ABSTRACT
Despite a long history of genetic engineering in Carica papaya L., genome editing protocols have not been previously validated for this species. To address this gap in the papaya improvement toolkit, we deployed CRIPSR/Cas9 to target the candidate Carica papaya L. phytoene desaturase (CpPDS) gene with the goal of inducing a visually scorable albino phenotype in edited tissue. A total of 73 plant lines stably transformed with pAC0025 (CRISPR construct targeting CpPDS) were obtained. Of these, 59 (81%) were fully albino. Genotyping of the targeted region within CpPDS was carried out for ten pAC0025 (CpPDS construct) lines, one untransformed control, and one line transformed with a negative control construct pAC0026 (no gRNA construct). Alignment of Sanger sequencing results revealed diverse edits in pAC0025-transformed plants including insertions of up to 415 bp, inversions and large deletions between gRNA target sites, and many smaller deletions. Mutations were observed at all three gRNA target sites. Overall, the high percentage of albino plants recovered in combination with the abundance of mutations observed across CpPDS gRNA target sites suggests an efficient genome editing protocol that could be used to improve papaya at the genetic level.
Introduction
Genome editing is revolutionising research in the biological sciences and is routinely facilitating precise, site-specific changes to target sequences within the species of interest. While multiple genome editing systems have been developed, the efficiency and flexibility of CRISPR-based strategies have catalysed their rise to predominance. The core components of these systems are a CRISPR-associated (Cas) protein and guide RNA (gRNA). While some Cas proteins have been modified to serve alternative functions, they traditionally act as non-specific endonucleases. Classic CRISPR applications utilise Cas9 from the bacterium Streptococcus pyogenes to induce blunt-end double-strand breaks (Jinek et al., Citation2012). These breaks result in mutations stemming from one of two native DNA repair processes: Non-homologous end joining (NHEJ) or homologous recombination (HR). The former is quite error prone, often resulting in deletions or insertions near the break site. These mutations have the potential to abolish or otherwise alter gene function. The efficiency, ease-of-use, and diverse applications of CRISPR/Cas9 have propelled its increasing popularity both in research and commercial biotechnology.
CRISPR/Cas has been successfully deployed in diverse plant species including corn (Svitashev, Schwartz, Lenderts, Young, & Cigan, Citation2016), wheat (Shan, Wang, Li, & Gao, Citation2014), cotton (Gao et al., Citation2017), tobacco (Chen et al., Citation2018), tomato (Brooks, Nekrasov, Lippman, & Van Eck, Citation2014), watermelon (Tian et al., Citation2017), and apple (Nishitani et al., Citation2016). An early step towards using CRISPR/Cas9 for either genetic studies or crop improvement is protocol development in the species of interest. In plants, the phytoene desaturase (PDS) gene is a common target for proof-of-concept studies (Chen et al., Citation2018; Fan et al., Citation2015; Nishitani et al., Citation2016; Tian et al., Citation2017). PDS encodes a key enzyme in the carotenoid biosynthesis pathway. In addition to its direct effect on carotenoid accumulation, it is vital to plastid development and chlorophyll biosynthesis, and may also affect gibberellic acid production (Qin et al., Citation2007). Plants lacking a functional PDS enzyme are albino and may exhibit other abnormalities including dwarfism and failure to reach physiological maturity. Due to this visually distinctive phenotype, PDS is a useful target for the development and optimisation of genome editing protocols in plants (Banakar et al., Citation2019; Fan et al., Citation2015; Gao et al., Citation2015; Hooghvorst, López-Cristoffanini, & Nogués, Citation2019; Kaur et al., Citation2018; Naim et al., Citation2018; Nakajima et al., Citation2017; Nishitani et al., Citation2016; Odipio et al., Citation2017; Pan et al., Citation2016; Tian et al., Citation2017; Wilson, Harrison, Armitage, Simkin, & Harrison, Citation2019).
Biotechnology is an especially vital tool in crops that have low genetic diversity, limiting a breeder’s ability to improve abiotic stress tolerance, disease resistance, and fruit quality through selection. One such species is Carica papaya L., a herbaceous perennial believed to have originated in Mesoamerica (Chávez-Pesqueira & Núñez-Farfán, Citation2017). Botanically, its fruits are large berries that range in size from a few grams to several kilograms per fruit. The ripe fruits are rich in nutrients including ascorbic acid (Vitamin C), provitamin A (beta-carotene), and in red-fleshed cultivars, lycopene, a potent antioxidant (Wall, Citation2006). Papaya is grown throughout tropical and subtropical regions worldwide, with production exceeding 13 million metric tons per year (Food and Agriculture Organization of the United Nations, Citation2020).
Papaya cultivation has been facing many challenges including pest and disease pressures and postharvest losses. Genetic engineering has been an important technology for addressing these issues. For example, papaya ringspot virus (PRSV) resistant varieties ‘SunUp’ and ‘Rainbow’ were some of the first commercially available speciality crops developed through genetic engineering (Gonsalves, Citation1998). Both biolistic and Agrobacterium-mediated transformation protocols have since been optimised for this species targeting a number of traits including herbicide tolerance (Cabrera-Ponce, Vegas-Garcia, & Herrera-Estrella, Citation1995), shelf life (López-Gómez et al., Citation2009), and tolerance to mites (McCafferty, Moore, & Zhu, Citation2006) and the fungal pathogen Phytophthora palmivora (Zhu, Agbayani, Jackson, Tang, & Moore, Citation2004). However, CRISPR/Cas-mediated genome editing has not been adopted for this crop though it has advantages over conventional transgenic approaches.
Here, we report the successful creation of C. papaya L. PDS knockouts with an albino phenotype utilising a CRISPR/Cas9 system as a proof of concept. This protocol should serve as a resource to accelerate genetic improvement in this species and enable innovation beyond the limits of existing genetic diversity.
Materials and Methods
gRNA Design
The putative CpPDS sequence, evm.model.supercontig_157.3, was retrieved from Phytozome v13 Carica papaya L. genome ASGPBv0.4. Three 20 nucleotide guide RNAs with NGG PAM sequences within the first two exons were chosen using CRISPOR based on high predicted specificity (MIT score ≥97) (Concordet & Haeussler, Citation2018). The relative positions of the three gRNA target sites in CpPDS are illustrated in . Guide RNA sequences were amplified using the plasmid pAGM9037 as a template, a target-specific forward primer (), and the critarev reverse primer (atgtacggccagcaacgtcg) (Grützner et al., Citation2021). PCR was carried out using ThermoFisher Platinum Taq DNA polymerase with an initial denaturation of 2 min for 94°C followed by 35 amplification cycles of 15s 94°C, 30s 66.7°C, and 45s 68°C, with a final 5 min extension at 68°C. PCR products (660 bp each) were purified using an EZNA Cycle Pure Kit (Omega Bio-tek, Inc., Norcross, GA).
