Abstract
Focusing on the case of the European Union's Sixth Framework Programme and drawing on a range of documentary material from the European Commission, Council of Ministers, Parliament and numerous committees, this paper analyses the cultural politics surrounding the debate about the funding of human embryonic stem cell science. First, it places HESC science within the context of the global struggle over the moral status of the human embryo and a conceptual understanding of the cultural politics produced by that struggle. Secondly, it outlines the primary components of cultural trading in HESC science, constructs a framework for the analysis of the EU's moral economy in this policy field and, using the same components, outlines a policy profile of the regulatory positions of the then 15 Member States as a context for the FP6 debates. Thirdly, it applies this framework to the cultural conflict surrounding FP6 and HESC science, the institutional manoeuvring that accompanied and to an extent channelled that conflict, and the contribution of bioethics to the operation of the EU's growing, if inefficient, moral economy in this field.
Introduction
On 10 June 2004 the European Commission issued the third call within the ‘Life Sciences, Genomics and Biotechnology for Health’ Priority of its Sixth Framework Programme (FP6: 2002–06). The designated work programme topics included the functional genomics of human embryonic stem cell differentiation, human stem cells for spinal cord injury, a European human embryonic stem cell registry, and a conference on the state of the art of stem cell research, focusing on the potential and limitations for curing severe diseases, aimed at patients (European Commission, Citation2004). The issuing of the call was a real and symbolic victory for the proponents of human embryonic stem cell (HESC) research, particularly the UK, following a long and often bitter political conflict spanning the European Commission, the Council of Ministers, the European Parliament, national governments and a host of associated agencies and policy networks. It is not, however, a final victory over the opponents of human embryo research nor a sure indication of the likely course of the hostilities that are continuing within the confines of the Seventh Framework Programme (FP7) which commenced in October 2006.
The struggle within the European Union (EU) over the future of HESC science forms part of a global contest for national advantage. Scientists engaged in the HESC field promise that their work will lead to therapies capable of dealing with one of the major challenges of modern medicine: irreversible organ and tissue failure. First created in 1998 by the research teams of James Thomson at the University of Wisconsin and John Gearhart at Johns Hopkins University, HESCs are unique in that they are ‘pluripotent’: that is they are undifferentiated cells that have the capacity to develop into almost all of the body's tissue types (Gearhart, Citation1998; Thomson et al., Citation1998). This plasticity sets them apart from the other types of stem cells derived from adult tissues, foetal tissues and umbilical cord blood and facilitates the claim that HESCs have the potential to generate an unlimited supply of transplantable tissues. Whole organ transplants, such as for heart or kidney disease, will no longer rely on a supply of donors, neurodegenerative disorders such as Parkinson's, Alzheimer's disease and multiple sclerosis will become treatable, and patients with chronic conditions such as diabetes will be given new tissues capable of replacing the function of pharmaceutical regimes. Tissue engineering and regenerative medicine, it is argued, will be revolutionized.
However, HESC science is not culturally neutral because the stem cell lines on which it is based are created through a process that requires the destruction of the human embryo. As a result, the scientific and economic progress of HESC science and its therapeutic applications is dependent on the nature of its engagement at national and international levels with key cultural values and beliefs concerning the moral status of the early human embryo. Depending on their social and religious contexts, these values may be more or less sympathetic to the new science and may or may not resonate positively with values supportive of the science such as those of ‘scientific progress’ and ‘population health gain’. Where the human embryo is accorded a high status, there may be cultural resistance to HESC science.
