Abstract
Objectives
To assess gout and asymptomatic hyperuricemia in Japan and review treatment conditions.
Methods
This retrospective cross-sectional study analyzed the prevalence of hyperuricemia and gout, and characteristics and treatment of patients with those conditions, using Japanese health insurance claims and medical check-up data collected from April 2016 through March 2017.
Results
Among 2,531,383 persons registered in the database, 1.1% (men 1.9%, women <0.1%) were diagnosed with gout and 2.6% (4.1%, 0.4%) with asymptomatic hyperuricemia. Medical check-ups showed 13.4% (19.6%, 1.0%) of patients with hyperuricemia (serum uric acid [sUA] > 7.0 mg/dL). Urate-lowering therapy (ULT) was prescribed for 80.7% of patients identified with gout and 72.4% identified with asymptomatic hyperuricemia. ULT adherence was satisfactory, but most patients were treated with low-dose ULT. Less than half of patients receiving ULT achieved the sUA target (≤6.0 mg/dL). In gout patients, the incidence of gout flare was 47.8% (0.74 flares/person-year).
Conclusions
Although hyperuricemia prevalence is similar in Japan and worldwide, gout is comparatively rare in Japan. Gout and asymptomatic hyperuricemia are often treated with low-dose ULT, and many patients fail to reach target sUA, suggesting that gout management is suboptimal in Japan. Patients would benefit from stricter focus on a treat-to-target approach for gout management.
Introduction
The prevalence of gout is increasing worldwide [Citation1,Citation2], and although this condition can be effectively managed by using currently available medications, treatment options are often underutilized [Citation3,Citation4]. Only a limited number of patients start urate-lowering therapy (ULT), treatment adherence is low [Citation5], and most patients experience recurrence of gout flare [Citation6].
Gout flare can effectively be prevented by maintaining serum uric acid (sUA) at 6.0 mg/dL or below [Citation7], a level nearly uniformly recommended by guidelines internationally [Citation8–10], which leads to dissolution of urate crystals in the body. This requires a ‘treat-to-target’ approach for ULT [Citation3,Citation11]. In this regard, real-world evidence from many countries shows that most gout patients fail to reach their sUA target [Citation5,Citation12,Citation13].
The initiation of ULT early in the clinical course of gout has been associated with a reduced risk of gout flare [Citation14,Citation15], and the development of gout at an early age appears to be a marker for gout severity [Citation16], suggesting that ULT should be introduced as early as possible. The 2016 European League Against Rheumatism (EULAR) gout management recommendations state that the introduction of ULT should be considered at the first diagnosis of gout, and strongly recommend early ULT initiation [Citation10]. Japanese guidelines suggest that ULT should be introduced even earlier, and should be considered in patients having asymptomatic hyperuricemia with sUA 8.0 mg/dL or above in the presence of comorbidities such as decreased renal function, urinary calculus, hypertension, ischemic heart disease, diabetes, or metabolic syndrome, as well as in patients with gouty arthritis and/or tophi [Citation8]. In clinical trials of the ULT febuxostat, the percentage of enrolled patients with gouty tophi was much lower in Japan than in the US (1.3% vs. 24.0%) [Citation17,Citation18]. These findings suggest that gout may be less severe in Japanese patients than in the US, which may reflect the benefit of introducing ULT soon after onset. However, several points remain unclear, including real-world information on ULT in Japanese patients with gout or asymptomatic hyperuricemia.
To better understand the treatment of patients with gout or asymptomatic hyperuricemia in Japan, we conducted a cross-sectional survey of Japanese medical insurance claims and medical check-up data. This survey included the prevalence of diagnosed gout and asymptomatic hyperuricemia, the number of actual prescriptions for ULT, the percentage of patients who achieved the treatment target for sUA, and the incidence of gout flare.
Materials and methods
Study design
This survey involved a retrospective cross-sectional study of information from an insurance claims database and data from medical check-ups from April 2016 to March 2017. The study was registered through the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000031503). Data were obtained from JMDC Inc.
JMDC collects information from multiple organizations that provide health insurance coverage to Japanese employees (the subscribers) and their dependents, and incorporates that information into the JMDC Claims Database [Citation19], including diagnostic codes and names of prescription drugs prescribed, and permission to provide some of that information to third parties. The database contains anonymized information from patients across medical facilities and through visits to multiple institutions, and accounts for approximately 10% of all members of Japanese health insurance organizations and approximately 2% of the Japanese population overall.