Table 1. Three gRNAs were used to build binary vector pAC0025 targeting CpPDS. Each forward primer was paired with the same critarev reverse primer (atgtacggccagcaacgtcg) to generate PCR amplicons for cloning into the appropriate acceptor vector. Guide RNA target sequences are indicated in all caps within the forward primer sequences. The level 1 acceptor vectors into which PCR products were cloned are indicated, which determine the position of the gRNA cassette in the finished plasmid.
Assembly of transcriptional units (level 1 MoClo constructs)
CRISPR/Cas9 vectors to be used for papaya transformation were constructed using golden gate modular cloning (MoClo) (Weber, Engler, Gruetzner, Werner, & Marillonnet, Citation2011). First, individual transcriptional units for a visual marker (GFP), hygromycin resistance (hptII), the Cas9 enzyme (zCas9i), and each of three gRNAs (gRNAs 1, 2, and 3 targeting CpPDS) were assembled in level 1 constructs. The GFP module pAC0001 was constructed using pICSL12006 (Cassava Vein Mosaic Virus promoter and 5’ UTR) (Engler et al., Citation2014), pICH41531 (GFP CDS), and pICH41421 (NOS 3’ UTR and terminator) (Engler et al., Citation2014) in the level 1 position 1 acceptor pICH47732 (Weber et al., Citation2011). The hptII module pAC0002 was constructed using pICH87633 (NOS promoter and 5’ UTR) (Engler et al., Citation2014), pICSL80036 (hptII CDS) (Lawrenson et al., Citation2015), and pICH41421 (NOS 3’ UTR and terminator) (Engler et al., Citation2014) in the level 1 position 2 acceptor pICH47742 (Weber et al., Citation2011). The zCas9i module pAC0004 was constructed using pICSL12006 (Cassava Vein Mosaic Virus promoter and 5’ UTR), pAGM47523 (maize-codon-optimised SpCas9 CDS with 13 introns and 2NLS) (Grützner et al., Citation2021), and pICH41432 (OCS 3ʹUTR and terminator) (Engler et al., Citation2014) in the level 1 position 3 acceptor pICH47751 (Weber et al., Citation2011). The modules pAC0011, pAC0012, and pAC0013 were constructed for transcription of gRNAs 1, 2, and 3, respectively. Each gRNA assembly contained the Arabidopsis thaliana U6 promoter (AtU6) contributed by the level 0 vector pAGM38869 (Grützner et al., Citation2021), purified PCR product, and appropriate level 1 acceptor plasmid (level 1 positions 4, 5, and 6; see ) (Weber et al., Citation2011). Level 1 golden gate reactions were carried out in 20 µL volumes using 10 units (1 µL) Eco31I (BsaI) (ThermoFisher, Waltham, MA), 400 units (1 µL) T4 DNA Ligase (New England Biolabs, Ipswich, MA), 1 mM ATP (New England Biolabs, Ipswich, MA), Buffer G at 1X (ThermoFisher, Waltham, MA), 100 ng of acceptor plasmid, and insert DNA at a ratio of 2 moles insert for every 1 mole acceptor plasmid. Reactions were incubated for three cycles of digestion and ligation (40°C for 10 min, 16°C for 10 min) followed by 10 min at 50°C for final digestion and 20 min at 80°C for enzyme inactivation before holding at 16°C. Golden gate reactions were transformed into chemically competent Top10 E. coli and level 1 plasmids multiplied in LB supplemented with 100 mg/L carbenicillin. Omega-Biotek’s EZNA Plasmid DNA Mini Kit I (Omega Bio-tek, Inc., Norcross, GA) was used for plasmid purification. Plasmids were confirmed via Sanger sequencing (Genewiz, South Plainfield, NJ) with primer I1f (GTGGTGTAAACAAATTGACGC) (Grützner et al., Citation2021).
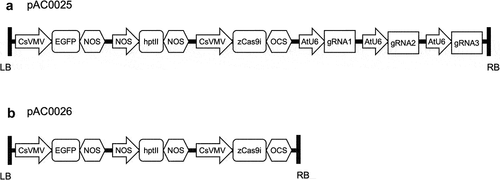
Assembly of multigene binary vectors (level 2 MoClo constructs)
Two level 2 binary vectors were assembled for plant transformation (). The first, pAC0025 was assembled using all previously described level 1 constructs: pAC0001 (GFP), pAC0002 (hptII), pAC0004 (zCas9i), pAC0011 (gRNA 1), pAC0012 (gRNA 2), and pAC0013 (gRNA 3), as well as end-linker pICH50927 in level 2 acceptor plasmid pAGM8031 (Weber et al., Citation2011). The second level 2 vector, pAC0026, was built to serve as a negative control for genome editing. pAC0026 was assembled using constructs pAC0001 (GFP), pAC0002 (hptII), pAC0004 (zCas9i), and end linker pICH50892 in the level 2 acceptor plasmid pAGM8031 (Weber et al., Citation2011). All gRNA expression cassettes were omitted from pAC0026. Level 2 golden gate reactions were carried out in 20 µL volumes using 10 units (1 µL) BpiI (ThermoFisher, Waltham, MA), 400 units (1 µL) T4 DNA Ligase (New England Biolabs, Ipswich, MA), 1 mM ATP (New England Biolabs, Ipswich, MA), Buffer G at 1X (ThermoFisher, Waltham, MA), 100 ng of acceptor plasmid, and insert DNA at a ratio of 2 moles insert for every 1 mole acceptor plasmid. Reactions were incubated for three cycles of digestion and ligation (37°C for 10 min, 16°C for 10 min) followed by 10 min at 37°C for final digestion and 20 min at 65°C for enzyme inactivation before holding at 16°C. Golden gate reactions were transformed into chemically competent Top10 E. coli and level 2 plasmids multiplied in LB supplemented with 100 mg/L spectinomycin. Omega-Biotek’s EZNA Plasmid DNA Mini Kit I (Omega Bio-tek, Inc., Norcross, GA) was used for plasmid purification. Plasmids were confirmed via Sanger sequencing (Genewiz, South Plainfield, NJ) with primers AC0365 (ACCTTGACAGTGACGACAAA) and AC0366 (CATGTGCATCCTCGGTCTC).
Figure 2. CRISPR construct T-DNA diagrams. LB indicates left border and RB indicates right border. A) pAC0025 T-DNA includes cassettes for the expression of GFP, selectable marker hptII conferring hygromycin resistance, zCas9i, and three gRNAs targeting CpPDS. B) pAC0026 (the negative control construct) lacks gRNA transcriptional units.
