65.3) pg/mL, at week 24 and 1.48 (0.15–15.7) pg/mL at week 48; n = 16). Quantification of plasma IL-18 was not possible in many samples because a high proportion of patients exceeded the plasma ULOQ (5000 pg/mL) at all time-points (73.7% (14/19), 37.5% (6/16), and 53.3% (8/15) at baseline, week 24 and week 48, respectively).
Safety
The median duration of exposure to canakinumab was 337 days and ∼65.0% of patients received treatment for ≥48 weeks. All patients experienced ≥1 AE during the study, with an exposure-adjusted event rate of AEs per 100 patient-years (EAER/100PY) of 983.0. The most commonly reported AEs were viral upper respiratory tract infection (36.8%; EAER/100PY, 129.3), headache (21.1%; EAER/100PY, 51.7), injection site reaction, and urticaria (each 21.1%; EAER/100PY, 25.9; ).
Table 3. AEs by primary system organ class (≥4 events) and preferred term (≥2 events).
Overall, eight (42.1%) patients experienced 16 SAEs during the study. Two (10.5%) patients discontinued treatment due to an SAE, sJIA flare. sJIA flare (‘Still’s disease’ or ‘JIA’ as preferred term of AE) was the most commonly reported SAE (21.1%, n = 4). Four patients experienced serious infections (influenza (n = 2), gastroenteritis (n = 1), pharyngitis (n = 1), and Epstein–Barr virus infection (n = 1)) and one patient had suspected MAS (Supplementary Table S1). However, it was adjudicated by the MASAC due to insufficient information. Additionally, two cases of potential MAS were adjudicated by the MASAC as having some clinical and/or laboratory features of MAS but with a possible alternative explanation (unlikely MAS).
No malignancies or anaphylactic reactions were reported. Overall, 4/19 (21.1%) patients had local injection site reactions, and none discontinued due to injection site reactions. Three (15.8%) patients had a grade 1 (mild), and one patient had a grade 0 local injection site reaction. No deaths occurred during the 48-week study.
Discussion
Herein, the efficacy and safety of canakinumab is reported for the first time in Japanese patients with sJIA. The majority of patients in the study had failed tocilizumab treatment before study entry.
These results demonstrate that canakinumab treatment was effective in achieving a rapid and sustained response in Japanese patients with sJIA. All patients achieved ACR pedi 30 at week 8 and 73.7% successfully tapered corticosteroids at week 28. For the ACR pedi 30 endpoint, the confounding effect of steroids on ACR response in four patients who received an increased dose of corticosteroids for treatment of AEs of sJIA flare should be considered; however, this would not impact on the overall conclusion that a treatment effect was seen in a high proportion of patients. The efficacy of canakinumab observed in sJIA patients in this study concurred with a previous report in which 83.7% of canakinumab-treated sJIA patients achieved ACR pedi 30 at day 15 [Citation22]. In addition, 44.5% of patients on corticosteroids at study entry successfully tapered their corticosteroid dose within 5 months, with 33% of patients discontinuing the corticosteroid treatment [Citation22].
Patients exhibited an early-onset ACR pedi response during the study, with 84.2% of patients achieving ACR pedi 70 by week 2 and ACR pedi 90 by week 4; 47.4% of patients achieved ACR pedi 100 at week 4. In the majority of patients, the ACR pedi 70/90/100 response levels were maintained and/or increased to week 48. Rapid and sustained efficacy response rates attained in this study exemplify the beneficial role of canakinumab in the treatment of Japanese patients with sJIA.
Achieving inactive disease, the ideal therapeutic target, helps the prevention of joint damage and disability, and may enhance physical function and QoL [Citation43,Citation44]. In a previous study, treatment with canakinumab demonstrated high inactive disease rates in ∼33.0% of patients on days 15 and 30. Similarly, in this study, a fast onset of action was demonstrated in 32.0% of patients who achieved inactive disease status by day 15, and the proportion of patients who achieved inactive disease increased to 75.0% at week 48.
Flares were observed in three patients during the 48 weeks of treatment, corroborating results from a previous study [Citation22]. The rapid treatment effect with canakinumab was further evidenced by a reduction in CRP levels and the number of joints affected, as well as the improvement in the PGA of disease activity at day 3. Parent/patient-reported outcome assessments, including overall wellbeing and pain, showed improvements that were clinically meaningful at the earliest time-point measured, week 2. The CHAQ disability score showed improvement at the first evaluation (week 2) which increased over time. The median change at week 48 of −1.1 (–100%) indicates a treatment effect of ∼5.8 times the MCID, compared with the cited MCID of −0.19 [Citation45]. These results suggest that treatment with canakinumab improves the functional ability of sJIA patients, in addition to improvement and maintenance of clinical indicators (active joint count, PGA, PPGA, and CRP).