This paper analyses the progress of the cultural conflict regarding the funding of HESC science by FP6 as reflected in the documents and debates of the European Commission, the Council of Ministers, the European Parliament and numerous committees. It shows how bioethics has facilitated the emergence of what is termed a ‘moral economy’ where values may be traded and cultural disputes routinized, though not necessarily resolved. Its approach is as follows. First, it places HESC science within the context of the global struggle over the moral status of the human embryo and a conceptual understanding of the cultural politics produced by that struggle. Secondly, it outlines the primary components of cultural trading in HESC science, constructs a framework for the analysis of the EU's moral economy in this policy field and, using the same components, outlines a policy profile of the regulatory positions of the then 15 Member States as a context for the FP6 debates. Thirdly, it applies this framework to the cultural conflict surrounding FP6 and HESC science, the manoeuvring by the institutions of the EU that accompanied and to an extent channelled that conflict, and the contribution of bioethics to the operation of the EU's growing, if inefficient, moral economy in this field.
Cultural politics and bioethics: international context and analytical perspective
HESC science generates cultural conflict not because its subject is stem cells but because its subject is human embryonic stem cells. HESCs are derived from the inner cell mass of five to six day old pre-implantation embryos called blastocysts. In order to derive HESCs, the outer membrane of the blastocyst is punctured and the inner cell mass with its pluripotent stem cells transferred to a petri dish containing a culture medium. The blastocyst is thus destroyed and the HESCs are cultivated in vitro to produce a stem cell line. Stem cell lines can then be modified so that they differentiate into the cells of particular tissues depending on the therapeutic objective of the investigation.
In this context, the global political struggle over the moral status of the human embryo, and the role of religion within that struggle, is not, of course, a new phenomenon but a continuing feature of the experience of medical science in such arenas as assisted reproductive technologies (ART), pre-implantation genetic diagnosis (PGD) and, most notably, abortion. In the case of the latter, there is plentiful data illustrating the international variation in national values. For example, the United Nations' (UN) Abortion Policies: A Global Review shows how the different national policies weight the moral status of the embryo against other cultural values. Thus, abortion is permitted upon request (individual choice) in 65% of developed countries, but only 14% of developing countries, and is permitted for economic and social reasons in 75% of developed countries and 19% of developing countries. Least contentious is abortion to save the woman's life which is permitted in nearly all developed (99%) and developing (96%) countries (United Nations, Citation2002). At the regional level of the European Union (EU), the 2005 Eurobarometer survey shows a similarly large variation of values regarding the human embryo (European Commission, Citation2005, p. 71). Although 53% of EU citizens believe that ‘protecting the dignity of any human unborn life will be very important for our society in ten years time’, this figure disguises a wide variation between countries like Malta (74%), Greece (73%) and Ireland (73%) at one end of the continuum and those like Bulgaria, Hungary and Lithuania (all 37%) at the other. The policies on abortion of Malta (illegal under all circumstances) and Ireland (permitted to save the woman's life) are consistent with their citizens' values.
At the national level, cultural divisions over the status of the human embryo have produced acrimonious and longstanding conflicts on abortion policy to the extent that in the United States (US) they have assumed the mantle of ‘culture wars’ with religion a significant player (Hunter, Citation1994; McConkey, Citation2001; see also Mulkay, Citation1997 on the United Kingdom (UK) debates). On both sides of the cultural divide, religious groups have provided a continuing source of political pressure as medical advance has brought the human embryo on to the policy agenda in new forms (Hoffmann & Johnson, Citation2005). However, in Europe it is primarily Catholicism that has acted as the main religious influence on both national and transnational policy formation on the human embryo (Githens, Citation1996; Minkenberg, Citation2002). Focusing on several types of embryo research including PGD, embryo production for research purposes, ESC lines, and therapeutic cloning, Fink identified a clear relationship between the proportion of Catholics in a European country and restrictive embryo research policies (Fink, Citation2005).
Given this context, how can we best conceptualize the cultural politics generated by the demands of HESC science and the contribution of bioethics to the consequent competition for control of political meaning? Social anthropology's approach to the concept of ‘culture’ and ‘cultural politics’ has evolved in ways that render it a useful tool for the political scientist engaged in understanding how values interact with the operation of power. Originally, anthropology treated ‘culture’ as if it were a set of ideas or meanings shared by a whole population of homogeneous individuals (Asad, Citation1973, Citation1979). ‘Culture’ thus became a bounded entity with a fixed identity that sustained itself in a static equilibrium (Gough, Citation1968). However, more recent approaches have challenged this interpretation and emphasized instead the dynamic nature of culture ‘as an active process of meaning making and contestation over definition, including itself’ (Street, Citation1993, p. 2). Clearly this approach resonates much more readily with the concept of ‘politics’ where the struggle for power is a given that is rarely, if ever, static. It makes it clear that culture is a resource that can form part of the political action in terms of both context and content.
