Abstract
This paper compares the attitudes of European and US scientists engaged in human genetics research about some of the ethical issues raised by recent advances in genetic testing, by the increasing likelihood of subsequent genetic therapies, and by hovering threats to the privacy of those tested in the face of concerns raised by individual and institutional third parties. Surveys of both groups indicate strong and fairly uniform support for attempts to discern and cure serious diseases or disorders and decisions to terminate pregnancies in which fetuses have serious genetic defects. But the data also indicate a number of issues where European and US respondents disagree and where that disagreement is characterised by a more cautious approach on the part of the European scientists. These data should provide a foundation for subsequent reflection and discussion within the scientific communities as well as within the society at large.
Introduction
While progress in genetic knowledge and technology is thought to have the potential to ‘profoundly improve human health’ (NHGRI, Citation1997), advancement in human genetics has also generated unprecedented social, psychological, and legal questions as well as a range of ethical issues to be considered (e.g., Lemke, Citation2004; Nussbaum, Citation2004). And genetic breakthroughs are proceeding more rapidly than the establishment of a consensus on the ethics of their use, leaving society ‘scrambling to match … values and policies to scientific advances’ (Frankel & Chapman, Citation2001). The question remains: How should the fruits of the genetic revolution be utilised?
To better understand the evolving field of genetic medicine and the ethical dilemmas that accompany it, to provide a foundation for reflection and discussion within the scientific and medical communities, and to furnish information for policy makers and the public at large, we conducted surveys of those with specialised knowledge of the field—US and European scientists who engage in human genetics research. As an in-depth survey of the views of these experts, our study obtained not only aggregated responses to closed-ended questions but also a compilation of insightful open-ended comments.
Ethical issues in biomedicine are varied and complex, involving questions of ‘justifiability, responsibility and accountability’ (ASHG, Citation2000) as well as moral acceptability—how to ‘put the common morality and medical traditions into a coherent package’ (Beauchamp & Childress, Citation2001). This paper will deal with ethical questions generated by new capabilities in genetic testing and diagnosis as well as ethical questions raised by the increasing likelihood of successful forms of gene therapy. Because ethical issues may differ from culture to culture, one might expect that the development of ethical thinking about emerging knowledge and technology might also vary. Accordingly, the paper will also compare and contrast attitudes of US and European researchers.
Survey method
Twelve-page questionnaires were mailed to the total 3,632 US members of the American Society of Human Genetics (ASHG) as well as 978 to the European membership of ASHG and pertinent sections of the European Molecular Biology Organisation (EMBO). A total of 1,236 completed US questionnaires were returned (in addition, 363 postcards from US scientists who had never engaged in human genetics research). A total of 324 completed European surveys were returned.
The total response rates were 38% and 33%, respectively. Though not ideal, these are understandable considering: (1) the lengthy and detailed nature of the questionnaire, which included solicitations of written comments; and (2) the fact that there were no monetary inducements and no follow-up mailings.Footnote1 In any case, there is no reason to think that there is any difference between respondents and non-respondents in the US compared to Europe, so that the comparative data should be reliable.
This paper will compare responses from the 816 US researchers who completed surveys only during 2002—a time frame which overlapped that of the European study—with responses from 324 European respondents, including scientists from 25 countries, the largest numbers being: Great Britain (24%), Germany (14%), France (13%), The Netherlands and Italy (8%), Switzerland (6%), Israel (5%), Spain and Sweden (4%), Denmark and Iceland (3.5%), and Ireland (3%). Respondents were encouraged throughout the survey to include written comments about their replies. Approximately two-thirds of the US group and one-half of the European respondents volunteered thoughtful insights.
Background of scientists
More than two-thirds of the respondents (70% of the 816 US respondents and 73% of the 324 Europeans) have been working in the field for more than ten years; 29% and 25%, respectively, between three and ten years; and only 1% for less time. Most (75% and 78%) are academic researchers, 12% of US and 7% of European scientists are in private industry, 10% and 13% respectively work in government. Some 56% of US respondents are male (43% female), while 68% of European respondents are male (31% female).
Results: ethics and genetic testing
Since the advent of genetic diagnostics, and now the completion of the Human Genome Project and its contribution to the expansion of genetic knowledge, new tests for genetic predispositions—both testing for defective genes in healthy individuals and prenatal testing for genetic defects in the fetus—have rapidly become available, creating choices that evoke an array of ethical questions. This section will deal with respondents' views on whether to test for a gene and under what conditions, what measures to take as a result of findings about a predisposition and on what basis, and who has the right to genetic information so generated and who does not. Quotation marks will be employed to indicate specific wording used in the survey questionnaire.