Japanese health insurance subscribers generally have a medical check-up every year. For this study, data were available from about one third of the population in the JMDC database.
Because this study uses anonymized administrative data, no ethics approval number, statement of patient consent, or clinical trial registration number is required.
Participants
The study population comprised health insurance subscribers who were in the JMDC Claims Database (the database population) and met the inclusion criteria.
Inclusion criteria were:
Uninterrupted subscription to the insurance program from April 2016 through March 2017.
Diagnosis of gout or asymptomatic hyperuricemia.
Age 18–65 years.
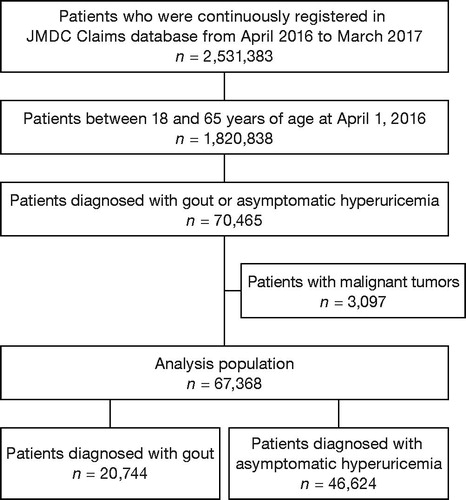
The patient was considered to have gout if the database showed Code M10 (Gout, ICD10 coding system) twice or more in separate months, Code M10 (Gout, ICD10 coding system) once or more with one or more prescriptions for M04 (Anti-gout preparations, ATC code) and gout diagnosis shown on the same insurance claim form, or Code M10 (Gout, ICD10 coding system) once or more and one or more prescriptions for M01A1 (Anti-rheumatics, non-steroidal plain, ATC code) or H02A2 (Oral corticosteroids, plain, ATC code) and gout diagnosis shown on the same insurance claim form. The patient was considered to have asymptomatic hyperuricemia if the definition of gout did not apply and the database showed Code E790 (Hyperuricaemia without signs of inflammatory arthritis and tophaceous disease, ICD10 coding system) twice or more in separate months, or Code E790 (Hyperuricaemia without signs of inflammatory arthritis and tophaceous disease, ICD10 coding system) once or more and one or more prescriptions for M04 (Anti-gout preparations, ATC code) and diagnosis for hyperuricaemia without signs of inflammatory arthritis or tophaceous disease, shown on the same insurance claim form (Supplementary Table S1). Patients with malignant tumors were excluded to avoid the confounding effect of hyperuricemia due to cancer chemotherapy. The definition of ‘malignant tumors’ in this study is provided in Supplementary Table S1.
Study measures
The following variables were analyzed.
Prevalence of diagnosed gout and asymptomatic hyperuricemia
Patient characteristics
Prescriptions for ULT: number of patients who received prescriptions, mean prescribed dose, treatment adherence
Proportion of patients achieving the sUA treatment target (6.0 mg/dL or below)
Incidence proportion and incidence rate for gout flare
The terms ‘patient characteristics’ and ‘gout flare’ in this study are defined in Supplementary Table S1.
Statistical methods
All patients who met the eligibility criteria were included in analysis. Data were analyzed separately for the subgroups with gout or asymptomatic hyperuricemia and for each ULT. Categorical variables were summarized with the number and proportion of patients, and continuous variables were summarized using descriptive statistics. The prevalence of diagnosed gout and of asymptomatic hyperuricemia was calculated for the database population, including subgroups by sex and age.
A definition of ULT is provided in Supplementary Table S1. The number of patients who were prescribed ULT, the mean prescribed dose, and the medication possession ratio (MPR) were analyzed from the insurance claims data; double counting was permitted for patients with prescriptions for multiple ULTs during the time period.
Findings for sUA were obtained from annual medical check-up data in the database. If data from multiple examinations were available for the same person, the most recent examination data were analyzed. Patients with prescriptions for multiple ULTs at the time of examination were excluded from sUA analysis. The proportion of patients achieving target sUA (6.0 mg/dL or below), and the 95% confidence interval, were calculated. In patients prescribed febuxostat or allopurinol, similar analysis was performed for each dosage. In patients with gout, the incidence proportion and incidence rate were also calculated for gout flare during the study period.
