ABSTRACT
Floral initiation in biennial-fruiting red raspberry is controlled by the interaction of temperature and photoperiod. To determine the threshold temperatures for short day (SD) floral initiation in early- and late-flowering cultivars, we exposed plants of ‘Glen Ample’, ‘Glen Mor’ and ‘Duo’ to 12°, 16° and 20°C in a daylight phytotron under naturally decreasing autumn daylength at Ås, Norway (59°40’N). While none of the cultivars ceased growing or initiated floral primordia at 20°C, ‘Glen Ample’ and ‘Glen Mor’ initiated buds at 12° and 16°C, whereas ‘Duo’ formed flower buds at 12°C only. Surprisingly, however, all plants flowered abundantly in spring after winter chilling in the dark at −1.5 ± 0.5°C for 7 months. We discuss two possible explanations for this unusual and novel flowering response. Fractional induction is well known in raspberry, and we visualise that in SD at 20°C, the SD requirement is fulfilled, while floral induction is still blocked by inappropriate temperature. A vernalisation-like response is alternatively suggested as this can take place at near-freezing temperatures in the dark. A combination of the two mechanisms is also possible and likely. We conclude, however, that the two floral induction processes are fundamentally different and controlled by different physiological mechanisms.
Introduction
Floral initiation in the biennial-fruiting red raspberry (Rubus idaeus L.) has been extensively studied and shown to be controlled by a pronounced interaction of temperature and photoperiod (Heide & Sønsteby, Citation2011; Hodnefjell et al., Citation2018; Moore & Caldwell, Citation1985; Sønsteby & Heide, Citation2008; Williams, Citation1960). Cultivars of diverse origin were shown to grow continuously without initiating floral primordia under both short day (SD) and long day (LD) conditions at 18–21°C. At 15°C they cease growing and initiate floral primordia at photoperiods shorter than 15 h, whereas at temperatures below 12°C they cease growing and initiate floral primordia regardless of the photoperiod. However, Heide and Sønsteby (Citation2011) demonstrated that 15°C is not an absolute threshold temperature for floral induction in biennial-flowering raspberries. Extended SD exposure under high radiation conditions resulted in marginal flowering in ‘Glen Ample’ even at 18°C. Furthermore, Hodnefjell et al. (Citation2018) demonstrated some genotypic differences in the critical temperature, with ‘Glen Ample’, ‘Balder’ and ‘Vene’ initiating floral primordia in SD at 16°C, whereas four other cultivars did not. It is also known that raspberry plants, even when vegetatively propagated, have a juvenile-like period during which they cannot flower (Sønsteby & Heide, Citation2008; Williams, Citation1960). Although this period is completed at approximately the 15th leaf stage, there may still be better flowering as the plants grow beyond that stage.
These studies show that 15°C is not an absolute critical temperature for flowering in biennial-fruiting red raspberry. The critical temperature varies somewhat between cultivars and can be modified by environmental factors. In this experiment, we compared the critical temperatures for flower initiation of early and late cultivars with that of the standard commercial ‘Glen Ample’.
Materials and methods
Plant material and cultivation
The cultivars ‘Glen Ample’, ‘Glen Mor’ and ‘Duo’ were used for the experiment. ‘Glen Ample’ and ‘Glen Mor’ were released from the James Hutton Institute, Dundee, Scotland, and ‘Duo’ from the Norwegian Berry Breeding program at Njøs Frukt- og Bærsenter, Leikanger, Norway. ‘Glen Mor’ was chosen for its early flowering and compact growth habit, as well as its resistance to root rot disease (James Hutton Ltd, Citation2020). ‘Duo’ was chosen for its late flowering and vigorous growth habit. The plants were propagated from root buds and raised as single-stem plants in a greenhouse at 21°C and continuous light during the summer of 2022 as described by Sønsteby and Heide (Citation2008). On 3 August, when the plants had developed approximately 20 leaves, they were distributed to three daylight phytotron compartments maintained at 12°C, 16°C and 20°C and exposed to natural daylengths at Ås, Norway (59°40’N, 10°45’E) for 9 weeks until 5 October. Then, one plant of each treatment was harvested and all buds along the entire length of the shoot were dissected to determine their floral stage. The remaining five plants were moved directly into a dark cold store maintained at 5°C. After 2 weeks, the temperature was lowered to −1.5°C ± 1.0°C which was maintained during winter. In the spring of 2023, the plants were moved into an open high tunnel for observations of flowering. Temperatures in the phytotron were controlled to ±1°C, and a water vapour pressure deficit of 530 Pa was maintained at all temperatures.