When to test, what can be achieved, and the patient's right to choose
Support for predisposition testing of adults is fairly high, particularly among US respondents: 72% of US and 58% of European scientists agree with ‘testing for a gene that suggests an increased risk of developing a serious disease’. When information that could be derived from testing does not coincide with medicine's ability to cure or prevent a disorder, slightly fewer, but still majorities (66% vs. 72% above for US respondents, and 54% vs. 58% for the Europeans), approve of ‘testing for a gene that denotes a serious disease for which there is no cure’. Whether a condition is curable or not seems, then, to have only a slight influence on approval of genetic testing. These conclusions are similar to findings about public approval for genetic testing in the US and Europe (Eurobarometer, Citation1990–2002; Milewa, Citation1999; NCGR, Citation1998; Singer, Van Hoewyk & Antonucci, Citation2005).Footnote2
Respondents' generally pro-active attitude—especially US respondents—stands in contrast to the more restrictive position taken by the US Committee on Assessing Genetic Risks, in which they recommend that testing for predispositions to late-onset monogenic diseases ‘should only be considered for treatable or preventable conditions of relatively high frequency’ (Andrews et al., Citation1994). The Committee's guideline emphasises the emotional price of positive test results when disease symptoms might not be imminent or severe—i.e., psychological burdens including emotional distress and anxiety. Other factors also might affect patient decisions not to be tested. Cost and likelihood of insurance reimbursement is one; fear about discriminatory consequences is another and will be discussed later.
Actual behavior on the part of physicians and patients, on the other hand, according to a number of health system analysts—‘Genetic tests fall short of public embrace’ (Kolata, Citation1998), for example—appears to reflect the disadvantages rather than the advantages of pursuing prognoses for what is not alterable: ‘There are hundreds of millions of dollars being made on DNA testing for treatable diseases, and [only] tens of millions for non-treatable diseases’ (Hercz, Citation1998).
Whether to take more extraordinary action—precautionary surgery—when certain genes indicative of tendencies toward breast or ovarian cancer are discovered (‘time bomb genes’, because women with these genes face a greater than 50/50 risk) was the basis of another question. Our survey asked about cases in which such genes were identified but where no symptoms were present: Do respondents agree with patients and doctors who opt for preventive surgery such as mastectomies or hysterectomies in such cases, in order to reduce the risk of the disease's occurrence or its severity of outcome? Some 54% of US researchers do (19% do not), compared with only 35% of European researchers (an equivalent 37% of whom do not). Similar proportions of both groups were not sure (27% and 28%, respectively).Footnote3
There is one important exception to the overall high advocacy of genetic testing. When we asked about the desirability of ‘mandatory premarital testing for genetic disorders, as is required for venereal diseases’, we found strong objection to any statutory requirement for testing. This principle of voluntariness is shared among scientists: 84% of US and 89% of European respondents oppose mandatory testing, with comments such as: ‘The decision to undergo genetic testing for any disease or susceptibility should be taken by the individual to be tested…[with] access to all available data regarding the benefits, limitations, and potential risks’ (European researcher).
The standard of non-obligatory testing is backed by advisory groups in both Europe and the US. The US Committee on Assessing Genetic Risks found no justification for state-sponsored mandatory public health programs involving genetic testing of adults, instead stipulating that it was ultimately the patient's ‘right to know’ or not to know (Andrews et al., Citation1994). Similarly, recommendations on genetic testing for health care purposes, by the Committee of Ministers of the Council of Europe Citation(1992), included two critical principles: self-determination of the persons concerned and the non-compulsory nature of tests: ‘Health service benefits, family allowances, marriage requirements, or other similar formalities…should not be made dependent on the undergoing of genetic tests’.
While respondents espouse patient autonomy by insisting that testing be voluntary, they make it clear, however, that testing ought to be done under professional supervision, as do the respective advisory panels. A high 81% of US and 95% of European researchers answer ‘no’ to the question of whether ‘genetic diagnostic kits should be available to the general public for their own use, independent of the professional support of a hospital, doctor, or genetic counselor’.
Genetic risk and planned births
The use of genetic testing in prenatal diagnosis evokes ethical questions because of its connection with possible pregnancy termination based on the results of such tests. Concerned about the use of prenatal genetic testing for identification of trivial characteristics or conditions, the US Committee for Assessing Genetic Risk's recommendation was for prenatal diagnosis to be offered ‘only for the diagnosis of genetic disorders and birth defects’ (Andrews et al., Citation1994), in effect attempting to reign in unlimited reproductive autonomy.
Our survey asked respondents to weigh the acceptability of terminating pregnancies in a variety of situations when in utero tests could determine high probabilities of certain outcomes. Differences between US and European researchers are small (a range of 0% to 9%), but findings do reveal a consistently slightly higher tendency on the part of US respondents to find therapeutic abortions acceptable for all of the reasons listed.
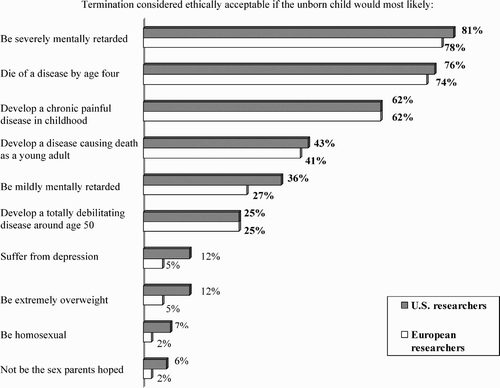
Responses vary by severity of outcome, where acceptance of termination is highest for conditions perceived as actual diseases or disorders—‘demonstrably injurious’, as one US researcher wrote. For example, 81% of US and 78% of European respondents approve of termination when severe mental retardation is involved, whereas only 36% and 27% respectively are in approval for mild retardation, and less than 12% and 5%, respectively, for either depression or serious weight problems.