Long-term use of high-doses of corticosteroids is associated with toxicity including poor bone development and growth in sJIA patients [Citation28,Citation29]. Therefore, corticosteroids are progressively tapered and then suspended in sJIA patients [Citation21,Citation27,Citation43,Citation46]. In this study, the majority of patients demonstrated a corticosteroid tapering effect at week 28 (87.5%), with 12.5% discontinuing and 55.6% tapering corticosteroids below 0.2 mg/kg/day. The proportion of patients that successfully tapered corticosteroids remained stable to week 48 (81.3%), with more patients being corticosteroid-free at week 48 (31.3%), which may be beneficial in protecting sJIA patients from corticosteroid-related side-effects.
Moreover, exploratory analyses demonstrated that canakinumab provided an improvement in disease activity accompanied by corticosteroid dose-reduction in Japanese patients with sJIA who had prior exposure to tocilizumab. sJIA is the heterogeneous disease and these data suggest that the cytokines which play a central role of sJIA is different in each patients.
Canakinumab inhibits IL-1β-stimulated IL-6 secretion [Citation47]. A marked reduction in plasma IL-6 concentration was observed from baseline to week 2 and levels remained low to week 48 in canakinumab-treated sJIA patients. In sJIA, IL-18 is one of the most studied cytokines as a biomarker of disease activity and/or prognosis [Citation48]. Put et al. [Citation49] demonstrated an elevated plasma IL-18 level in patients with active sJIA compared with patients with inactive disease or healthy controls, where the level of IL-18 in the majority of patients exceeded 10,000 pg/mL. However, in this study, plasma IL-18 was not measurable in many patients because the value was above the ULOQ of 5000 pg/mL at baseline and subsequent time-points. Therefore, the effect of canakinumab on plasma IL-18 levels remains inconclusive. Further assessment of IL-18 levels with canakinumab use is necessary.
The safety profile of canakinumab observed in this study is consistent with the previous canakinumab studies [Citation22,Citation35]. Infections, especially of the upper respiratory tract were the most frequently reported AEs (about 75.0%). Serious AEs were reported in eight (42.1%) patients and largely associated with disease activity and infections (influenza, gastroenteritis, pharyngitis, and Epstein–Barr virus infection). All SAEs resolved except for sJIA flare in three patients. The study drug was discontinued due to an SAE in two patients; however, both events were of sJIA flare, indicating loss of efficacy and no safety issues leading to study drug discontinuation occurred until 48 weeks. The results of the adjudication of MAS cases do not suggest an effect of canakinumab on the development of MAS, and is consistent with previous reports [Citation35,Citation50]. There were no severe injection-site reactions, and these results were similar to previous studies [Citation34,Citation35].
Limitations
This study had limitations with regard to its small sample size and the non-controlled open-label study design. Another limitation is the short observation period; long-term studies are ongoing to assess long-term efficacy and safety of canakinumab in Japanese patients with sJIA.
Conclusion
Canakinumab (4 mg/kg q4w s.c.) rapidly induced and maintained a treatment response in a high proportion of Japanese sJIA patients, with or without prior use of tocilizumab, and was associated with tapering of oral corticosteroids in the majority of patients. The safety data from the 48 week treatment period showed no new safety findings compared with the known safety profile of canakinumab.
Author contributions
NS and TK conceived and planned the study. KN, RH, HU, ST, NI, TI, MS, MT, and SY substantially contributed to analysis and interpretation of data. KN took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Supplemental Material
Download MS Word (31 KB)Acknowledgements
The authors thank Amit Agarwal, Novartis Healthcare Pvt. Ltd, India for medical writing support.
Conflict of interest
ST: Consultancy fee from Bristol-Myers Squibb. Speaking fees, and honoraria from Chugai, Abbvie, Ono, Takeda, Tanabe-Mitsubishi, Sanofi, Glaxo-Smith-Kline.
NI: Consultancy fee from Tanabe-Mitsubishi Pharm, GlaxoSmithKline. Speaking fees and honoraria from Mitsubishi Tanabe Pharma Corporation, Novartis Pharma K.K., Janssen Pharmaceutical K.K., AbbVie GK.
MS: Speaking fees and honoraria from Novartis Pharma K.K.
NS, TK: Employees of Novartis Pharma K.K.
The rest of the authors declare that they have no competing interests.
Additional information
Funding
References
- Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369(9563):767–78.
- Takei S. Systemic JIA as an autoinflammatory disease. Inflamm Regen. 2011;31(1):52–62.