Once ‘culture’ is seen as a contested process of meaning making, other questions about key terms and concepts arise. In her discussion of the politicization of culture, Wright suggests we need to ask:
How are these concepts used and contested by differently positioned actors who draw on local, national and global links in unequal relations of power? How is the context framed by implicit practices and rules or do actors challenge, stretch or interpret them as part of the contest too? In a flow of events, who has the power to define? How do they prevent other ways of thinking about these concepts from being heard? How do they manage to make their meanings stick, and use institutions to make their meanings authoritative? With what material outcomes? (Wright, Citation1998, p. 9)
This politicized view of culture presents cultural values as part and parcel of the political process. Culture is no longer absolute, nor separate nor non-negotiable but part of the enduring competition for power by conflicting interests. Like all forms of politics, the struggle for control of the meaning to be attributed to significant cultural issues is susceptible to mechanisms that, through systems of rules productive of outcomes, enable the routinization of that struggle. Particularly where cultural values stand in the way of scientific and economic advance, a method that brings the cultural opposition from behind the barricades to the negotiating table is likely to be welcomed by governments desirous of solutions. Once at the table that opposition may find that its values have no special ontological status but are converted and defined merely as counters in what may be termed a moral currency that can be traded in the political marketplace. If the moral currency is dependable enough, if it has international credibility and if it is generally convertible then the exchange of values the currency facilitates can be described as a global moral economy. The currency must of course remain neutral and must be seen not as having its own interest but as constituting the impartial means for achieving fruitful moral trade. If a currency is successful in encouraging global moral trade then this is likely to be marked by an increase in its own political value.
As the emerging currency of the international moral economy, bioethics presents itself as a neutral technique that uses ‘tools for measurement that transcend culture’ and are able to produce ‘a single, correct solution for each ethical problem which is largely independent of person, place or time’ (Bosk, Citation1999, p. 63). Bioethics is thus an eminently transferable coinage since its value is derived from its apparent impartial functionality for the governance of science rather than from any localized source of historic or cultural authority such as religion. But the growing hegemony of bioethics over the governance of new health technologies in the global moral economy is due also to its adaptive qualities. Although the genesis of bioethics was associated with the institutions of medical education and medical ethics, bioethics rapidly developed alliances with other knowledge territories, thus broadening the sources of its epistemic power. In this context Lopez observes that ‘its legitimacy and authority are in part secured through the way it is embedded in an ecology of social sanctioned knowledges (e.g. law, medicine, economics, moral and political philosophy, and political liberalism), as well as practices of governance and self-government’ (Lopez, Citation2004, p. 888). Although the embedded nature of bioethics means that it is not a homogenous epistemic community, this quality enhances rather than undermines its power.
The political flexibility of bioethics is also demonstrated in its ability to evolve in order to maintain its governance lead as the arbiter of cultural tensions. Thus, for example, in 1994 Knoppers and Chadwick identified five basic principles underlying what they termed the ‘international consensus’ on research on the human genome: autonomy, privacy, justice, equity and quality out of respect for human dignity (Knoppers & Chadwick, Citation1994). Nine years later they concluded that, as a result of new genetic research, these existing principles had been superseded by new international trends in ethics that should be collated and codified as the principles of reciprocity, mutuality, solidarity, citizenry and universality (Knoppers & Chadwick, Citation2005). From the perspective of Knoppers and Chadwick as leading bioethicists, the ad hoc evolution of bioethics through the adaptation of its initial principles to local technological development at the national level was acceptable but needed order imposed upon it. To be globally viable, bioethics must transcend the national cultural context as medical science has done through the creation of an international identity based on common professional standards and conceptual frameworks, transnational disciplinary societies and networks, and the international mobility of its workforce. For without an international bioethics order, the means of value exchange and trading would be uncertain and the functionality of bioethics as the currency of the global moral economy undermined.