All analyses were performed using SAS version 9.4.
Results
Study population
Subject disposition is shown in . The subjects were 2,531,383 patients registered in the JMDC Claims Database during 12 consecutive months from April 2016 to March 2017. Of those, 1,820,838 were between 18 and 65 years of age on 1 April 2016; this number became the denominator population for calculating the prevalence of gout and asymptomatic hyperuricemia. The study population was 67,368 patients who met the eligibility criteria.
Prevalence of diagnosed gout and asymptomatic hyperuricemia
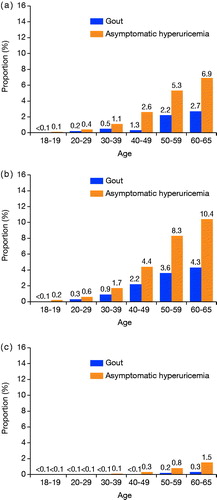
The prevalence of diagnosed gout was 1.1% (20,744/1,820,838) overall: 1.9% (20,038/1,068,005) for men and <0.1% (706/752,833) for women. The prevalence of diagnosed asymptomatic hyperuricemia was 2.6% (46,624/1,820,838) overall: 4.1% (43,751/1,068,005) men and 0.4% (2,873/752,833) women (Supplementary Table S2). Both gout and asymptomatic hyperuricemia were identified more frequently in men than in women. Prevalence increased proportionally with age in both men and women ().
Proportion of hyperuricemic individuals having sUA level greater than 7.0 mg/dL, and proportion diagnosed with gout or asymptomatic hyperuricemia
A subpopulation was created of 613,097 persons whose sUA level was measured at medical check-ups during the study period. Within that subpopulation, hyperuricemic individuals having sUA greater than 7.0 mg/dL consisted of 13.4% overall (82,380/613,097), 19.6% of men (80,263/409,073), and 1.0% of women (2,117/204,024). Among these hyperuricemic individuals, gout was diagnosed in 4.6% overall (3,805/82,380), 4.7% of men (3,786/80,263) and 0.9% of women (19/2,117); asymptomatic hyperuricemia was diagnosed in 10.2% overall (8,433/82,380), 10.3% of men (8,274/80,263), and 7.5% of women (159/2,117). This proportion was particularly low in younger subjects (Supplementary Table S3).
Patient characteristics
Within the total number of patients diagnosed with gout or asymptomatic hyperuricemia (n = 67,368), mean age was 49.9 (standard deviation [SD] 9.2) years. The group was predominately male (94.7%). The most common comorbidity was hyperlipidemia (59.7%), followed by hypertension (53.7%) and type 2 diabetes (28.9%). The percentage of patients with renal dysfunction was low (8.5%). Among patients with gout or asymptomatic hyperuricemia for whom the estimated glomerular filtration rate (eGFR) could be calculated from medical check-up data (n = 29,025), eGFR mean was 70.84 (SD 16.00) mL/min/1.73m2 ().
Table 1. Patient characteristics.
Prescriptions for ULT
ULT was prescribed for 80.7% (16,740/20,744) of patients with diagnosed gout and 72.4% (33,763/46,624) with diagnosed asymptomatic hyperuricemia. Among ULT prescriptions (n = 50,503), febuxostat accounted for 48.6% of patients (n = 24,527), allopurinol for 41.1% (n = 20,741), benzbromarone for 12.3% (n = 6,236), topiroxostat for 3.9% (n = 1,952), and probenecid for 0.4% (n = 204).
The median values for MPR were higher for allopurinol and benzbromarone (85.8% and 80.6%, respectively), followed by febuxostat (76.2%), probenecid (68.9%), and topiroxostat (46.9%). Mean prescribed dose was 17.3 (SD 8.5) mg/day for febuxostat, 135.8 (SD 55.2) mg/day for allopurinol, 44.9 (SD 19.6) mg/day for benzbromarone, 51.1 (SD 25.7) mg/day for topiroxostat, and 503.2 (SD 259.3) mg/day for probenecid ().
Table 2. Days of prescription, MPR, and mean dose of ULT for patients with gout and asymptomatic hyperuricemia.