- Takei S, Yamashita S, Kato T. Nation-wide survey for patients with juvenile idiopathic arthritis in Japan. Annual Report on Children with Chronic Refractory Diseases from the Japanese Ministry of Health, Labor and Welfare 2008. p. 102–13.
- Fujikawa S, Okuni M. Clinical analysis of 570 cases with juvenile rheumatoid arthritis: results of a nationwide retrospective survey in Japan. Acta Paediatr Jpn. 1997;39(2):245–9.
- Okamoto N, Yokota S, Takei S, Okura Y, Kubota T, Shimizu M, et al. Clinical practice guidance for juvenile idiopathic arthritis (JIA) 2018. Mod Rheumatol. 2019;29(1):41–59.
- Martini A, Ravelli A, Di Fuccia G, Rosti V, Cazzola M, Barosi G. Intravenous iron therapy for severe anaemia in systemic-onset juvenile chronic arthritis. Lancet. 1994;344(8929):1052–4.
- Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377(9783):2138–49.
- Schneider R, Laxer RM. Systemic onset juvenile rheumatoid arthritis. Baillieres Clin Rheumatol. 1998;12(2):245–71.
- Henderson CJ, Cawkwell GD, Specker BL, Sierra RI, Wilmott RW, Campaigne BN, et al. Predictors of total body bone mineral density in non-corticosteroid-treated prepubertal children with juvenile rheumatoid arthritis. Arthritis Rheum. 1997;40(11):1967–75.
- De Benedetti F, Alonzi T, Moretta A, Lazzaro D, Costa P, Poli V, et al. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammation. J Clin Invest. 1997;99(4):643–50.
- Simon D, Lucidarme N, Prieur AM, Ruiz JC, Czernichow P. Effects on growth and body composition of growth hormone treatment in children with juvenile idiopathic arthritis requiring steroid therapy. J Rheumatol. 2003;30(11):2492–9.
- Ravelli A, Minoia F, Davi S, Horne A, Bovis F, Pistorio A, et al. 2016 classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann Rheum Dis. 2016;75(3):481–9.
- Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201(9):1479–86.
- Gattorno M, Piccini A, Lasiglie D, Tassi S, Brisca G, Carta S, et al. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2008;58(5):1505–15.
- De Benedetti F, Martini A. Is systemic juvenile rheumatoid arthritis an interleukin 6 mediated disease? J Rheumatol. 1998;25(2):203–7.
- Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80(2):227–36.
- Chen O, Shan N, Zhu X, Wang Y, Ren P, Wei D, et al. The imbalance of IL-18/IL-18BP in patients with systemic juvenile idiopathic arthritis. Acta Biochim Biophys Sin (Shanghai). 2013;45(4):339–41.
- Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken). 2011;63(4):465–82.
- Malattia C, Martini A. Glucocorticoids in juvenile idiopathic arthritis. Ann N Y Acad Sci. 2014;1318:65–70.
- Toplak N, Blazina S, Avcin T. The role of IL-1 inhibition in systemic juvenile idiopathic arthritis: current status and future perspectives. Drug Des Devel Ther. 2018;12:1633–43.
- Ringold S, Weiss PF, Beukelman T, Dewitt EM, Ilowite NT, Kimura Y, et al. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Care Res (Hoboken). 2013;65(10):1551–63.
- Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367(25):2396–406.
- Yokota S, Imagawa T, Mori M, Miyamae T, Aihara Y, Takei S, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371(9617):998–1006.
- DeWitt EM, Kimura Y, Beukelman T, Nigrovic PA, Onel K, Prahalad S, et al. Consensus treatment plans for new-onset systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2012;64(7):1001–10.
- Woerner A, Uettwiller F, Melki I, Mouy R, Wouters C, Bader-Meunier B, et al. Biological treatment in systemic juvenile idiopathic arthritis: achievement of inactive disease or clinical remission on a first, second or third biological agent. RMD Open. 2015;1(1):e000036.
- De Benedetti F, Brunner H, Ruperto N, Schneider R, Xavier R, Allen R, et al. Catch-up growth during tocilizumab therapy for systemic juvenile idiopathic arthritis: results from a phase III trial. Arthritis Rheumatol. 2015;67(3):840–8.
- Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat NM, Horneff G, et al. Canakinumab in patients with systemic juvenile idiopathic arthritis and active systemic features: results from the 5-year long-term extension of the phase III pivotal trials. Ann Rheum Dis. 2018;77(12):1710–9.
- Stanbury RM, Graham EM. Systemic corticosteroid therapy-side effects and their management. Br J Ophthalmol. 1998;82(6):704–8.
- Mushtaq T, Ahmed SF. The impact of corticosteroids on growth and bone health. Arch Dis Child. 2002;87(2):93–6.