Completed level 2 constructs pAC0025 and pAC0026 were transformed into Agrobacterium tumefaciens strain GV3101 by electroporation. Briefly, 0.5 µL plasmid with 50 µL electro-competent Agrobacterium were added to an ice-cold electroporation cuvette, electroporated at 2.2 kV for 6 ms, then incubated with 1 mL LB media at 28°C for 3.5 hours with shaking at 225 rpm prior to plating on LB media supplemented with 10 mg/L rifampicin, 50 mg/L gentamycin, and 100 mg /L spectinomycin. Plates were incubated at 28°C for 48 to 72 hours to facilitate the growth of transformed bacterial colonies.
Papaya tissue culture and transformation
University of Florida papaya accession T5-2562 (a large-fruited, gynodioecious breeding line) was selected for transformation. Glassine bags were secured around hermaphroditic flowers just prior to anthesis to ensure self-pollination. Green fruits were harvested 85–94 days later, surface sterilised by soaking in 1.5% sodium hypochlorite with two drops Tween 20 per litre for 1 h and opened under aseptic conditions in a laminar flow hood. White, immature seeds were removed and zygotic embryos extracted with the aid of a dissecting scope. Embryos were plated directly on callus induction media (0.5X MS Salts, 1X MS Vitamins, 60 g/L sucrose, 10 mg/L 2,4-D) solidified with 0.8% agar for at least 4 weeks or until callus proliferation was evident. Thereafter, callus tissue was spread in a thin layer on filter paper atop callus induction media, incubated in darkness at ~25°C and subcultured monthly. Tissue was subsequently transferred to liquid multiplication media (0.5X MS Salts, 1X MS Vitamins, 60 g/L sucrose, 2 mg/L 2,4-D) and maintained in liquid culture from at least 7 weeks to 6 months prior to transformation. Five to 10 g (fresh weight) tissue per 75 mL media was maintained in each 250 mL volume flask. Flasks were aerated on a shaker at 100 rpm without direct light and subcultured every 14 days. Four days prior to transformation, liquid cultures were transferred to fresh liquid multiplication media. On the day of co-cultivation, papaya cultures were passed through a sterile 2 mm-square nylon mesh, and only tissue passing through the filter was used for transformation.
Papaya transformation was carried out using the method described previously (Zhu, Fitch, & Moore, Citation2006) with minor modifications. Single colonies of Agrobacterium tumefaciens GV3101 strains carrying CRISPR plasmids were first used to inoculate 4 mL starter cultures of LB with antibiotics (rifampicin 10 mg/L, gentamycin 50 mg/L, and spectinomycin 100 mg/L). Starter cultures were incubated at 28°C with agitation at 225 rpm. After approximately 20 hours, 1 mL of starter culture was used to inoculate each 50 mL liquid culture. The larger cultures were incubated for an additional 18 to 20 hours, or until an OD600 between 0.5 and 0.8 was reached. Cultures were then induced by adding acetosyringone (20 μM final concentration) and incubated for an additional 2 to 6 hours. Final OD600 was confirmed to be <1.0. Between 1.00 and 1.30 g filtered papaya tissue was placed in a 50 mL conical vial containing 0.50 g autoclaved 600 mesh carborundum and 20 mL liquid multiplication media, the mixture vortexed for 60 seconds at speed setting 7 (FisherBrand analog vortex mixer), and tissue was washed 3 to 4 times with 25 mL multiplication media. To begin co-cultivation, 5 mL of Agrobacterium culture was added to the tissue, incubated at room temperature for 10 minutes, decanted, and papaya tissue was drained on sterile filter paper. Finally, papaya tissue was spread in a thin layer on top of a piece of sterile filter paper on multiplication media solidified with 0.8% agar and incubated for 2 days in the dark at 25°C. To end co-cultivation, tissue was transferred to a sterile 50 mL conical vial and washed 3 to 4 times with 25 mL multiplication media with 500 mg/L carbenicillin and 100 mg/L cefotaxime. Tissue was then spread across a new piece of sterile filter paper on multiplication media, 0.8% agar, supplemented with 500 mg/L carbenicillin and 100 mg/L cefotaxime. Four weeks after transformation, filter paper and papaya tissue were moved to fresh multiplication media with antibiotics as well as 50 mg/L hygromycin for selection. Tissue was incubated on selective multiplication media and subcultured monthly for 3 to 4 months before transfer to MBN shoot regeneration media (1X MS Salts, 1X MS Vitamins, 30 g/L sucrose, 200 µg/L BA, and 200 µg/L NAA), 0.8% agar with 50 mg/L hygromycin in 100 mm x 20 mm plates and placed under lights with 16 hours light/8 hours dark cycles. Transformations were repeated for a total of six replicate plates each for empty GV3101, empty GV3101 hygromycin selection control, pAC0026, and pAC0025.
Characterisation of mutants
After 3 to 4 months on MBN shoot regeneration media, all regenerating plants were visually scored as either dark green (wild-type), pale green, or albino. Genomic DNA was extracted from 10 putative PDS mutants (pAC0025-transformed lines) as well as one untransformed plant and one plant line transformed with the negative control construct pAC0026 based on a previously described method (Edwards, Johnstone, & Thompson, Citation1991). Briefly, ~100 mg of leaf or stem tissue was placed in a 2 mL screw-cap microcentrifuge tube with 300 µl extraction buffer (200 mM Tris.Cl pH 8, 250 mM NaCl, 25 mM EDTA, 0.5% SDS) and two stainless steel beads. Samples were ground in a paint shaker for 90 seconds, centrifuged briefly until a speed of 18,000 x g was reached, and a half-volume (150 ul) of 3 M sodium acetate buffer (pH 5.2) was added to each sample prior to incubation at −20°C for 10 minutes. Tubes were centrifuged at 18,000 x g for 15 minutes before transferring 200 uL supernatant to new 1.5 mL microcentrifuge tubes along with an equal volume (200 uL) isopropanol and incubated for 5 minutes at room temperature. DNA was pelleted by centrifuging at 18,000 x g for 15 minutes, supernatant discarded, and pellets were washed with 70% ethanol and resuspended in 500 uL nuclease-free water. To confirm the presence of T-DNA in putatively transformed plants, 287 bp of the GFP CDS was amplified via PCR with primers AC0368 (CAACTACAACAGCCACAACG) and AC0369 (ACTTGTACAGCTCGTCCATG) using Apex Taq RED Master Mix (Genesee Scientific, San Diego, CA) and thermal cycling conditions as follows: Initial denaturation for 5 min at 95°C followed by 30 amplification cycles of 30s 95°C, 30s 55°C, and 20s 72°C, with a final 10 min extension at 72°C. PCR was also carried out using primers AC0418 (TTGAGCTCGTCAGAGCAAAGAT) and AC0419 (TGTGTCAAAAACAGGGCAACC) to amplify the genomic CpPDS region targeted by gRNAs for subsequent sequencing. PCRBIO HS VeriFi reagents were used for amplification (PCR Biosystems, Wayne, PA) with an initial denaturation for 1 min at 95°C, 35 amplification cycles of 15s 95°C, 15s 58°C, and 72°C 30s, and a 5 min final extension at 72°C. PCR product was ligated into a pJET1.2 blunt-end cloning vector (ThermoFisher, Waltham, MA), and transformed into Top10 E. coli. Plasmid was purified from ≥5 bacterial clones per plant line and sent for Sanger sequencing (Genewiz, South Plainfield, NJ) with primer AC0419. Sequence alignments were carried out using Geneious Prime v11 (Auckland, NZ).