National pressures and ethical boundaries
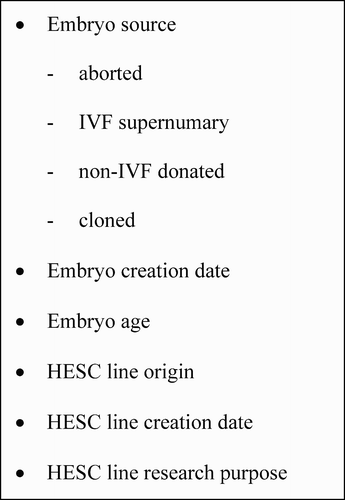
The moral economy of the EU in respect of HESC science can be analysed in terms of the components derived from the building blocks of HESC science. These are thus simultaneously scientific and ethical objects ().
Through the use of ethical arguments for or against the use of particular components, or combinations of components, hierarchies of meaning are created and choices implied about the preferred future path of HESC science. Given the scientific, economic and cultural pressures at work in the field, over time positions are rarely static and cultural trading occurs in the sense that a government or authority may, in response to these pressures, alter its support for particular components, or a set of components, in order to achieve political advantage or a workable political compromise. In addition, new scientific possibilities combine with the inventiveness of bioethicists to generate new cultural components in the discourse about the nature of desirable policies.
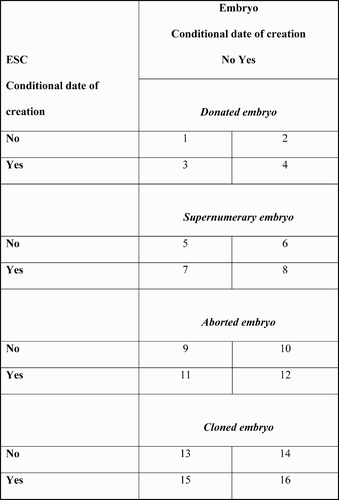
In 2003, a European Commission survey of the then 15 Member State policies at the time of the debate about HESCs and FP6 revealed five major regulatory policy positions when expressed in terms of the above components, positions from which we can assume their cultural bargaining would begin ( and ).
Table 1. Regulations in EU Member States regarding human embryonic stem cell research (March 2003)
In cultural terms, the policy positions are constructed so that as one moves from 1 to 5 the moral status (value) attached to the human embryo diminishes and the value attached to its scientific, commercial and social utility correspondingly increases. In regulatory policy terms, the same continuum can be described as extending from ‘restrictive’ (of scientific freedom through protection of the embryo) to ‘liberal’ (facilitative of science, industry and certain social interests)—though these terms of course have their own value connotations. In political terms, the continuum shows the increasing impact of scientific, industrial and pro-HESC social interests as one moves from policy 1 to policy 5.