Responses also vary by the length of time before the negative effect occurs, where acceptance is highest when onset is early in life—for example, dying of a disease before age four (where the acceptance rate for termination is 76% and 74%, respectively) versus dying of a disease as a young adult (43% and 41%). Opposition to termination steadily increases, then, as the age of disease onset increases, although it rarely reaches a majority: dying of a disease by age four (only 10% and 11%, respectively, say unacceptable), developing a chronic painful disease in childhood (15% and 14%), developing a disease causing death as a young adult (32% and 30%), and developing a totally debilitating disease around age 50 (51% and 48%).
The strongest objections, however, involve conditions that would not necessarily be characterised as diseases, that would not necessarily be perceived as negative, that have a considerable societal component, or that are thought to be arbitrary—sex selection (89% and 95%, respectively, say unacceptable), homosexuality (83% and 92%), obesity (71% and 78%), and depression (71% and 77%). These traits fall instead into the categories of improvement or enhancement, or what is deemed socially desirable or advantageous. One European respondent commented: ‘These techniques should be used only to cure disease and never for improving human capabilities’.
Among these characteristics, sex preference is the only one that can actually be determined in utero at this time. Just as 89% of US and 95% of European respondents take issue with terminating a pregnancy because of the sex of the fetus (only 5% and 2% respectively find it acceptable), the US Committee on Assessing Genetic Risks (Andrews et al., Citation1994) took a stand against the use of fetal diagnosis for the sole purpose of determining sex and the subsequent use of abortion for the purpose of preferential sex selection: ‘[It] represents a misuse of genetic services that is inappropriate and should be discouraged by health professionals’.
Ethical lines become most blurred for situations that cannot be clearly categorised. For a disease causing death as a young adult, 43% and 41% respectively say termination is ethically acceptable. For mild mental retardation, while 36% of US and 27% of European respondents feel termination is acceptable, there is equal or higher opposition: 36% and 41%, respectively.
Other aspects of this cure-versus-enhancement debate will be taken up again in the discussion of gene therapy. But first we'll turn our attention to respondents' perspectives on the important matter of collecting and protecting genetic information acquired through genetic testing.
Confidentiality, privacy, and concerns about discrimination
Genetic information collected about individuals, through voluntary testing or from participation in research studies, is highly sensitive. The information on predisposition that genetic tests provide can enable not only the opportunity for treatment or prevention but also the possibility of stigmatisation and discrimination. Testing, therefore, raises serious privacy concerns.
Apprehension about institutional access to genetic information, particularly by employers and health and life insurers, is widespread and universal in our surveys results. Studies of public opinion reveal similar apprehension and distrust (Eurobarometer, Citation2002; Milewa, Citation1999; NCGR, Citation1998; Singer, Corning & Lamias, Citation1998). A 1999 survey by the American Management Association demonstrated a basis in fact in the US: 30% of large and midsize companies required some form of genetic information about their employees, and 7% actually used that information in hiring and promoting (Martindale, Citation2001). In the United Kingdom, the insurance industry has locked horns with the government over using genetic information in assessing applications for life insurance (Milewa, Citation1999). And the Council of Europe Citation(1992) has advocated that insurers ‘should not have the right to require genetic testing or to enquire about results of previously performed tests, as a pre-condition for the conclusion or modification of an insurance contract’ and that employments ‘should not be made dependent on the undergoing of genetic testing’.
Respondents to our survey, in overwhelming numbers, perceive disclosures of genetic information to two critical institutions—health insurers and employers—as ‘threats to privacy rights’: 92% of US and 90% of European scientists, regarding the former; and 92% and 89%, respectively, regarding the latter. More generally, they are concerned with possible threats to privacy in regard to collecting, storing, and accessing sensitive data, specifically inadequate consent procedures (69% and 76%, respectively), access to computerised data (77% and 71%), and the existence of DNA databanks (44% and 42%). Additionally, they overwhelmingly advocate passing laws to prevent genetic discrimination. Over 87% of US and 90% of European respondents support legislation ‘prohibiting employers from collecting genetic information on prospective and/or present employees’, and 86% of both groups favour ‘prohibiting health insurers from requiring genetic information on applicants’.
Besides possible emotional disadvantages, then, such fear of misuse of tests by certain institutional third parties may apparently have an effect on the utilisation of testing. While in utero testing for inherited genetic disorders, to ensure a healthy baby, is in great demand in the US, genetic tests that have become available for adult-onset afflictions (e.g., breast or colon cancer) have been met with the ‘surprising dearth of interest’ referred to above (Kolata, Citation1997). And in one breast cancer study, one-third of women contacted for possible inclusion refused to participate specifically because of fear of losing jobs or insurance if a genetic defect was discovered (Martindale, Citation2001). The possible ‘exploitation of genetic information’ is found to raise anxieties across all European Union countries as well, particularly in the North (Eurobarometer, Citation2002).