Results
Identification of transformed papaya lines
One-hundred eighty-two plant lines were recovered through somatic embryogenesis, consisting of 73 lines transformed with the CRISPR construct targeting CpPDS pAC0025, 78 lines transformed with the no-gRNA control construct pAC0026, and 31 untransformed lines as an additional negative control. In the presence of 50 mg/L hygromycin in tissue culture media, untransformed papaya tissue did not grow and eventually turned brown and senesced (). Transformed lines were identified after 3 to 4 months on selective media as they remained pale yellow in colour and increased in size (). Transgenic status of regenerating papaya lines was further confirmed by observation of GFP via fluorescence microscopy with a Leica MZ10 F dissection scope (excitation 425 nm, Wetzlar, DE) and was highly correlated with hygromycin-resistant lines (). After transfer of transformed tissue to MBN shoot regeneration media, somatic embryos germinated and continued to increase in size ().
Figure 3. Embryogenic papaya tissue 20 weeks after co-cultivation with Agrobacterium tumefaciens. A) No-selection control showing healthy papaya tissue growing on multiplication media without hygromycin. B) Selection control with untransformed papaya tissue on selective multiplication media supplemented with 50 mg/L hygromycin showing characteristic tissue browning. C) Papaya tissue co-cultivated with Agrobacterium harbouring construct pAC0025 (binary vector with gRNAs targeting CpPDS) with putatively transformed calli actively growing on selective multiplication media circled in red.
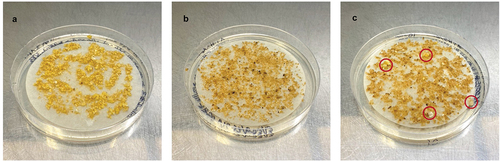
Figure 4. GFP expression in papaya transformed with the plasmid pAC0025. A) Embryogenic papaya culture 34 days post co-cultivation with Agrobacterium. Somatic embryos were primarily at the globular stage. B) Late torpedo (top) and early cotyledon (bottom) stage somatic embryos 131 days post co-cultivation with Agrobacterium.
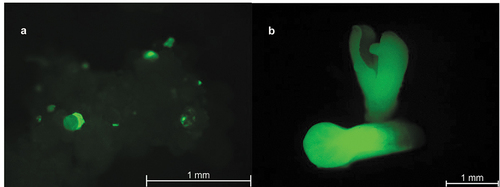
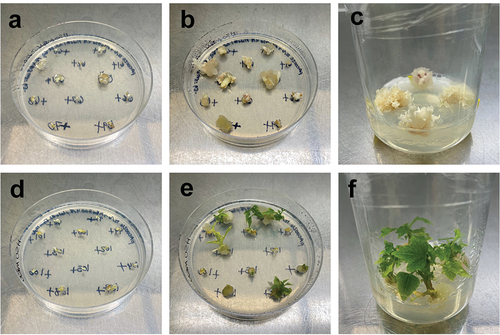
Figure 5. Putative papaya transformants growing on MBN shoot regeneration media with pAC0025 targeting CpPDS (A, B, and C) or negative control vector pAC0026 (D, E, and F). A) pAC0025 transformants immediately after transfer from multiplication media. B) pAC0025 transformants after 4 weeks under lights. C) pAC0025 transformants after 10 weeks under lights. D) pAC0026 transformants immediately after transfer from multiplication media. E) pAC0026 transformants after 4 weeks under lights. F) pAC0026 transformants after 8 weeks under lights.
Transformed papaya phenotypes
Transformed papaya plants could be classified into three categories based on their morphology in vitro. Plants were either dark green (wild-type), pale green, or albino (). The dark green phenotype corresponds to that of typical unedited papaya plants in tissue culture. Pale green plants were relatively few and could grow into dark green plants over time. Albino plants were most likely the result of successful disruption of the CpPDS gene, as these are not commonly observed in papaya tissue culture after embryo germination in the presence of light. Regeneration was discontinued after three to four months on MBN shoot regeneration media, as development of edited plants stalled. Albino plants displayed many signs of abnormal development, including short stems and leaves that failed to expand ( & ).
Estimation of genome editing efficiency
A total of 73 plant lines stably transformed with pAC0025 (CRISPR construct targeting CpPDS) were obtained. Of these, 59 were fully albino. Of the remaining 14 lines, 7 were pale green and 7 were dark green (wild-type) in appearance. Additionally, 78 papaya lines transformed with the negative control construct pAC0026 were also recovered. None of these were albino and only three had a pale green phenotype. The remaining 75 plant lines (96%) were dark green. Similarly, all 31 untransformed control lines were dark green. As albinos were only observed among plants transformed with pAC0025, and albinism is a classical sign of PDS disruption in plants, we infer that these plant lines represent successful editing events. The percentage of pAC0025-transformants with an albino phenotype, 81%, can therefore be used to approximate genome editing efficiency. This may be a conservative estimate, however, as some edits to CpPDS may not abolish its function.
PCR screening of transformed papaya lines
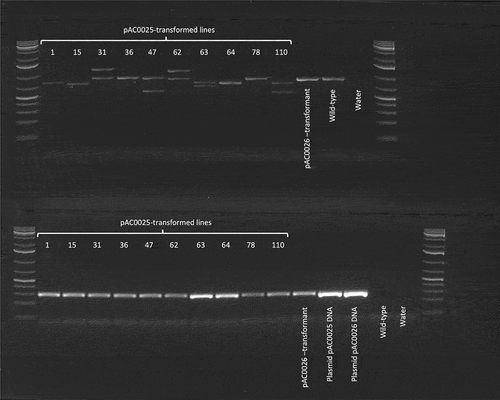
PCR analysis of CpPDS was carried out for 12 regenerated papaya lines to identify large insertions or deletions mediated by CRISPR/Cas9. Regenerated papaya lines included 10 plant lines transformed with pAC0025 (CpPDS CRISPR construct), one untransformed control, and one line transformed with pAC0026 (no-gRNA construct). Amplification of a region at the 5’ end of CpPDS encompassing all three gRNA target sites revealed large deletions and insertions present in lines 31, 47, 62, 63, and 110 that were readily detected upon separation of the PCR products by gel electrophoresis (). Other edit types including small insertions, small deletions, and sequence inversions could not be readily detected using agarose electrophoresis. Amplification of the expected 287 bp PCR amplicon of a partial GFP CDS confirmed integration of T-DNA in all eleven transformed lines ().