Experimental design and data collection
The experiment was laid out in a split-plot design with temperatures as main plots and cultivars as sub-plots. Each treatment was started with six plants of each cultivar, and the results of each plant were treated as replications in the statistical analysis. During cultivation in the phytotron, the positions of the plant trollies were completely re-randomised every day in connection with daily fertigation of the plants. Plant growth was monitored by weekly recordings of shoot height and number of leaves in each plant. The flowering stages of the dissected buds were scored according to the 6-stage scale used by Sønsteby and Heide (Citation2008). Flowering of the overwintered plants was recorded as the number of flowering plants and the number of flowering nodes in each plant. The data were subjected to analysis of variance (ANOVA) by standard procedures using a Minitab® Statistical Software program package (Release 17.2.1 MiniTab, MiniTab Inc., State College, PA, USA). Percentage values were subjected to an arc sin transformation before the ANOVA.
Results
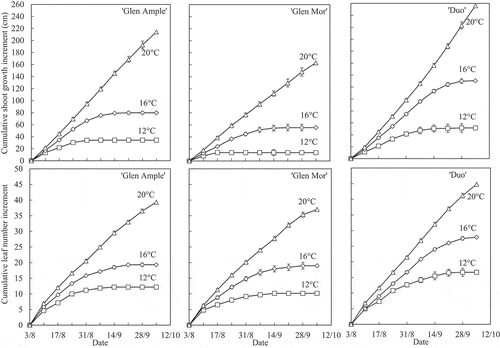
Growth performance of the plants in the phytotron is presented in . While shoot growth rate differed between the three cultivars, being lowest in ‘Glen Mor’ and highest in ‘Duo’, the difference in the rate of leaf formation was significant only between ‘Duo’ and the two Glen cultivars. Both shoot extension and leaf production increased with increasing temperature in all cultivars, while the duration of growth varied between cultivars and temperatures. At 12°C, growth ceased on 17 August (after 2 weeks of cultivation) in ‘Glen Mor’, and on 31 August and 14 September, in ‘Glen Ample’ and ’Duo’, respectively. At 16°C, however, the cessation was delayed by 4 weeks in ‘Glen Mor’ and by 2 weeks in ‘Glen Ample’ and ‘Duo’. At 20°C, all cultivars grew at almost constant rates throughout the 9-week period. In general, cessation of leaf formation followed the same time pattern as cessation of growth, although with 1 or 2 weeks delay due to the delayed appearance of the last initiated leaves.
Figure 1. Effects of temperature on growth of three red raspberry cultivars in naturally decreasing daylengths at Ås, Norway during August and September. Data are the means ± SE of six plants per treatment.