While the potential for genetic discrimination on the part of institutional third parties has created a good deal of public debate and has led to demands for legal protections, a more private and complex aspect of confidentiality of genetic information has been less visible but equally contentious. It involves a balance between: (1) the strict confidentiality that has traditionally guided the patient-physician relationship and the protection of personal medical data (restated to apply to genetic information, by the US Committee on Assessing Genetic Risk and the Council of Europe); and (2) the competing health needs of other family members. These individuals include blood relatives, as well as spouses, partners or fiancé(e)s, and their prospective children—all third parties who could be at risk and, therefore, subject to harm.
When faced with a decision as to which is a doctor's foremost duty—‘to safeguard a patient's confidentiality’, when he or she is diagnosed with a serious disease, or to protect family members who could benefit from knowing such test results—patient confidentiality takes precedence among our respondents: 71% of US and 65% of Europeans chose the former, while only 13% and 16% respectively chose ‘a relative's right to know’.
However, European and US advisory panels have advised, as a matter of principle, that patients ‘should disclose to relatives general information relevant to ensuring the health of these relations’ (Andrews et al., Citation1994):
… in the case of a severe genetic risk for other family members, consideration should be given [by patients], in accordance with national legislation and professional rules of conduct, to informing family members about matters relevant to their health or that of their future children. (Council of Europe, Citation1992)
In the US, two state court decisions (Florida and New Jersey) have moved toward expanding the legal duty of physicians to warn third parties about genetically inherited diseases (McAbee, Sherman & Davidoff-Feldman, Citation1998). The two cases sent conflicting signals, however, as to whether doctors can be held liable for not warning a patient's relatives. And since physicians generally have little information on or access to relatives, in the end, it still appears to remain up to the patient.
In sum, the rights of related and at-risk third parties have begun to erode the long-standing strong support for patient confidentiality. One US researcher wrote: ‘Patient confidentiality has been written in stone as most important’; but then vacillated, saying, ‘at the risk of others suffering because of that, I don't know’; and concluded that the focus should be on ‘counseling to try to get the patient to see how it affects others’. In any case, this contentious area needs to be monitored in coming years.
An interesting finding, then, in the context of this principle of patient confidentiality and respondents' attitudes against revealing sensitive genetic information occurs in matters of adoption, where the reverse appears to be the case, most markedly for US scientists. Some 84% believe ‘diagnosis of a disease or high risk of a disease’ should be provided to prospective adoptive parents, compared to a much smaller majority (65%) of European scientists. A majority (58%) of US respondents endorse disclosing information on ‘carrier status as well’, compared with far fewer (30%) Europeans. On the whole, only 27% of European respondents and an even lower 8% of Americans say that genetic information should not be provided to prospective adoptive parents—that ‘disclosing such information would discriminate against a child being considered for adoption’.
Explanations for this finding may include the fact that patient autonomy is not an absolute right and that compelling reasons may ‘trump that right’ (Tancredi, Citation1994); that the rights of the adopting parents—who are considered the ‘clients’ in this situation—have to be protected (as is typically the case in decisions concerning terminating pregnancies, where ‘right to life’ is not attributed to fetuses (Deutsche Welle-World, Citation2004)); and finally, the viewpoint that in comparable situations in which couples use sperm or egg donation or surrogate motherhood, prospective parents expect to screen donors or surrogates for potentially deleterious genetic traits (Holtzman, Citation1989).
Results: ethics and gene therapy
Health system watchers allude to our being on the brink of a biomedical ‘revolution’ in which gene therapy would be the outcome: ‘All diseases … have a genetic component, and the best possible treatment in many cases would be to repair the genetic defect that permits the disease’ (Wade, Citation1999). This section will deal with respondents' reactions to ethical questions raised by the increasing potential for successful forms of gene therapy: the purposes to which future somatic cell gene therapy would be put, the likelihood and advisability of being able to modify the genetic makeup of children, and the acceptability of germ-line (or inheritable genetic modifications) therapy.
Pragmatic issues: ‘high promise, low fulfillment’
Controversies surround the field regarding accountability and responsibility in gene therapy research and expectations for the fruits of such work, about which we sought survey respondents' reactions. Distinct majorities (65% of US and 72% of European) agree that there exist ‘unrealistic expectations of questions that can be answered from genetic data’, what critics inside the field have even referred to as a ‘continuous positive “spin” that is unusual for most medical research’ (Rosenberg & Schechter, Citation2000).