Figure 7. PCR amplification of genomic DNA from putatively edited plants and controls. The first and last lanes in each row were loaded with GeneRuler 1kb+ DNA ladder. Lane labels reflect the source of genomic DNA utilised in each PCR reaction. ‘pAC0025-transformed lines’ indicates PCR reactions utilising gDNA from papaya plants transformed with pAC0025, the CRISPR/Cas9 plasmid targeting CpPDS. Lanes labelled ‘pAC0026 transformant’ indicate PCR with gDNA from a plant transformed with the no-gRNA negative control plasmid pAC0026. Lanes labelled ‘Plasmid pAC0025 DNA’ and ‘Plasmid pAC0026 DNA’ correspond to PCR reactions utilising purified plasmid DNA as template. In ‘Wild-type’ lanes gDNA from an untransformed papaya plant was used as PCR template, and ‘water’ lanes served as a negative control with no DNA template added to the PCR reactions. Partial CpPDS sequence (top row) wild-type amplicon size 909 bp and partial GFP CDS from T-DNA insertion (bottom row) expected amplicon size is 287 bp.
CpPDS sequence analysis of putatively edited papaya lines
Sequence analysis of CpPDS was carried out for the same 12 papaya lines described above. Alignment of CpPDS Sanger sequencing reads to the wild-type sequence revealed diverse edits in pAC0025-transformed plants, including insertions of up to 415 bp, inversions and large deletions between gRNA target sites, and many smaller deletions ( & ). The most common edits were large deletions between gRNA target sites, which were observed in at least one allele of all edited lines sequenced except for line 36. Deletion of the sequence between gRNA sites 1 and 2 was especially common, accounting for six out of nine edited plant lines and 10 out of 18 of the edited CpPDS alleles sequenced. Deletions spanning sites targeted by gRNA 1 and 3 as well as 2 and 3 were also present.
Table 2. CpPDS genotypes of plant lines transformed with pAC0025. “-“ indicates a deletion, ‘+’ an insertion, and ‘inversion’ denotes that the sequence between cut sites has been excised, reversed, and re-ligated. ‘WT’ indicates wild-type sequence, or the absence of mutations.
Table 3. Sequence alignment of CpPDS genotypes at all three gRNA target sites. ‘WT’ indicates wild-type sequence. ‘Al.’ represents allele, described here as ‘A’ or ‘B’. For plant lines found to be homozygous for CpPDS, alleles are identical and represented as ‘A/B’. PAM sites are denoted by underlined characters, italicised sequences represent gRNA targets, “-“ indicates a deletion, ‘+’ an insertion, and grey shading designates an inversion.
Mutations were typically observed at all three gRNA target sites. However, in one of the lines (pAC0025 line 78), no edits were found. This plant also had a dark green/wild-type phenotype indicating that while it was positive for the presence of the GFP CDS, CpPDS has not been successfully edited. In the case of the two alleles harbouring large insertions, line 31 allele A, and line 62 allele B, the foreign DNA sequences were found to be derived from the binary vector pAC0025. One line (line 78) was found to be homozygous for wild-type CpPDS, which is consistent with its wild-type (dark green) appearance. Phenotypes intermediate between wild-type and albino were also observed. Genotyping of two pale green mutant lines, line 47 and line 110, revealed that each had one PDS allele that had sustained mutations predicted to alter the translation reading frame. However, in both cases the second allele differed from wild-type only by a 309 bp deletion between gRNA target sites 1 and 3. While this large deletion would result in a loss of 74 amino acids encoded by exons 1 and 2 as well as a single amino acid substitution, it should not disrupt normal translation of the remaining protein sequence. The apparent accumulation of reduced levels of chlorophyll in lines 47 and 110 suggests that the function of the CpPDS enzyme is impaired but not abolished in these lines. Of the remaining seven lines sequenced, two (lines 1 and 15) appear to be homozygous mutants, with a single edited CpPDS allele detected in each. All other edited lines characterised are biallelic, with both CpPDS alleles bearing distinct mutations.
Discussion
Efficient genome editing systems for crop plants represent a key resource for the development of new varieties in addition to facilitating basic research in genetics and molecular biology. Despite well-established transformation protocols (Cai et al., Citation1999; Fitch, Manshardt, Gonsalves, Slightom, & Sanford, Citation1990, Citation1992; Zhu et al., Citation2006), and the publication of the genome in 2008 (Ming et al., Citation2008), genome editing has not been previously documented in C. papaya L. Here, we describe successful CRISPR/Cas9-mediated genome editing in papaya targeting the CpPDS gene. This approach can now be deployed for the improvement of agronomically relevant traits in the tropical fruit crop C. papaya L.
In the present study, functional disruption of the targeted gene, phytoene desaturase (PDS), appeared to occur in 59 out of 73 independent transformation events, suggesting an editing efficiency of ≥80%. The PDS gene was selected as a target for genome editing protocol development in papaya due to the ease with which knockouts can be identified based on their albino phenotype. Unfortunately, disruption of PDS does not impart any useful improvements from an applied perspective, limiting its utility to proof-of-concept studies. In fact, PDS mutants are typically extremely weak. It is commonly reported that complete PDS knockouts cannot be fully regenerated through the rooting and acclimatised to soil stages. This was our observation in papaya, and the phenomenon has been previously documented in coffee (Breitler et al., Citation2018), melon (Hooghvorst et al., Citation2019), and banana (Kaur et al., Citation2018). Despite these drawbacks, PDS has proven to be a very useful target for CRISPR/Cas9 protocol development in plants (Chen et al., Citation2018; Fan et al., Citation2015; Nishitani et al., Citation2016; Tian et al., Citation2017), as it facilitates rapid approximation of editing efficiency based on phenotypic data alone.