Bud dissections at the end of the 9-week period (on 5 October) revealed that no floral buds had been initiated at 20°C, whereas there were large differences in the flower development stages of the cultivars at 12° and 16°C (). At 12°C, all the cultivars had initiated floral buds, although they were less advanced in ‘Duo’ than in the two Glen cultivars which had advanced flower bud primordia even at the terminal bud position. While the buds of ‘Glen Ample’ were more advanced over a wide range of bud positions, ‘Glen Mor’ had initiated floral buds a few more nodes towards the base of the cane. At 16°C, however, ‘Glen Mor’ had more flower buds which also were more advanced than those of ‘Glen Ample’. At 12°C, the most advanced buds were found around the seventh position from the top, whereas at 16°C, the most advanced buds were found a few nodes higher up, and the topmost buds were always vegetative. In ‘Duo’, all flower buds were located at higher bud positions than in the other cultivars at 12°C, whereas all buds were vegetative at 16°C. Outdoors in spring, the advancement of flowering of the plants with flower primordia was still evident. Surprisingly, however, all plants grown at 20°C did also flower profusely (). In ‘Glen Mor’ and ‘Duo’ the number of flowers per plant was even higher in these plants. As usual, anthesis was advanced by low previous temperature in the phytotron, whereas the percentage of dead and non-breaking buds generally increased with increasing autumn temperature because of top freezing after the abrupt transfer of actively growing plants to near-freezing temperatures in the fall. However, an opposite temperature response was generally found for the percentage of flowering nodes, although the temperature trend was modified by variations in the percentage of non-breaking nodes. All these temperature and cultivar effects and their interactions were generally highly significant (). The number of flowers per lateral and per plant increased with increasing temperature in the phytotron, an effect that was associated with greater final shoot length at high temperature and a higher proportion of long laterals at the lower nodes after tip-freezing.
Figure 2. Effects of temperature on profiles of flower development stages of lateral buds along the entire shoot of three red raspberry cultivars after growth for 9 weeks at 12°C and 16°C as shown. The data represent one single plant per treatment. No flower buds were observed at 20°C. Buds at stages ≥ 2 are indicated in yellow.
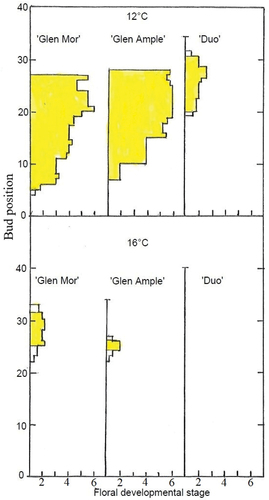
Table 1. Effects of temperature under naturally decreasing autumn daylength on growth and flowering of three red raspberry cultivars during a 9-week treatment period, followed by cold storage for 7 months and forcing in an open high tunnel in the following spring.
Discussion
The results demonstrated marked differences in both growth rate and floral initiation patterns among the three cultivars (). Shoot growth rate increased with increasing temperature in all cultivars, being highest in ‘Duo’ and lowest in ‘Glen Mor’. In contrast, the rate of leaf initiation differed only between ‘Duo’ and the two Glen cultivars. Hence, the taller plants of ‘Glen Ample’ compared with ‘Glen Mor’ were due only to longer internodes of the former cultivar. The duration of growth increased with increasing temperature in all cultivars and was associated with a delay in the appearance of floral buds. This was especially evident in the vigorous growing ‘Duo’ which failed to initiate floral buds at 16°C. At 20°C, however, none of the cultivars initiated floral buds during the 9-week treatment period.
These results are consistent with our present knowledge of the critical temperatures for SD floral induction in biennial-fruiting red raspberry. While most cultivars have a critical temperature of 15°C (Heide & Sønsteby, Citation2011; Williams, Citation1960), other cultivars such as ‘Glen Ample’, ‘Balder’ and ‘Vene’ were found to marginally initiate primordia even at 16°C (Heide & Sønsteby, Citation2011; Hodnefjell et al., Citation2018). The present results show that ‘Duo’ belongs to the former and ‘Glen Mor’ to the latter group. This response supports the assumption that vigorous growth and late floral initiation are associated with elevated temperature thresholds for floral initiation.
A highly surprising finding was, however, that plants without floral primordia in autumn flowered profusely in spring after 7 months of chilling at −1.5°C. This occurred in all cultivars and shows that an extended chilling in the dark induces flowering in apparently vegetative plants. Such a floral induction response is unprecedented in the literature, and we can only speculate about the underlying mechanism(s).