Many respondents, however, maintain that it is not human genetics professionals who have created such exaggerated expectations for gene therapy; rather that it is less knowledgeable outsiders: e.g., ‘The media and other non-genetic individuals are responsible for…unrealistic expectations, by writing stories, books, films and unrealistic usage of the technology in such a way the public believes it is real’ (US researcher). Still others believe that geneticists ‘are not always quick to restrain public and media over-interpretation of genetic research’ (European researcher). The biotechnology industry, which has much invested in gene therapy, has also been accused of contributing to the hype.
But why would research into gene therapy evoke unrealistic expectations more than other areas of medical research? The reason is likely because of the far-reaching possibilities of relief (if the theory works) both for those affected by pervasive, presently untreatable diseases and for those who might be affected in the future, among a public desperate for hope. The language of human genetics, while helping to make the science more comprehensible for the lay public, may also heighten expectations—references to ‘code of life’, ‘book of man’, ‘blueprint for a human being’. Furthermore, some writers believe that the ‘exuberant optimism’ discussed above has ‘only increased the frustration and disappointment, as one major human gene-therapy trial after another failed to demonstrate any clinical efficacy’ (Leiden, Citation1999).
In its April 2000 statement on gene therapy, the American Society of Human Genetics' Board of Directors sought to re-focus:
The considerable promise of gene therapy must not be lost in the wake of premature claims and tragic consequences in some clinical trials. The appropriate course is to proceed with a greater commitment to rigorous critical evaluation and a heightened sense of responsibility to the patients who entrust their life and health to us. (ASHG, Citation2000)
Somatic cell gene therapy
Although somatic cell gene therapy is increasingly coming to be viewed as a natural and logical extension of current techniques for treating disease, the future of gene therapy is still speculative. While more than a third of respondents did not venture predictions, the rest are fairly optimistic. Half of all US and 44% of all European researchers believe in the likelihood that gene therapy will one day become ‘as routine and pervasive as immunisations or antibiotics—the day when healing genes are able to enter the bloodstream and go directly to the cell that needs help’. Just 14% of each group think it will be ‘untenable’. This overall, long-range confidence in the ultimate capabilities of human genetic engineering is further demonstrated by the finding that 71% of US and 54% of European scientists agree with the suggestion that gene therapy treatment should start ‘earlier in the disease process’ (instead of being regarded as a last-chance therapy when alternatives have not been helpful), so that it might have a ‘better chance of preventing deterioration of the patient's condition’.
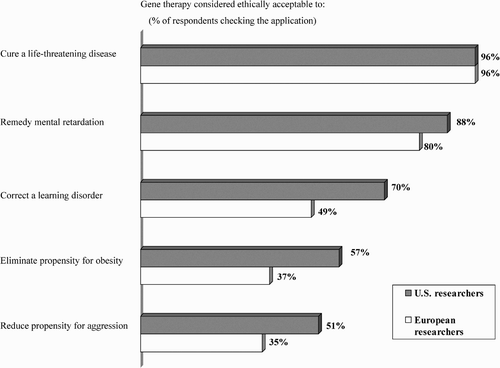
As for the ethics of future human gene therapy, support is almost unanimous: 96% of both groups would support such intervention to cure a life-threatening disease, ‘if it were to become safe, effective and cost-efficient’. Findings are somewhat lower, but still high, regarding applying gene therapy to the unborn child in utero. Some 85% of US and 81% of European respondents perceive this objective as ethically acceptable ‘if only serious disease is targeted and the risk-benefit ratios for mother and fetus are sufficient’ (only 5% and 13% respectively say unacceptable).
However, these responses are in relation to an ideal potential for gene therapy. Therefore, in order to gauge viewpoints for the time being, we asked respondents to weigh two options for preventing genetic disease or disorder in prospective children: current practices of prenatal diagnosis followed by abortion as well as in vitro pre-implantation testing followed by selection/discard (less risky and less expensive, but allowing only disease-free embryos or fetuses to continue development); as opposed to gene therapy to treat such diseases or disorders, which though presently limited, more risky and more expensive, involves treatment of affected embryos or fetuses rather than termination. Under present conditions, results demonstrate a lowered emphasis on gene therapy. The larger proportion (46% and 42%, respectively), agrees more with ‘continuing with present methods, which are less risky and less costly’. Nevertheless, nearly 30% of each group of respondents agrees more with ‘moving toward gene therapy in order to move away from termination’. (Some 7% and 17% respectively are not comfortable with either; 18% and 11% answered ‘not sure’.)
Support for gene therapy also rapidly drops off if it is to be used for other than cures for life-threatening diseases or conditions of mental retardation. Respondents were asked to check the admissibility of different somatic cell gene therapies (if and when the technique was to become a dependable reality):
As shows, among US respondents, there is reduced support for correcting learning disorders, eliminating the propensity for obesity, and reducing tendencies toward aggression, but all of these still gain majority support, whereas there is a precipitous drop to 22% and below for what appear to be seen as enhancements rather than cures for ailments: intelligence, baldness, and sleep dependence. Among European respondents, the distinction begins with utilising gene therapy to correct learning disorders (just less than a majority acceptability). In fact, European researchers demonstrate attitudes regarding acceptability of utilising gene therapy for learning disorders, eliminating propensity for obesity, and reducing tendencies toward aggression that are approximately 20 percentage points below those of their US counterparts. For the three least acceptable applications—to improve intelligence, eliminate baldness, and decrease sleep dependence—the differences are around 10%.