- Yokota S, Itoh Y, Morio T, Origasa H, Sumitomo N, Tomobe M, et al. Tocilizumab in systemic juvenile idiopathic arthritis in a real-world clinical setting: results from 1 year of postmarketing surveillance follow-up of 417 patients in Japan. Ann Rheum Dis. 2016;75(9):1654–60.
- Horneff G, Schulz AC, Klotsche J, Hospach A, Minden K, Foeldvari I, et al. Experience with etanercept, tocilizumab and interleukin-1 inhibitors in systemic onset juvenile idiopathic arthritis patients from the BIKER registry. Arthritis Res Ther. 2017;19(1):256.
- Alten R, Gram H, Joosten LA, van den Berg WB, Sieper J, Wassenberg S, et al. The human anti-IL-1 beta monoclonal antibody ACZ885 is effective in joint inflammation models in mice and in a proof-of-concept study in patients with rheumatoid arthritis. Arthritis Res Ther. 2008;10(3):R67.
- Kuemmerle-Deschner JB, Ramos E, Blank N, Roesler J, Felix SD, Jung T, et al. Canakinumab (ACZ885, a fully human IgG1 anti-IL-1beta mAb) induces sustained remission in pediatric patients with cryopyrin-associated periodic syndrome (CAPS). Arthritis Res Ther. 2011;13(1):R34.
- Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360(23):2416–25.
- Ruperto N, Quartier P, Wulffraat N, Woo P, Ravelli A, Mouy R, et al. A phase II, multicenter, open-label study evaluating dosing and preliminary safety and efficacy of canakinumab in systemic juvenile idiopathic arthritis with active systemic features. Arthritis Rheum. 2012;64(2):557–67.
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–2.
- Ruperto N, Ravelli A, Pistorio A, Malattia C, Cavuto S, Gado-West L, et al. Cross-cultural adaptation and psychometric evaluation of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ) in 32 countries. Review of the general methodology. Clin Exp Rheumatol. 2001;19:S1–S9.
- Ruperto N, Martini A. Networking in paediatrics: the example of the Paediatric Rheumatology International Trials Organisation (PRINTO). Arch Dis Child. 2011;96(6):596–601.
- Ruperto N, Giannini EH, Pistorio A, Brunner HI, Martini A, Lovell DJ. Is it time to move to active comparator trials in juvenile idiopathic arthritis? A review of current study designs. Arthritis Rheum. 2010;62(11):3131–9.
- Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40(7):1202–9.
- Wallace CA, Ruperto N, Giannini E, et al.; Childhood Arthritis and Rheumatology Research Alliance; Pediatric Rheumatology International Trials Organization; Pediatric Rheumatology Collaborative Study Group. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31:2290–4.
- Ravelli A, Magni-Manzoni S, Pistorio A, Besana C, Foti T, Ruperto N, et al. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr. 2005;146(5):598–604.
- Grevich S, Shenoi S. Update on the management of systemic juvenile idiopathic arthritis and role of IL-1 and IL-6 inhibition. Adolesc Health Med Ther. 2017;8:125–35.
- Consolaro A, Negro G, Lanni S, Solari N, Martini A, Ravelli A. Toward a treat-to-target approach in the management of juvenile idiopathic arthritis. Clin Exp Rheumatol. 2012;30:S157–S62.
- Brunner HI, Klein-Gitelman MS, Miller MJ, Barron A, Baldwin N, Trombley M, et al. Minimal clinically important differences of the childhood health assessment questionnaire. J Rheumatol. 2005;32(1):150–61.
- Woo P. Systemic juvenile idiopathic arthritis: diagnosis, management, and outcome. Nat Clin Pract Rheumatol. 2006;2(1):28–34.
- Chakraborty A, Tannenbaum S, Rordorf C, Lowe PJ, Floch D, Gram H, et al. Pharmacokinetic and pharmacodynamic properties of canakinumab, a human anti-interleukin-1β monoclonal antibody. Clin Pharmacokinet. 2012;51(6):e1.
- Gohar F, Kessel C, Lavric M, Holzinger D, Foell D. Review of biomarkers in systemic juvenile idiopathic arthritis: helpful tools or just playing tricks? Arthritis Res Ther. 2016;18:163.
- Put K, Avau A, Brisse E, Mitera T, Put S, Proost P, et al. Cytokines in systemic juvenile idiopathic arthritis and haemophagocytic lymphohistiocytosis: tipping the balance between interleukin-18 and interferon-γ. Rheumatology (Oxford). 2015;54(8):1507–17.
- Grom AA, Ilowite NT, Pascual V, Brunner HI, Martini A, Lovell D, et al. Rate and clinical presentation of macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis treated with canakinumab. Arthritis Rheumatol. 2016;68(1):218–28.