In asymptomatic hyperuricemia patients, MPR for ULT was higher and the mean prescribed dose of ULT was lower than in gout patients. For example, with febuxostat the median MPR was 69.0% and mean dose was 18.9 (SD 9.4) mg/day in patients with gout, and 79.5% and 16.5 (SD 8.0) mg/day, respectively, in patients with asymptomatic hyperuricemia. With allopurinol, those numbers were 78.1% and 145.6 (SD 59.2) mg/day vs. 88.5% and 131.3 (SD 52.7) mg/day, respectively (Supplementary Table S4).
Proportion of target sUA achievement
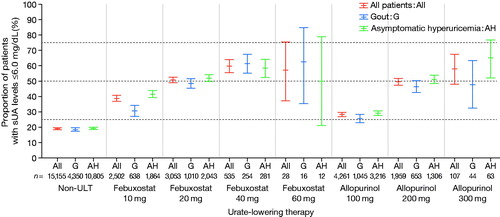
Among patients with gout or asymptomatic hyperuricemia whose sUA levels were obtained from medical check-up data (n = 30,013), the proportion of patients who achieved their sUA target (6.0 mg/dL or below) was calculated ( and Supplementary Table S5). The target was achieved in 19.0% of patients who were not prescribed ULT (2,875/15,155), 44.3% for all patients under treatment with ULT (6,588/14,858), 46.8% for febuxostat (2,908/6,214), 35.4% for allopurinol (2,290/6,475), 70.0% for benzbromarone (1,241/1,773), 36.7% for topiroxostat (127/346), and 44.0% for probenecid (22/50).
Figure 3. Proportion of achievement of target sUA under ULT prescription in patients with gout or asymptomatic hyperuricemia. Point estimate and 95% confidence interval were plotted for the proportion of achievement of target sUA. ULT: urate-lowering therapy; sUA: serum uric acid.
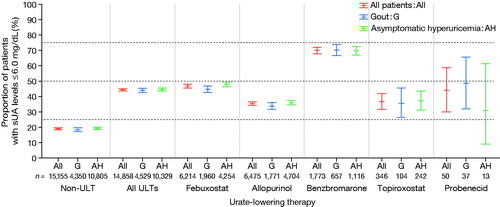
and Supplementary Table S6 show the proportion of patients who achieved their sUA target (6.0 mg/dL or below), stratified by prescribed dose, for ULT with either febuxostat or allopurinol in patients with gout or asymptomatic hyperuricemia. Achievement of the sUA target tended to increase dose-dependently, both with febuxostat and with allopurinol, although a relatively small number of patients were treated with febuxostat 60 mg or allopurinol 300 mg, so the confidence interval was large for those dose levels.
Incidence of gout flare
The incidence proportion and incidence rate of gout flare in diagnosed gout (n = 20,744) during the study period was 47.8% (9,923/20,744) and 0.74 flares/person-year (15,448 flares/20,744 person-year), respectively.
Discussion
In previous research using the JMDC Claims Database, gout prevalence in 2014 was reported at 1.66% in Japanese men [Citation20]. The present data, from April 2016 to March 2017, showed a prevalence of 1.9% in men. These figures, although trending higher than two years previously, remain lower than the reported prevalence of 4–5% in the US, UK, and the Asian countries of Taiwan, Hong Kong, and Singapore [Citation1,Citation2]. Meanwhile, our findings for hyperuricemic individuals with sUA level greater than 7.0 mg/dL, using data from medical check-ups, showed a prevalence of 19.6% in men, consistent with worldwide trends (about 20%) [Citation1,Citation21,Citation22]. While the prevalence of hyperuricemia is thus about the same in Japan as in other countries, the prevalence of gout is comparatively lower in Japan. One hypothesis links this lower level of gout to the likelihood of early ULT intervention; such intervention can begin at the stage of asymptomatic hyperuricemia rather than waiting until after the first occurrence of gout flare based on recommendations in the Japanese guidelines [Citation8]. The data also showed that only 10.2% of hyperuricemic individuals were actually diagnosed with asymptomatic hyperuricemia in daily practice, and this percentage was even lower in younger subjects, suggesting that there may be many young subjects with asymptomatic hyperuricemia who could benefit from treatment.