Based on the percentage of albino mutants among plants transformed with pAC0025, we estimate a genome editing efficiency of over 80% has been achieved in papaya. This rate is consistent with the range of editing efficiencies that have been reported in other species. While CRISPR/Cas9 editing efficiencies as high as 100% have been previously documented in plant species including tomato, grapes, watermelon, and rice (Brooks et al., Citation2014; Ren et al., Citation2016; Tian et al., Citation2017; Zhou, Liu, Weeks, Spalding, & Yang, Citation2014), such high editing rates are not ubiquitous. Others have reported edits in fewer than half of transformed plants (Lawrenson et al., Citation2015; Ma et al., Citation2019). Genome editing efficiency is influenced by parameters including gRNA target sequence and construct design. The later encompasses choice of regulatory elements affecting Cas9 and gRNA expression level (i.e. promoters and terminators), as well as the Cas9 coding sequence utilised. In all constructs described here, Pol II-type promoter, CsVMV, was selected to drive Cas9 expression paired with a strong OCS terminator. This combination has yielded successful editing in Arabidopsis thaliana, as has the CsVMV promoter paired with Cas9 in Brassica oleracea (Castel, Tomlinson, Locci, Yang, & Jones, Citation2019; Lawrenson et al., Citation2015). RNA polymerase III-type promoters are typically used for expression of gRNAs, with U6 or U3 being the most common (Zhang, Gao, Wang, & Zhao, Citation2017). Cas9 codon optimisation, the number of nuclear localisation signals, and the addition of introns have all been shown to have some effect, with introns having the greatest impact (Castel et al., Citation2019; Grützner et al., Citation2021). The Cas9 CDS utilised in the present study (zCas9i) is codon-optimised for Zea mays and features 2 NLS and 13 introns (Grützner et al., Citation2021), and has been shown to be efficient for high-level multiplexing in A. thaliana (simultaneous 12x knockout) and Nicotiana benthamiana (simultaneous 8x knockout) (Stuttmann et al., Citation2021). Successful editing in papaya using zCas9i further demonstrates its utility beyond model species.
Guide RNA selection is another factor known to play a large role in experimental outcomes as different target sequences can result in very variable editing rates. While algorithms predicting gRNA efficacy have been developed and this score is featured in many design tools, strong evidence of their accuracy in plants is lacking (Naim et al., Citation2020). A common strategy for selecting efficient gRNAs is to first evaluate their activity empirically in a protoplast or leaf agroinfiltration assay with transient expression or direct delivery of CRISPR/Cas9 components (Gao et al., Citation2015; Tian et al., Citation2017). Forgoing preliminary gRNA evaluation, satisfactory efficacy was achieved in papaya by selecting multiple gRNAs based on predicted specificity targeting 5’ exons of the putative PDS gene.
Uncommon edits were observed as revealed through sequencing of edited and regenerated papaya lines. This included large insertions of vector DNA as has been previously documented in plant species including rice (Banakar et al., Citation2019), potato (Johansen et al., Citation2019), and wheat (Arndell et al., Citation2019). However, it is notable that in this study CRISPR/Cas9 components were delivered exclusively via Agrobacterium tumefaciens-mediated transformation with a binary vector. These results contrast with previous findings in rice suggesting that large random insertions are a feature of biolistic delivery systems specifically (Banakar et al., Citation2019). CRISPR/Cas9-mediated sequence inversions observed here have also been previously reported in rice (Banakar et al., Citation2019; Liang, Zhang, Lou, & Yu, Citation2016), maize (Schwartz et al., Citation2020), and Arabidopsis (Schmidt, Pacher, & Puchta, Citation2019); encompassing both bombardment-based and Agrobacterium-mediated transformation approaches. The prevalence of fragment dropout between gRNA target sites as observed in seven out of nine edited lines genotyped is consistent with previous findings in other plant species when multiple gRNAs with target sites in proximity to one another are simultaneously deployed (Naim et al., Citation2020).
The CRISPR/Cas9-mediated genome editing protocol described here represents a new resource for papaya improvement through biotechnology. Potential genome editing targets in papaya are numerous, but examples include the ACC oxidase gene for prolonged shelf life (López-Gómez et al., Citation2009) and the eukaryotic initiation factor 4E (eIF4E) gene, which could confer resistance to potyviruses (Chandrasekaran et al., Citation2016). Despite the promise of this technology, significant hurdles remain. A key practical limitation of the protocol described here is that it yields plants which are not only genome edited, but also transgenic. While it is possible to eventually obtain plants harbouring only the edit(s) and no foreign DNA using conventional breeding methods, this may significantly lengthen the product development timeline or pose regulatory challenges. For these reasons, a ‘transgene-free’ CRISPR/Cas9-mediated genome editing protocol that could rely on transient expression of the Cas enzyme and gRNAs (Zhang et al., Citation2016), or the delivery of preassembled Cas-gRNA complexes known as ribonucleoprotein particles (RNPs) (Woo et al., Citation2015), may also be desirable.
Conclusions
Here, we report successful genome editing of the PDS gene in the species C. papaya L. We observed a high rate of editing, with 81% of plant lines transformed with pAC0025 (CpPDS CRISPR construct) displaying a complete albino phenotype. Genotyping a subset of transformed lines confirmed that mutations were prevalent at all three gRNA target sites, and included insertions, deletions, and inversions. The results also revealed that CpPDS is essential for chlorophyll synthesis in papaya. The validation of a CRISPR/Cas9 genome editing system in C. papaya L. provides a valuable tool for plant breeders seeking to improve fruit quality, disease resistance, and other traits, in addition to supporting basic genetics research.
Data availability
Plasmid and sequencing data are available in Supplemental Data.