Cure versus enhancement
In addition to inferring where respondents make ethical distinctions between using genetic testing and gene therapy to cure disease on the one hand and affect enhancements on the other, we asked outright: ‘How morally acceptable is the use of genetic techniques to improve human capabilities in prospective children rather than solely to cure disease’? Large majorities (67% of US and 74% of European researchers) say enhancement is unacceptable. Among US scientists, 35% say ‘not generally acceptable’ and 32% ‘not at all acceptable’, but in Europe, greater numbers (46%) say ‘not at all acceptable’ (28% ‘not generally acceptable’).
The study next sought to probe the finer distinctions in the debate over ‘perfecting’ prospective children—which particular qualities might be deemed legitimate candidates for genetic modification and which should not. Respondents were asked which traits they perceived as ethically acceptable among the possible characteristics parents might one day be in a position to ‘choose’ for a baby.
The only quality that gained more than minimal support is one that involves an effect on others. Reducing a ‘negative’ personality trait, such as violence, was chosen by 40% of US respondents; however, while highest among European scientists as well, only 25% believe it would be appropriate to do so. Improved intelligence gains some acceptance: 25% and 17% respectively. Other items garner little or no acceptance: increasing a ‘positive’ personality trait, such as caring (19%, 9%); influencing weight (16%, 4%), musical ability or artistic talent (14%, 6%), height (12%, 3%), athleticism (11%, 3%), strength (10%, 1%), gender (9%, 2%), skin color (6%, 1%), hair or eye color (6%, 1%).
One European respondent summarised attitudes: ‘We have no right to be so deterministic with our descendants’. Nevertheless, a pattern of greater openness to such treatments by US compared with European researchers is repeated here again. Such debate may be considered premature, but public discussion about ‘designer’ babies, or what biotech critic Jeremy Rifkin has called the ‘ultimate shopping experience’ (Lemonick, Citation1999), has already begun.
Germ-line gene therapy
In contrast to somatic cell gene therapy, which is aimed at individuals or prospective children, inheritable genetic modifications (IGM), traditionally called germ-line treatment, would entail correcting genetic flaws in the reproductive DNA of human embryos, thereby eliminating the defect not only from the individual-to-be but also from succeeding generations.
Compared with the 96% of all respondents who support somatic cell gene therapy to cure life-threatening diseases, fewer view germ-line intervention to prevent them as ethically acceptable, ‘if and when gene repair/replacement were to become a safe and validated technique’. While there is still a clear majority (64%) of US scientists who support possible future germ-line gene therapy (18% do not), a far smaller proportion of European researchers (39%) take this perspective (43% do not).
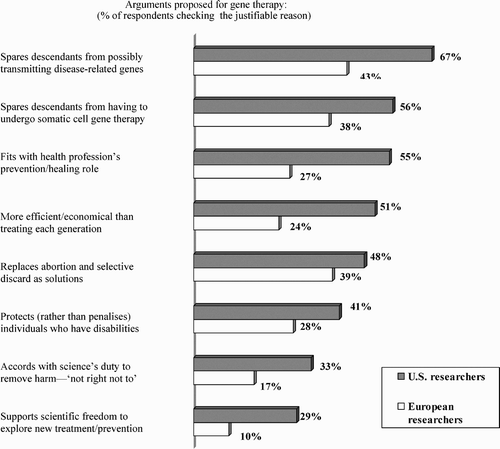
The survey next measured the extent to which different reasons provide justification for interceding in this manner, and here again European respondents exhibit a much more moderate approach:
In view of the rationale perceived to be the most relevant, it appears that acceptability rests largely on what has been described as the ‘duty of the present generation to protect … the genetic quality of the next’ (Pollack, Citation1998), by selectively reducing the genetic load passed on to future generations. In this respect, germ-line genetic engineering could be seen as advancing this biomedical goal even more than somatic cell therapy would. Then why this decreased support for a therapy that might ultimately be more efficient, especially in the context of a question that contains the qualifier, ‘if it were safe and validated’?
Clues to researchers' attitudes may be contained in their responses to reasons presented that might warrant prohibiting germ-line gene therapy. Highest is the prospect of unforeseen negative effects (64% and 68%, respectively). Other sources of concern involve respondents' rejection of enhancement (54%, 52%) and questionable, ‘malevolent’ uses to which the technology might be put (42%, 48%). Still other reasons include: excessive cost (31%, 28%), reduction in diversity of the human genome (25%, 27%), the belief that germ-line intervention is an unnecessary method when alternatives are available (22%, 34%), interfering with evolution/natural selection (17%, 23%), a child's right to have a genome that has not been tampered with (15%, 21%), and that it goes beyond limits humans ought not exceed or is contrary to religious beliefs (10%, 18%). On the whole, European respondents are more likely to agree with the array of ethical arguments against germ-line gene therapy than are US scientists.