Similar to other countries, the xanthine oxidase inhibitors febuxostat and allopurinol are the most commonly prescribed ULTs in Japan. Topiroxostat, another xanthine oxidase inhibitor, is used only in Japan [Citation23]. In this study, febuxostat was prescribed in 48.6% of cases and allopurinol in 41.1%, compared with benzbromarone in 12.3%, topiroxostat in 3.9%, and probenecid in 0.4%. MPR median values exceeded 80% for allopurinol and benzbromarone, indicating good adherence, but not for febuxostat (76.2%), probenecid (68.9%), or topiroxostat (46.9%). This was a cross-sectional study, so the MPR values may have been relatively low in patients who were first-time users of newer ULTs or who were switched from an older ULT during the year of the study period.
The number of comorbidities was slightly higher in patients with asymptomatic hyperuricemia than with gout (), possibly because Japanese treatment guidelines recommend consideration of ULT in patients having asymptomatic hyperuricemia with sUA 8.0 mg/dL or above in the presence of comorbidities such as decreased renal function, urinary calculus, hypertension, ischemic heart disease, diabetes, or metabolic syndrome.
MPR for ULTs tended to be higher in asymptomatic hyperuricemia than in gout, possibly because patients with asymptomatic hyperuricemia tended to have more comorbidities than patients with gout and would thus normally visit the hospital or clinic more frequently and take other medications concomitantly. This could improve their MPR for ULTs and thus be a confounding factor. These findings are strongly supported by previous research outside Japan, in which treatment adherence in gout patients increased proportionally to the number of comorbidities experienced by each patient [Citation24]. The higher MPR in asymptomatic hyperuricemia also suggests that in Japan a relatively high number of asymptomatic patients may be receiving ULT before their condition progresses to symptomatic gout, possibly contributing to the lower prevalence of gout in Japan than in other countries.
In this study, the mean dose was 17.3 mg/day for febuxostat and 135.8 mg/day for allopurinol, considerably lower than the approved maintenance dosage in Japan (40–60 mg/day and 200–300 mg/day, respectively). To prevent gout flare at the start of treatment, the Japanese package insert stipulates that ULT is to be initiated at a low dose and then gradually increased, because a stepwise dose increase of febuxostat can effectively reduce sUA while minimizing gout flare at the start of ULT [Citation25]. In this regard, the low mean dose seen in Japan suggests that many Japanese physicians start ULT at an approved initial dosage, 10 mg of febuxostat or 100 mg of allopurinol, and then continue treatment at that same low dose, with no dose escalation. Of course the present study is based on a cross-sectional survey, so data included both the maintenance dose and the escalation dose, the initial dose was lower than the maintenance dose, and a simple average could not accurately represent the actual clinical situation. The mean prescribed dose for benzbromarone was 44.9 mg/day, in comparison to the approved maintenance dosage of 50–150 mg/day. This suggests that the real-world benzbromarone dose was much closer to the prescribed dose than was the case for allopurinol or febuxostat. Meanwhile, in a retrospective study of 871 patients with gout who were treated at a specialized hospital for gout management [Citation26], the mean prescribed dose was higher for allopurinol and lower for benzbromarone than the mean prescribed dose in our study. This indicates considerable difference between prescription levels in real-world clinical practice and in specialized medical facilities.
The sUA target (6.0 mg/dL or below) was achieved by less than half of the patients under treatment with ULT (44.3% of patients with gout or asymptomatic hyperuricemia overall). Target sUA was achieved in 46.8% of patients using febuxostat and 35.4% using allopurinol. These low proportions may be due to the mean prescribed doses for febuxostat and allopurinol, which were lower than the approved maintenance dosages. The proportion of patients achieving the sUA target was highest (70.0%) for benzbromarone, apparently related to the higher mean prescribed dose for that drug. Since these results appear to be confounded by indication (reason for prescription), our study does not provide a direct comparison between the effectiveness of individual drugs. However, its results clearly support the real-world effectiveness of ULT. Dose-dependent increases in the achievement of target sUA were noted both for febuxostat and allopurinol, reaching 59.8% for febuxostat 40 mg and 57.9% for allopurinol 300 mg. This suggests that dose escalation to the approved maintenance dosage would increase the number of patients who reach their target sUA.