One possible mechanism is the principle of fractional floral induction which has been demonstrated in a variety of both SD and LD plants (Bernier et al., Citation1981; Thomas & Vince-Prue, Citation1997). This is the summation of the effect of two or more inductive periods of sub-critical duration when separated by intercalated non-inductive periods. Such fractional induction was demonstrated in ‘Malling Promise’ and ‘Glen Ample’ raspberry (Sønsteby & Heide, Citation2008; Williams, Citation1960) when SD induction was interrupted by an intercalated LD period. An analogous response was demonstrated in strawberry by Sønsteby and Heide (Citation2017). In such plants, where flowering is controlled by the interaction of photoperiod and temperature (Heide, Citation1977; Heide & Sønsteby, Citation2011), it can be visualised that under inductive SD but non-inductive temperature conditions, the photoperiodic requirement may be fulfilled while floral induction is still blocked by inappropriate temperatures. Some flowering genes might have been activated in such fractionally induced plants, which then may only need an additional low temperature impulse for realisation of floral induction.
Another possible explanation for the phenomenon might be that the chilling response is a vernalisation-like process. This possibility is supported by the demonstration of vernalisation in the annual-fruiting raspberry cultivars ‘Autumn Bliss’ (Carew et al., Citation2001) and ‘Polka’ (Sønsteby et al., Citation2009). In small plants raised from adequately chilled roots, an additional exposure to 6–7°C for 7–10 weeks reduced both the time and number of leaves before flowering compared with plants grown continuously at 24°C. The authors concluded that these results are consistent with a vernalisation-like advancement of flowering that is distinct from the effect of chilling on bud dormancy release (cf. Chouard, Citation1960).
It should be kept in mind, however, that vernalisation, which is defined as induction or promotion of flowering by low temperature (Bernier et al., Citation1981), does not lead directly to floral initiation but is a preparatory process that enables the plants to initiate flowering under subsequent inductive conditions (Chouard, Citation1960; Thomas & Vince-Prue, Citation1997). Thus, a vernalisation process would imply that flower buds might not be initiated before the plants are exposed to inductive photoperiods in spring. However, since flowering time was not highly different in plants with and without floral primordia in the autumn (), our results are inconclusive in that respect. Regrettably, since we did not expect the non-induced plants to flower, we did not check the floral initiation status of the plants before they were moved outside in spring.
On the other hand, the likelihood of a vernalisation process is favoured by the fact that it has an optimum at near-freezing temperatures and readily takes place in complete darkness in a variety of plants if their carbohydrate and energy supply is adequate (Bernier et al., Citation1981; Chouard, Citation1960; Thomas & Vince-Prue, Citation1997). It should be kept in mind that extended chilling is necessary for bud break and flowering along the entire length of an overwintering raspberry cane (Heide & Sønsteby, Citation2011; Mazzitelli et al., Citation2007; Palonen et al., Citation2015). Thus, the long cane ‘Glen Ample’ plants used by Sønsteby et al. (Citation2009), which yielded almost 4 kg of fruit per plant, were stored at −1°C for more than 6 months before forcing, while Palonen et al. (Citation2015) found that the cropping potential of raspberry long cane plants increased steadily with increasing duration of storage at −1°C for up to 20 weeks. Taken together, all these results indicate that winter chilling is not only necessary for dormancy release in raspberry winter buds but also enhances flowering and cropping potential of partially induced buds. Such a dual effect of winter chilling concurs with the early ideas of Chouard (Citation1960) and makes it quite feasible that the unexpected flowering could have been brought about by a vernalisation-like process. Furthermore, the two suggested possible mechanisms are not mutually exclusive but can be merged to provide a unified model involving both a fractional photoperiodic induction and an additional vernalisation-like low-temperature effect.
It is evident, however, that the two temperature-related processes are fundamentally different and utilise different physiological mechanisms. Thus, although both processes were enhanced by low temperatures, the primary induction process required SD whereas the winter chill effect took place in complete darkness. The final flowering in spring was, however, indirectly enhanced by high temperature during the SD induction period due to enhancement of shoot growth and the associated increase in node numbers and, hence, the potential number of flowering cites. However, further investigations are needed for a full understanding of this novel flowering phenomenon.
Conclusion
We conclude that, none of the tested raspberry cultivars initiated floral buds in SD at 20°C in autumn, while they all flowered abundantly in spring after chilling in the dark for seven months. We suggest that the combination of fractional flower induction and dark vernalisation may possibly explain this novel flowering response.