In September 2000, the American Association for the Advancement of Science (AAAS) called for a moratorium on inheritable genetics modifications technology and urged scientists to focus on changing somatic cells only—alterations which will not be passed to the next generation. In order to plan for future directives for IGM approaches, the AAAS also strongly recommended that it be used only for therapeutic, rather than enhancement, objectives.
Variation in ethical viewpoints
Are there any factors other than the main variable that might be producing the differences discussed here? The backgrounds of the two groups—US and European researchers—differ in one main way—there are 12% more male and fewer female respondents among the European cohort. However, when we control for gender, there are no meaningful differences between responses of male and female respondents either in the US or in Europe.
Another concern is with the effects of German respondents' attitudes on the European results overall, since we have found that German researchers are generally more guarded than their European counterparts in regard to ethical issues in human genetics (Rabino, Citation2005). However, when we extract the responses from German scientists from the totals, again we continue to find similar types and still considerable degrees of differences between the two groups.
Discussion and conclusions
European human genetics researchers appear, in general, to be more reserved than US scientists in their support for genetic testing and for potential actions that might follow test results. A slimmer majority of Europeans compared with Americans support testing adults for genes indicative of serious disease, whether a cure exists or not (58% vs. 72% and 54% vs. 66%). In addition, fewer European researchers would support preventive surgery where there are genetic indicators but no actual symptoms (35% vs. 54%).
Where the two groups of respondents are more similar regarding genetic testing is in their shared strong opposition to using pre-natal test results to control for non-disease consequences, such as sex selection—though even here the number of European scientists who oppose such measures consistently exceeds the number of Americans.
On the subject of gene therapy, European researchers join the Americans in strong support for its potential use to cure life-threatening genetic diseases or to remedy mental retardation. But, as with genetic testing, as soon as it is proposed that such therapies be used for less severe conditions, ethical concerns arise and support drops—but much more precipitously among Europeans than among Americans, e.g., from 96% to cure a life-threatening disease to 49% to correct a learning disorder, compared to a drop among US respondents from 96% to 70%. European respondents are also consistently much less in favor of germ-line gene therapy and less supportive of all the reasons posed to justify it.
These differences, both large and small, are seen in contrast to issues for which US and European scientists are in almost complete agreement. One is in the area of therapeutic abortions. The two groups demonstrate comparable high degrees of acceptance of termination for genetically identified diseases and lower levels of acceptability when asked about termination for less serious or non-disease issues, such as mild retardation, depression, homosexuality, and sex selection (though support does drop more among Europeans).
Areas of total consensus include: opposition to mandating premarital testing and opposition to institutional access to genetic information—by employers and health insurers. Where respondents do diverge, regarding patient confidentiality, is when it runs counter to a need to know on the part of other family members or non-related, at-risk third parties. A slightly larger majority of US scientists tend to opt for protecting personal genetic data in the face of health concerns of other family members. But when it comes to adoption, that caution reverses, and a much larger percentage of American respondents are willing to give prospective adoptive parents access to such data.
In sum, European researchers are more cautious than are their US colleagues concerning most of the proposed uses of genetic testing and gene therapy, although both groups strongly support adult and prenatal testing for the benefit of discovering serious diseases as well as subsequent abortions in cases of severely negative in utero tests; and both groups strongly support gene therapy as a potential treatment for serious diseases or disorders.
In a paper reporting human genetics researchers' attitudes toward stem cell and therapeutic cloning controversies, we found similar tendencies of US researchers to be more accepting of certain technologies as well as more prone to action, as compared with their European counterparts (Rabino, Citation2004). We are not in a position to offer cultural or other reasons for these differences, except that they appear to confirm stereotypes about American society being more aggressive in general. It may also be that US scientists are more concerned about their competitive position, as we found in an earlier study (Rabino, Citation1993). We leave it, then, to those more familiar with wider, comparative societal conditions to suggest other knowledgeable explanations of these similarities and differences.
Finally, we should note that both European and US scientists used the open-ended comments to state the necessity for the general public to have greater knowledge about genetics and genetic technology, the scarcity of which has been revealed in studies of the general public in the US and Europe (Singer et al., Citation2005; Eurobarometer, Citation2002). Respondent comments echo the recommendation of the Council of Europe Citation(1992) that the subject be included in ‘curricula for general and further education, both at school and at university level, and in professional training’.
Acknowledgments
The author wishes to thank the respondents of the European Molecular Biology Organisation (EMBO) and the American Society of Human Genetics (ASHG) for their participation. I wish to thank as well Dr. Frederick Seitz, President Emeritus, the Rockefeller University—consultant to the study; and Dr. June Riess Goldner for her expertise in the construction of the survey and its analysis. Without the generous backing of the Richard Lounsbery Foundation, this study would not have been possible.
Notes
1. For a recent article on return rates for mailed surveys under varying conditions, see Trussell and Lavrakas Citation(2004).
2. See Wertz & Fletcher Citation(Unpublished observation) for an earlier international study involving clinicians' attitudes toward genetic testing, 1995.