Gout flare is extremely painful and constitutes a severe disease burden for many patients. Recurrence of gout flare has been reduced by maintaining sUA at 6.0 mg/dL or below in gout patients [Citation7], and the target sUA for gout patients is widely accepted as 6.0 mg/dL or below. In contrast, there has been little debate over an appropriate target sUA for patients with asymptomatic hyperuricemia. Further discussion is anticipated as the importance of intervention in asymptomatic hyperuricemia becomes more widely recognized. In this regard, a recent study clearly showed a significant decrease in the incidence of gout flare with febuxostat treatment compared to placebo in stage 3 chronic kidney disease (CKD) patients with asymptomatic hyperuricemia [Citation27]. This is the first evidence that providing ULT to patients with asymptomatic hyperuricemia, with the objective of maintaining sUA at 6.0 mg/dL or below, can be beneficial for the future prevention of gout flare. In Japan there have been no previous reports from real-world clinical practice on the incidence of gout flare in patients with gout. In patients identified as having gout, our study showed an annual incidence proportion of 47.8% for gout flare and incidence rate of 0.74 gout flares/person-year. In retrospective cohort research in patients with identified gout, using US medical claims databases from health insurance providers, the incidence proportion was 55.1% for gout flare during a 12-month follow-up period and the mean incidence rate was 0.73 flares/person-year [Citation28], similar to our findings. The present study clearly shows that stepwise dose increases are not being implemented optimally in actual clinical settings. It seems likely that gout management could be achieved by a treat-to-target approach to sUA, implementing appropriate stepwise increases in ULT dose to achieve and maintain sUA of 6.0 mg/dL or below.
A major strength of this study is the use of a large-scale database of insurance information, analyzing data from more than 60,000 patients with gout or asymptomatic hyperuricemia. In addition, Japan is one of the few nations in the world that provides treatment for asymptomatic hyperuricemia; these database findings describe the current status of ULT and control of sUA levels in that study population. The study also has limitations. Insurance claims data are collected for the purpose of payment/reimbursement (not research), which limits the validity of definitions of terms such as gout, asymptomatic hyperuricemia, and comorbidity. The data show the number of prescriptions written by physicians, but not whether patients actually used the drugs. Results may be further confounded by factors that cannot be measured by insurance claims, such as patient health awareness and lifestyle, social and economic factors, and the physician’s clinical practice. There is an additional limitation of incomplete data; because the JMDC data are from health insurance societies whose members work primarily for Japanese companies, very little data are available on persons 65 years of age and above, and no data on persons 75 years and above. The present study excluded data from persons 66 years and older, so it is difficult to generalize from these findings to the general Japanese population. The limitation of using medical check-up data means that information on sUA is obtained from only a certain portion of patients, that on the day of the medical check-up the patient might have reduced his or her use of ULT or other regular medications, and that these measurements are generally taken only once a year. In addition, this was a cross-sectional study with evaluation at a single time point, so it was not possible to estimate the causal relationship between exposure and outcome.
In conclusion, although the prevalence of hyperuricemia is similar in Japan and around the world, gout is comparatively rare in Japan. Gout and asymptomatic hyperuricemia are often treated with low-dose ULT, and many patients fail to reach their target sUA, suggesting that gout management is suboptimal in Japan. Patients would benefit from stricter focus on a treat-to-target approach for gout management.
Supplemental Material
Download PDF (69 KB)Acknowledgments
Support for study planning and information on data handling for the database were provided by Gen Terashima, JMDC Inc. Review of the statistical analysis plan, clinical study report, and manuscript was provided by Hirotaka Mano, Pharmaceutical Development Administration Department, Teijin Pharma Limited. Medical writing support was provided by EDIT, Inc. (Tokyo, Japan) and was funded by Teijin Pharma Limited.
Conflict of interest
RK, AN and HH are employees of Teijin Pharma Limited, and HY reports grants and personal fees from Teijin Pharma Limited.
References
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136–41.
- Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: Prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–62.
- Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388(10055):2039–52.
- Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: A nationwide population study. Ann Rheum Dis. 2015;74(4):661–7.
- Roddy E, Zhang W, Doherty M. Concordance of the management of chronic gout in a UK primary-care population with the EULAR gout recommendations. Ann Rheum Dis. 2007;66(10):1311–5.
- Neogi T, Hunter DJ, Chaisson CE, Allensworth-Davies D, Zhang Y. Frequency and predictors of inappropriate management of recurrent gout attacks in a longitudinal study. J Rheumatol 2006;33(1):104–9.
- Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: Evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum. 2004;51(3):321–5.