At one end of the continuum of regulatory positions, the UK's Human Fertilisation and Embryology (Research Purposes) Regulations 2001 extends the Human Fertilisation and Embryology Act 1990 to permit the use of embryos, regardless of source, in research to increase knowledge about serious diseases and their treatment. At the other, the Irish constitution of 1937 as amended in 1983 provides that ‘the State acknowledges the right to life of the unborn and, with due regard to the equal right to life of the mother, guarantees in its laws to respect, and as far as practicable, by its laws to defend and vindicate that right’ (European Commission, Citation2003b, p. 42). As shows, in between there lies a variety of Member State positions and non-positions constructed by states seeking to reconcile conflicting cultural pressures from civil society, science and industry. For example, in an attempt to remove the human embryo from the political equation and thus distance themselves from the act of embryo destruction necessary for ESC creation, Germany, Austria and Denmark have allowed the importation of ESC lines while internally prohibiting their procurement from human embryos. In addition, Germany's Stem Cell Act 2002 requires that the ESCs were derived from supernumerary embryos before 1 January 2002 in the country of origin (European Commission, Citation2003b, p. 40).Footnote1 Commenting on the ethical contortions involved in the policy of ESC lines importation, the European Group on Ethics in Science and New Technologies (EGE) noted ‘a tendency to accept double morality where there is no coherence between different positions adopted by country’. It continued: ‘one could expect that to consider research on human embryos to derive stem cells as unethical, might imply the prohibition of the import for research of embryonic stem cells derived from human embryos as well as of the use of potential therapeutic applications resulting from such research, which is not always the case’ (EGE, Citation2002, para. 1.21). In the difficult world of human embryo politics, ‘double morality’ may well be the ethical price that has to be paid for a political compromise.
As the agenda setting debate for FP6 got under way, and as the move to establish genomics and biotechnology for health as its first priority became evident, so the cultural tensions inherent in this range of policy positions became manifest. The political atmosphere was already well heated, particularly in the European Parliament. As early as March 1989 a Parliamentary resolution on the ethical and legal problems of genetic engineering had called for legislation prohibiting any gene transfer to human germ line cells and defining the legal status of the human embryo in order to provide unequivocal protection of genetic identity (European Parliament, Citation1989). Other resolutions followed in 1993, 1997 and 1998 opposing cloning of the human embryo, supporting the Council of Europe's Convention on Human Rights and Biomedicine and calling on Member States to introduce a legally binding ban on the cloning of human beings (European Parliament, Citation1993, Citation1997, Citation1998).
In this context, the publication on 16 August 2000 of the UK Department of Health's Stem Cell Research: Medical Progress with Responsibility not only raised the political temperature dramatically, it also succeeded in focusing the European Parliament's attention by recommending that research on human embryos for therapeutic purposes including somatic cell nuclear replacement (SCNR), also known as ‘therapeutic cloning’, should be permitted: a very permissive approach when compared to other EU countries. National cultural differences now had a specific target issue and, with the FP6 awaiting approval and contingent upon parliamentary support, a powerful institutional vehicle for registering conflicting cultural values.Footnote2
The subsequent EU debate about HESC research and FP6 constituted a struggle for control of the policy discourse and thus of the policy agenda. Within the debate emerged a range of more detailed positions, again constructed from the units of cultural trading listed in and using combinations outlined in . Each ethical cell in the matrices can be regarded as potential agenda setting territory and thus as a political resource that through the operation of the moral economy may be exchanged for, or coupled with, other cells as trading takes place within the policy discourse.
Institutional struggle and policy discourse
FP6
Given the diversity of Member State policy positions on the moral status of the human embryo, it was to be expected that the UK's report advocating greater freedoms for ESC research would prove provocative and ethically challenging. Responding to that report, on 7 September 2000 the European Parliament passed a resolution opposed to both reproductive and therapeutic cloning. Therapeutic cloning (, cells 13–16) was seen as ‘irreversibly crossing a boundary in research norms’ and as contrary to public policy as adopted by the European Union (European Parliament, Citation2000). Much of the debate was couched in emotive and categorical terms with little suggestion from the opponents of ESC research that negotiation was either possible or proper. In its report Ethical Aspects of Human Stem Cell Research and Use published two months later, the EGE took a more sophisticated view and began the process of establishing an ethical continuum of types of human embryo and ESC research, using the kinds of criteria employed in , and suggesting that some criteria are more acceptable than others (EGE, Citation2000). While it regarded spare (supernumerary) embryos as an appropriate source for stem cell research, in an interesting conditional formulation it deemed ‘the creation of embryos with gametes donated for the purpose of stem cell procurement [as] ethically unacceptable, when spare embryos represent a ready alternative’ (EGE, Citation2000, para. 2.7; , cells 5–8 plus a conditional acceptance of cells 1–4). Meanwhile, ‘the creation of embryos by somatic cell nuclear transfer for research on stem cell therapy would be premature’ since there are alternative sources (EGE, Citation2000, para. 2.7, emphasis added; , cells 13–16). Embedded in this discourse are notions, first, of embryo status contingent upon source and, secondly, of ethics as a developmental process that moves from ‘premature’ to, presumably, mature.