Acknowledgments
The authors acknowledge Maria Brym, Wanda Montas, Pam Moon, Dr. Alfred Huo, and Dr. Francesco Cappai for technical guidance, Dr. Jonathan Crane for providing immature papaya fruit, and Dr. Patricio Muñoz for contributing bacterial strains. The MoClo Toolkit (Addgene kit # 1000000044) and plasmid pAGM47523 (Addgene plasmid # 153221; http://n2t.net/addgene:153221; RRID: Addgene_153221) were gifts from Sylvestre Marillonnet. The MoClo Plant Parts Kit (Addgene kit # 1000000047) and plasmid pICSL80036 (Addgene plasmid # 68259; http://n2t.net/addgene:68259; RRID: Addgene_68259) were gifts from Nicola Patron.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arndell, T., Sharma, N., Langridge, P., Baumann, U., Watson-Haigh, N.S., & Whitford, R. (2019). gRNA validation for wheat genome editing with the CRISPR-Cas9 system. BMC Biotechnology, 19, 1–12. doi:10.1186/s12896-019-0565-z
- Banakar, R., Eggenberger, A.L., Lee, K., Wright, D.A., Murugan, K., Zarecor, S., Wang, K. (2019). High-frequency random DNA insertions upon co-delivery of CRISPR-Cas9 ribonucleoprotein and selectable marker plasmid in rice. Scientific Reports, 9, 1–13. doi:10.1038/s41598-019-55681-y
- Breitler, J.-C., Dechamp, E., Campa, C., Zebral Rodrigues, L.A., Guyot, R., Marraccini, P., & Etienne, H. (2018). CRISPR/Cas9-mediated efficient targeted mutagenesis has the potential to accelerate the domestication of Coffea canephora. Plant Cell, Tissue and Organ Culture, 134, 383–394. doi:10.1007/s11240-018-1429-2
- Brooks, C., Nekrasov, V., Lippman, Z.B., & Van Eck, J. (2014). Efficient gene editing in tomato in the first generation using the clustered regularly interspaced short palindromic repeats/CRISPR-associated9 system. Plant Physiology, 166, 1292–1297. doi:10.1104/pp.114.247577
- Cabrera-Ponce, J.L., Vegas-Garcia, A., & Herrera-Estrella, L. (1995). Herbicide resistant transgenic papaya plants produced by an efficient particle bombardment transformation method. Plant Cell Reports, 15, 1–7. doi:10.1007/BF01690242
- Cai, W., Gonsalves, C., Tennant, P., Fermin, G., Souza, M., Sarindu, N., Gonsalves, D. (1999). A protocol for efficient transformation and regeneration of Carica papaya L. In Vitro Cellular & Developmental Biology-Plant, 35, 61. doi:10.1007/s11627-999-0011-3
- Castel, B., Tomlinson, L., Locci, F., Yang, Y., & Jones, J.D. (2019). Optimization of T-DNA architecture for Cas9-mediated mutagenesis in Arabidopsis. PLoS ONE, 14, e0204778. doi:10.1371/journal.pone.0204778
- Chandrasekaran, J., Brumin, M., Wolf, D., Leibman, D., Klap, C., Pearlsman, M., … Gal-On, A. (2016). Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Molecular Plant Pathology, 17, 1140–1153. doi:10.1111/mpp.12375
- Chávez-Pesqueira M., & Núñez-Farfán J. (2017). Domestication and Genetics of Papaya: A Review. Frontiers in Ecology and Evolution, 5, Article 155. doi:10.3389/fevo.2017.00155
- Chen, L., Li, W., Katin-Grazzini, L., Ding, J., Gu, X., Li, Y., … Deng, Z. (2018). A method for the production and expedient screening of CRISPR/Cas9-mediated non-transgenic mutant plants. Horticulture Research, 5, 13. doi:10.1038/s41438-018-0023-4
- Concordet, J.-P., & Haeussler, M. (2018). CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Research, 46, W242–W245. doi:10.1093/nar/gky354
- Edwards, K., Johnstone, C., & Thompson, C. (1991). A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Research, 19, 1349. doi:10.1093/nar/19.6.1349
- Engler, C., Youles, M., Gruetzner, R., Ehnert, T.-M., Werner, S., Jones, J.D., … Marillonnet, S. (2014). A golden gate modular cloning toolbox for plants. ACS Synthetic Biology, 3, 839–843. doi:10.1021/sb4001504
- Fan, D., Liu, T., Li, C., Jiao, B., Li, S., Hou, Y., & Luo, K. (2015). Efficient CRISPR/Cas9-mediated targeted mutagenesis in Populus in the first generation. Scientific Reports, 5, 12217. doi:10.1038/srep12217
- Fitch, M.M., Manshardt, R.M., Gonsalves, D., Slightom, J.L., & Sanford, J.C. (1990). Stable transformation of papaya via microprojectile bombardment. Plant Cell Reports, 9, 189–194. doi:10.1007/BF00232177
- Fitch, M.M., Manshardt, R.M., Gonsalves, D., Slightom, J.L., & Sanford, J.C. (1992). Virus resistant papaya plants derived from tissues bombarded with the coat protein gene of papaya ringspot virus. Nature Biotechnology, 10, 1466–1472. doi:10.1038/nbt1192-1466
- Food and Agriculture Organization of the United Nations, (2020). FAOSTAT statistics database. Rome, Italy: FAO. Retrieved from https://www.fao.org/faostat/ 26 October 2021
- Gao, W., Long, L., Tian, X., Xu, F., Liu, J., Singh, P.K., … Song, C. (2017). Genome editing in cotton with the CRISPR/Cas9 system. Frontiers in Plant Science, 8, 1364. doi:10.3389/fpls.2017.01364
- Gao, J., Wang, G., Ma, S., Xie, X., Wu, X., Zhang, X., &Xia, Q. (2015). CRISPR/Cas9-mediated targeted mutagenesis in Nicotiana tabacum. Plant Molecular Biology, 87, 99–110. doi:10.1007/s11103-014-0263-0
- Gonsalves, D. (1998). Control of papaya ringspot virus in papaya: A case study. Annual Review of Phytopathology, 36, 415–437. doi:10.1146/annurev.phyto.36.1.415
- Grützner, R., Martin, P., Horn, C., Mortensen, S., Cram, E.J., Lee-Parsons, C.W., &Marillonnet, S. (2021). High-efficiency genome editing in plants mediated by a Cas9 gene containing multiple introns. Plant Communications, 2, 100135. doi:10.1016/j.xplc.2020.100135
- Hooghvorst, I., López-Cristoffanini, C., & Nogués, S. (2019). Efficient knockout of phytoene desaturase gene using CRISPR/Cas9 in melon. Scientific Reports, 9, 1–7. doi:10.1038/s41598-019-53710-4
- Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J.A., & Charpentier, E. (2012). A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science, 337, 1225829. doi:10.1126/science.1225829
- Johansen, I.E. Liu, Y., Jørgensen, B., Bennett, E.P., Andreasson, E., Nielsen, K.L., … Petersen, B.L. (2019). High efficacy full allelic CRISPR/Cas9 gene editing in tetraploid potato. Scientific Reports, 9, 1–7. doi:10.1038/s41598-019-54126-w
- Kaur, N., Alok, A., Shivani, Kaur, N., Pandey, P., Awasthi, P., & Tiwari, S. (2018). CRISPR/Cas9-mediated efficient editing in phytoene desaturase (PDS) demonstrates precise manipulation in banana cv. Rasthali genome. Functional & Integrative Genomics, 18, 89–99. doi:10.1007/s10142-017-0577-5