3. For purposes of clarity of presentation of all remaining findings, where figures do not add up to 100%, the missing values are ‘not sure’ responses.
References
- American Society of Human Genetics (ASHG), 2000. Statement on gene therapy, American Journal of Human Genetics 67 (2000), pp. 272–3.
- Andrews, L., et al., 1994. Assessing Genetic Risks: Implications for Health and Social Policy. Washington, DC: National Academy Press; 1994.
- Beauchamp, T., and Childress, J., 2001. Principles of Biomedical Ethics. Oxford: Oxford University Press; 2001.
- Council of Europe, 1992. Recommendation on genetic testing and screening for health care Purposes, International Digest of Health Legislation 43 (1992), p. 284.
- Eurobarometer, 1990–2002. "Social values, science & technology". European Commission; 1990–2002.
- Deutsche Welle-World, 2004. European court rules against fetus rights, (2004), available at website <www.dw-world.de>.
- Frankel, M., and Chapman, A., 2000. Human Inheritable Genetic Modifications: Assessing Scientific, Ethical, Religious, and Policy Issues. Washington, DC: American Association for the Advancement of Science (A. A. A. S.)); 2000.
- Hercz, R., 1998. The gene machine, Canadian Business (1998), pp. 60–4.
- Holtzman, N., 1989. Proceed with Caution: Predicting Genetic Risks in the Recombinant DNA Era. Baltimore, MD: Johns Hopkins University Press; 1989.
- Kolata, G., 1997. Advent of testing for breast cancer leads to fears of disclosure and discrimination, The New York Times (1997), pp. C1–3.
- Kolata, G., 1998. Genetic testing falls short of public embrace, The New York Times (1998), p. A16.
- Leiden, J. M., 1999. Gene therapy enters adolescence, Science 285 (1999), pp. 1215–16.
- Lemke, T., 2004. Disposition and determinism—genetic diagnostics in a risk society, The Sociological Review 52 (2004), pp. 550–66.
- Lemonick, M., 1999. Designer babies, Time (1999), pp. 64–6.
- Lewin, T., 2000. Boom in gene testing raises questions of sharing results, The New York Times (2000), p. A1.
- Martindale, D., 2001. Pink slip in your genes, Scientific American (2001), pp. 19–20.
- McAbee, G., Sherman, J., and Davidoff-Feldman, B., 1998. Physician's duty to warn third parties about the risk of genetic diseases, Pediatrics (1998), pp. 140–2.
- Milewa, T., 1999. Public opinion and regulation of the new human genetics: a critical review, (1999), available on Public Health Genetics Unit website <www.phgu.org>.
- National Center for Genome Resources (NCGR), 1998. National survey of public and stakeholders attitudes and awareness of genetic issues, Genetics & Public Issues Program report. Santa Fe, New Mexico. 1998.
- National Human Genome Research Institute (NHGRI), 1997. "Ethical, legal, and social implications of the Human Genome Project, Office of Communications, NHGRI". Bethesda, Maryland. 1997.
- Nussbaum, M., 2004. Genética e justiça: tratando a doença, Impulso: Revista a Ciéncias Sociais a Humanas 15 (2004), pp. 25–34.
- Pollack, A., 1998. Gene therapy's focus shifts from rare illnesses, The New York Times (1998), p. F1.
- Rabino, I., 1993. How European and US genetic engineering scientists view the impact of public attention on their field: a comparison, Science, Technology, and Human Values 19 (1993), pp. 23–46.
- Rabino, I., 2004. Survey report: stem cell and cloning controversies, Genetic Engineering News 24 (2004), pp. 4–6.
- Rabino, I., 2005. Survey of European scientists on ethics of scientific advancements: Germans more guarded vis-à-vis human genetics, Genetic Engineering News (2005), pp. 6–12.
- Rosenberg, L. E., and Schechter, A., 2000. Gene therapist, heal thyself, Science 287 (2000), p. 1751.
- Singer, E., Corning, A., and Lamias, M., 1998. Trends: genetic testing, engineering, and therapy: awareness and attitudes, Public Opinion Quarterly (1998), pp. 633–64, winter.
- Singer, E., Van Hoewyk, J., and Antonucci, T., 2005. US attitudes toward genetic testing, 1990–2000, International Journal of Public Opinion Research (2005), pp. 111–25, spring.
- Tancredi, L., 1994. "Collection and disclosure of genetic information". In: Frankel, M., and Teich, A., eds. The Genetic Frontier: Ethics, Law, and Policy. Washington, DC: AAAS; 1994. pp. 47–51.
- Trussell, N., and Lavrakas, P., 2004. The influence of incremental increases in token cash incentives on mail survey response, Public Opinion Quarterly (2004), pp. 349–67, fall.
- Wade, N., 1999. Gene therapy passes important test, in monkeys, The New York Times (1999), p. F1.
- Wertz, D., and Fletcher, J., Unpublished observation. Ethics and genetics: an international survey, (Unpublished observation), 1995.