The EGE report signalled a move by some actors involved in the construction of the policy discourse to attempt to change the debate from one characterized by static and opposing ethical positions to one where successive refinements of position were normal and negotiation possible: in other words, it sought to establish the basic requirements of a moral economy. As time drew nearer for Parliament to consider the Commission's Framework Programme Six proposal, and as the critics of human embryo research made it clear that they would use this as an opportunity for expressing their opposition, so the objective need for negotiating room increased. Although the subsequent parliamentary debate on the First Reading of the proposal in November 2001 suggests that little had changed, and that categorical statements of broad ethical positions were still the norm, the amendments incorporated into the proposal prove otherwise. The amendments meant that FP6 would not fund ‘research activity aiming at human cloning for reproductive purposes’ or ‘the creation of embryos for research purposes including somatic cell nuclear transfer’ (therapeutic cloning—, cells 13–16). However, it would fund, and here is the compromise, ‘research on “supernumerary” early-stage (i.e. up to 14 days) human embryos (embryos genuinely created for the treatment of infertility so as to increase the success rate of IVF but no longer needed for that purpose and when destined for destruction)’ (European Parliament, Citation2001a: Article 3; , cells 5–8).
The success of these amendments indicates that there is in the situation a sub-text of covert political negotiation and cultural trading around the ethical components of embryo source and embryo age (up to 14 days).Footnote3 Under pressure from the conflicting political constituencies of the Framework Six Programme, the policy discourse was beginning to evolve and to suggest that some types of embryos are ethically more important than others. In an attempt to facilitate this evolution and as part of the search for a way through the thickets of the ethical debate, in December 2001 the European Parliament set up the Temporary Committee on Human Genetics and Other New Technologies of Modern Medicine to report on the ethical, legal, economic and social implications of human genetics. In the event, its activities served to stimulate the involvement of new civil society policy networks in the discussion and legitimize the inclusion of fresh ethical dimensions. As the debate on its final report on 29 November 2001 demonstrates, ethical collisions in the parliamentary arena were at this stage more achievable than were compromise positions (European Parliament, Citation2001b).
In contrast, in the separate arena of the EGE a moral economy was clearly at work: the expert agenda of human embryo research was experiencing a further process of ethical refinement in response to scientific and industrial demands for greater regulatory protection of their HESC investments. By 2002, there had been 500 patent applications worldwide referring to embryonic stem cells—of which one-quarter had been granted—but the EU's position on whether patents on human embryonic stem cells should or could be granted under the conditions of its 1998 Patent Directive remained unresolved (EGE, Citation2002, para. 1.16). The Directive was clear that industrial and commercial exploitation of human embryos is excluded from patenting but unclear about the patentability of cells obtained from embryos, regardless of embryo source (EGE, Citation2002, para. 1.21). Reflecting on this issue, the Group stated its opinion that ‘patenting of inventions allowing the transformation of unmodified stem cells from human embryonic origin into genetically modified stem cell lines or specific differentiated stem cell lines for specific therapeutic or other uses, is ethically acceptable as long as the inventions fulfil the criteria of patentability’ (EGE, Citation2002, para. 2.5). However, this liberalization of the ethics of patenting was balanced by the EGE's view on therapeutic cloning where, drawing on its earlier Opinion Ethical Aspects of Human Stem Cell Research and Use, it called for ‘a cautious approach, excluding the patentability of the process of creation of a human embryo by cloning for stem cells’ (EGE, Citation2002, para. 2.5).