- Lawrenson, T., Shorinola, O., Stacey, N., Li, C., Østergaard, L., Patron, N.,&Harwood, W. (2015). Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease. Genome Biology, 16, 1–13. doi:10.1186/s13059-015-0826-7
- Liang, G., Zhang, H., Lou, D., & Yu, D. (2016). Selection of highly efficient sgRNAs for CRISPR/Cas9-based plant genome editing. Scientific Reports, 6, 1–8. doi:10.1038/srep21451
- López-Gómez, R., Cabrera-Ponce, J.L., Saucedo-Arias, L.J., Carreto-Montoya, L., Villanueva-Arce, R., Díaz-Perez, J.C., … Herrera-Estrella, L. (2009). Ripening in papaya fruit is altered by ACC oxidase cosuppression. Transgenic Research, 18, 89–97. doi:10.1007/s11248-008-9197-0
- Ma, C., Liu, M., Li, Q., Si, J., Ren, X., & Song, H. (2019). Efficient BoPDS gene editing in cabbage by the CRISPR/Cas9 system. Horticultural Plant Journal, 5, 164–169. doi:10.1016/j.hpj.2019.04.001
- McCafferty, H.R., Moore, P.H., & Zhu, Y.J. (2006). Improved Carica papaya tolerance to carmine spider mite by the expression of Manduca sexta chitinase transgene. Transgenic Research, 15, 337–347. doi:10.1007/s11248-006-0005-4
- Ming, R., Hou, S., Feng, Y., Yu, Q., Dionne-Laporte, A., Saw, J.H., &Lewis, K.L. (2008). The draft genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus). Nature, 452, 991–996. doi:10.1038/nature06856
- Naim, F., Dugdale, B., Kleidon, J., Brinin, A., Shand, K., Waterhouse, P., & Dale, J. (2018). Gene editing the phytoene desaturase alleles of Cavendish banana using CRISPR/Cas9. Transgenic Research, 27, 451–460. doi:10.1007/s11248-018-0083-0
- Naim, F., Shand, K., Hayashi, S., O’Brien, M., McGree, J., Johnson, A.A., … Waterhouse, P.M. (2020). Are the current gRNA ranking prediction algorithms useful for genome editing in plants? PloS ONE, 15, e0227994. doi:10.1371/journal.pone.0227994
- Nakajima, I., Ban, Y., Azuma, A., Onoue, N., Moriguchi, T., Yamamoto, T., … Endo, M. (2017). CRISPR/Cas9-mediated targeted mutagenesis in grape. PLoS ONE, 12, e0177966. doi:10.1371/journal.pone.0177966
- Nishitani, C., Hirai, N., Komori, S., Wada, M., Okada, K., Osakabe, K., … Osakabe, Y. (2016). Efficient genome editing in apple using a CRISPR/Cas9 system. Scientific Reports, 6, 31481. doi:10.1038/srep31481
- Odipio, J., Alicai, T., Ingelbrecht, I., Nusinow, D.A., Bart, R., & Taylor, N.J. (2017). Efficient CRISPR/Cas9 genome editing of phytoene desaturase in Cassava. Frontiers in Plant Science, 8, 1780. doi:10.3389/fpls.2017.01780
- Pan, C., Ye, L., Qin, L., Liu, X., He, Y., Wang, J., … Lu, G. (2016). CRISPR/Cas9-mediated efficient and heritable targeted mutagenesis in tomato plants in the first and later generations. Scientific Reports, 6, 1–9. doi:10.1038/srep24765
- Qin, G., Gu, H., Ma, L., Peng, Y., Deng, X.W., Chen, Z., & Qu, L.-J. (2007). Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid, and gibberellin biosynthesis. Cell Research, 17, 471–482. doi:10.1038/cr.2007.40
- Ren, C., Liu, X., Zhang, Z., Wang, Y., Duan, W., Li, S., & Liang, Z. (2016). CRISPR/Cas9-mediated efficient targeted mutagenesis in Chardonnay (Vitis vinifera L.). Scientific Reports, 6, 1–9. doi:10.1038/srep32289
- Schmidt, C., Pacher, M., & Puchta, H. (2019). Efficient induction of heritable inversions in plant genomes using the CRISPR/Cas system. The Plant Journal, 98, 577–589. doi:10.1111/tpj.14322
- Schwartz, C., Lenderts, B., Feigenbutz, L., Barone, P., Llaca, V., Fengler, K., & Svitashev, S. (2020). CRISPR–Cas9-mediated 75.5-Mb inversion in maize. Nature Plants, 6, 1427–1431. doi:10.1038/s41477-020-00817-6
- Shan, Q., Wang, Y., Li, J., & Gao, C. (2014). Genome editing in rice and wheat using the CRISPR/Cas system. Nature Protocols, 9, 2395. doi:10.1038/nprot.2014.157
- Stuttmann, J., Barthel, K., Martin, P., Ordon, J., Erickson, J.L., Herr, R., &Keilwagen, J. (2021). Highly efficient multiplex editing: One-shot generation of 8×Nicotiana benthamiana and 12× Arabidopsis mutants. The Plant Journal, 106, 8–22. doi:10.1111/tpj.15197
- Svitashev, S., Schwartz, C., Lenderts, B., Young, J.K., & Cigan, A.M. (2016). Genome editing in maize directed by CRISPR–Cas9 ribonucleoprotein complexes. Nature Communications, 7, 13274. doi:10.1038/ncomms13274
- Tian, S., Jiang, L., Gao, Q., Zhang, J., Zong, M., Zhang, H., … Liu, F. (2017). Efficient CRISPR/Cas9-based gene knockout in watermelon. Plant Cell Reports, 36, 399–406. doi:10.1007/s00299-016-2089-5
- Wall, M.M. (2006). Ascorbic acid, vitamin A, and mineral composition of banana (Musa sp.) and papaya (Carica papaya) cultivars grown in Hawaii. Journal of Food Composition and Analysis, 19, 434–445. doi:10.1016/j.jfca.2006.01.002
- Weber, E., Engler, C., Gruetzner, R., Werner, S., & Marillonnet, S. (2011). A modular cloning system for standardized assembly of multigene constructs. PLoS ONE, 6, e16765. doi:10.1371/journal.pone.0016765
- Wilson, F.M., Harrison, K., Armitage, A.D., Simkin, A.J., & Harrison, R.J. (2019). CRISPR/Cas9-mediated mutagenesis of phytoene desaturase in diploid and octoploid strawberry. Plant Methods, 15, 1–13. doi:10.1186/s13007-019-0428-6
- Woo, J.W., Kim, J., Kwon, S.I., Corvalán, C., Cho, S.W., Kim, H., … Kim, J.-S. (2015). DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nature Biotechnology, 33, 1162–1164. doi:10.1038/nbt.3389
- Zhang, T., Gao, Y., Wang, R., & Zhao, Y. (2017). Production of guide RNAs in vitro and in vivo for CRISPR using ribozymes and RNA polymerase II promoters. Bio-Protocol, 7, e2148. doi:10.21769/BioProtoc.2148
- Zhang, Y., Liang, Z., Zong, Y., Wang, Y., Liu, J., Chen, K., … Gao, C. (2016). Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nature Communications, 7, 12617. doi:10.1038/ncomms12617
- Zhou, H., Liu, B., Weeks, D.P., Spalding, M.H., & Yang, B. (2014). Large chromosomal deletions and heritable small genetic changes induced by CRISPR/Cas9 in rice. Nucleic Acids Research, 42, 10903–10914. doi:10.1093/nar/gku806
- Zhu, Y.J., Agbayani, R., Jackson, M.C., Tang, C., & Moore, P.H. (2004). Expression of the grapevine stilbene synthase gene VST1 in papaya provides increased resistance against diseases caused by Phytophthora palmivora. Planta, 220, 241–250. doi:10.1007/s00425-004-1343-1
- Zhu, Y.J., Fitch, M.M., & Moore, P.H. (2006). Papaya (Carica papaya L.). In: K. Wang (Ed.), Agrobacterium Protocols Volume 2 (pp.209–217). Totowa, NJ: Humana Press. Methods in Molecular Biology, 344. doi: 10.1385/1-59745-131-2:209.