- Yamanaka H. The Guideline Revising Committee of the Japanese Society of gout and nucleic acid metabolism. Essence of the revised guideline for the management of hyperuricemia and gout. Japan Med Assoc J. 2012;55(4):324–9.
- Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64(10):1431–46.
- Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castaneda-Sanabria J, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76(1):29–42.
- Kiltz U, Smolen J, Bardin T, Cohen Solal A, Dalbeth N, Doherty M, et al. Treat-to-target (T2T) recommendations for gout. Ann Rheum Dis. 2017;76(4):632–8.
- Pandya BJ, Riedel AA, Swindle JP, Becker LK, Hariri A, Dabbous O, et al. Relationship between physician specialty and allopurinol prescribing patterns: A study of patients with gout in managed care settings. Curr Med Res Opin. 2011;27(4):737–44.
- Hatoum H, Khanna D, Lin SJ, Akhras KS, Shiozawa A, Khanna P. Achieving serum urate goal: A comparative effectiveness study between allopurinol and febuxostat. Postgrad Med. 2014;126(2):65–75.
- Rothenbacher D, Primatesta P, Ferreira A, Cea-Soriano L, Rodriguez LA. Frequency and risk factors of gout flares in a large population-based cohort of incident gout. Rheumatology. 2011;50(5):973–81.
- Dalbeth N, Saag KG, Palmer WE, Choi HK, Hunt B, MacDonald PA, et al. Effects of febuxostat in early gout: A randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 2017;69(12):2386–95.
- Yamanaka H. Gout and hyperuricemia in young people. Curr Opin Rheumatol. 2011;23(2):156–60.
- Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, et al. An allopurinol-controlled, randomized, double-dummy, double-blind, parallel between-group, comparative study of febuxostat (TMX-67), a non-purine-selective inhibitor of xanthine oxidase, in patients with hyperuricemia including those with gout in Japan: Phase 3 clinical study. J Clin Rheumatol. 2011;17(2):S13–S8.
- Becker MA, Schumacher HR, Jr., Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450–61.
- Kimura S, Sato T, Ikeda S, Noda M, Nakayama T. Development of a database of health insurance claims: Standardization of disease classifications and anonymous record linkage. J Epidemiol. 2010;20(5):413–9.
- Hakoda M, Kasagi F. Increasing trend of asymptomatic hyperuricemia under treatment with urate-lowering drugs in Japan. Mod Rheumatol. 2019;29(5):880–4.
- Kumar AUA, Browne LD, Li X, Adeeb F, Perez-Ruiz F, Fraser AD, et al. Temporal trends in hyperuricaemia in the Irish health system from 2006-2014: A cohort study. PLoS One. 2018;13(5):e0198197.
- Liu R, Han C, Wu D, Xia X, Gu J, Guan H, et al. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: A systematic review and meta-analysis. Biomed Res Int. 2015;2015:762820.
- Hosoya T, Sasaki T, Ohashi T. Clinical efficacy and safety of topiroxostat in Japanese hyperuricemic patients with or without gout: A randomized, double-blinded, controlled phase 2b study. Clin Rheumatol. 2017;36(3):649–56.
- Scheepers L, van Onna M, Stehouwer CDA, Singh JA, Arts ICW, Boonen A. Medication adherence among patients with gout: A systematic review and meta-analysis. Semin Arthritis Rheum. 2018;47(5):689–702.
- Yamanaka H, Tamaki S, Ide Y, Kim H, Inoue K, Sugimoto M, et al. Stepwise dose increase of febuxostat is comparable with colchicine prophylaxis for the prevention of gout flares during the initial phase of urate-lowering therapy: Results from FORTUNE-1, a prospective, multicentre randomised study. Ann Rheum Dis. 2018;77(2):270–6.
- Yamanaka H, Kamatani N, Kashiwazaki S. Comparative study of allopurinol and benzbromarone on the kinetics of uric acid metabolism. Hyperuricemia and Gout 1994;2:103–11.
- Kimura K, Hosoya T, Uchida S, Inaba M, Makino H, Maruyama S, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: A randomized trial. Am J Kidney Dis. 2018;72(6):798–810.
- Jackson R, Shiozawa A, Buysman EK, Altan A, Korrer S, Choi H. Flare frequency, healthcare resource utilisation and costs among patients with gout in a managed care setting: A retrospective medical claims-based analysis. BMJ Open. 2015;5(6):e007214.