Acknowledgements
The authors acknowledge the skilful technical assistance by Rodmar Rivero with dissection of apices, and by Kari Grønnerød and Mirjana Sadojevic with plant cultivation and sampling of data.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bernier, G., Kinet, J. M., & Sachs, R. M. (1981). The physiology of flowering (Vol. I). CRC Press.
- Carew, J. G., Mahmood, K., Darby, J., Hadley, P., & Battey, H. (2001). The effects of low temperatures on the vegetative growth and flowering of the primocane fruiting raspberry ‘Autumn bliss’. The Journal of Horticultural Science and Biotechnology, 76(3), 264–270. https://doi.org/10.1080/14620316.2001.11511361
- Chouard, P. (1960). Vernalization and its relation to dormancy. Annual Review of Plant Physiology, 11(1), 191–238. https://doi.org/10.1146/annurev.pp.11.060160.001203
- Heide, O. M. (1977). Photoperiod and temperature interactions in growth and flowering of strawberry. Physiologia Plantarum, 40(1), 21–26. https://doi.org/10.1111/j.1399-3054.1977.tb01486.x
- Heide, O. M., & Sønsteby, A. (2011). Physiology of flowering and dormancy regulation in annual- and biennial-fruiting red raspberry (Rubus idaeus L.) – a review. The Journal of Horticultural Science and Biotechnology, 86(5), 433–442. https://doi.org/10.1080/14620316.2011.11512785
- Hodnefjell, R., Heide, O. M., Rivero, R., Remberg, S. F., & Sønsteby, A. (2018). Control of growth cessation and floral initiation in red raspberry (Rubus idaeus L.) cultivars of diverse origin in controlled and natural environments. Scientia Horticulturae, 233, 412–420. https://doi.org/10.1016/j.scienta.2018.02.011
- James Hutton Ltd. (2020). https://www.huttonltd.com/services/plant-varieties-breeding-licensing/raspberry/glen-mor
- Mazzitelli, L., Hancock, R. D., Haupt, S., Walker, P. G., Pont, S. D. A., McNicol, J., Cardle, L., Morris, J., Viola, R., Brennan, R., Hedley, P. E., & Taylor, M. A. (2007). Co-ordinated gene expression during phases of dormancy release in raspberry (Rubus idaeus L.) buds. Journal of Experimental Botany, 58(5), 1035–1045. https://doi.org/10.1093/jxb/erl266
- Moore, J. N., & Caldwell, J. D. (1985). Rubus. In A. H. Halevy (Ed.), CRC handbook of flowering (Vol. IV, pp. 226–238). CRC Press.
- Palonen, P., Pohjola, M., & Karhu, S. (2015). Cropping potential of raspberry long cane plants as affected by their growing condition and the duration of storage. The Journal of Horticultural Science and Biotechnology, 90(6), 738–746. https://doi.org/10.1080/14620316.2015.11668740
- Sønsteby, A., & Heide, O. M. (2008). Environmental control of growth and flowering of Rubus idaeus L. cv. Glen Ample. Scientia Horticulturae, 117(3), 249–256. https://doi.org/10.1016/j.scienta.2008.05.003
- Sønsteby, A., & Heide, O. M. (2017). Flowering performance and yield of established and recent strawberry cultivars (Fragaria x ananassa) as affected by raising temperature and photoperiod. The Journal of Horticultural Science and Biotechnology, 92, 367–375. https://doi.org/10.1080/14620316.2017.1283970
- Sønsteby, A., Myrheim, U., Heiberg, N., & Heide, O. M. (2009). Production of high yielding red raspberry long canes in a Northern climate. Scientia Horticulturae, 121(3), 289–297. https://doi.org/10.1016/j.scienta.2009.02.016
- Thomas, B., & Vince-Prue, D. (1997). Photoperiodism in plants (2nd ed.). Academic Press.
- Williams, I. H. (1960). Effects of environment on Rubus idaeus L. V. Dormancy and flowering of the mature shoot. Journal of Horticultural Science, 335(3), 214–220. https://doi.org/10.1080/00221589.1960.11513985