It is clear that at this stage the search for practical ethical solutions to cultural conflict around HESCs and thus the activation of the moral economy was progressing much more swiftly in the expert arena of the bioethicists than in the parliamentary and Council arenas of the politicians. Nonetheless, it was in the latter two arenas that a way forward had to be found if FP6 was to be funded. Institutional struggle was about to begin in earnest. In an interesting and, in the view of the opponents of human embryo research, challenging manoeuvre, at the Second Reading of the FP6 proposal in June 2002 Parliament voted through the overarching Framework Programme and transferred the issue of the criteria for embryo and human ESC research to the process for approval of the relevant Specific Research Programme (European Parliament and European Council, Citation2002). This meant that the Parliament was not directly involved in the decision making because under EU procedures the Specific Programme details are a ‘technical issue’ and can be decided upon by Council without the agreement of Parliament. However, the advantage gained by this institutional move appeared to have been short lived when in the September of the same year, under pressure from Austria, Italy, Germany and Ireland, the Council decided on a package of measures in response to the opposition concerns. This reiterated the ban on therapeutic cloning research and, furthermore, stipulated that there should be: a moratorium on the EU funding of human embryo and human ESC research until December 2003; a report on human embryonic stem cell research as the basis for an inter-institutional seminar on bioethics; and, taking into account the seminar's outcome, further guidelines on the principles that should guide Community funding of such research to be produced by December 2003 (European Council, Citation2002).Footnote4
The increasing salience of novel modes of organized ethical engagement (Temporary Committee on Human Genetics, EGE Opinions, inter-institutional seminar, development of ethical funding guidelines, ethical review of projects) are indicators of the intensifying search for practical means that would enable a moral economy to work and thus promote the inclusion of cultural factors in the EU's transnational governance of the life sciences. In its March 2003 progress report on Life Sciences and Biotechnology—a Strategy for Europe, the Commission observed that:
Public authorities at large have to take into consideration concerns about the conditions under which fundamental choices are made in this field [of life sciences]. For its part the Commission is committed to ensuring that the ethical, legal, social and wider cultural aspects, as well as the different underlying ways of thinking, are taken into account at the earliest possible stage in Community-funded research. In particular, the issues of human cloning and human embryonic stem cell research have provoked intense public and political debate. Ethical and social debate must continue to be a natural part of the research and development process involving society as much as possible. (European Commission, Citation2003a, p. 3, emphasis in original)
However, the widespread recognition that cultural values are a legitimate component of the transnational governance of the life sciences did not readily lead to a parallel acceptance of the new mechanisms for the resolution of cultural conflict. Following the Commission's exhaustive report on human embryonic stem cell research and the inter-institutional seminar drawing on its findings in April 2003 (European Commission, Citation2003b, Citation2003c), the terms and constituency of the debate were undoubtedly enhanced—but so also was the difficulty of finding a sustainable compromise position. Cultural trading was taking place but no agreement could yet be reached.
Driven to a search for the lowest common ethical denominator, in June 2003 the Commission proposed a set of ethical guidelines that included the selection of embryos for research on the basis of the criteria of embryo source and date of embryo creation. Community funding was to be restricted to the derivation of human embryonic stem cell lines ‘from human embryos created as a result of medically-assisted in vitro fertilization designed to induce pregnancy and were no longer to be used for that purpose’ (supernumary embryos) and created before 27 June 2002, the date of approval of the overarching Framework Programme 6 (, cells 6 and 8) (European Commission, Citation2003b, pp. 4–5). Unsurprisingly, international scientists objected strongly to the date of embryo creation criterion being applied because of what they saw as its impact on the freedom and quality of their research (Research Europe, Citation2003). Under the terms of the consultation procedure, the European Parliament debated the Commission's proposal in November 2003 and, agreeing with the scientific view, not only removed the 27 June 2002 restriction but also enlarged the embryo source criterion to include those produced by spontaneous or therapeutic abortion (, cells 9–12) as well as supernumerary embryos from IVF treatment (European Parliament, Citation2003a). This amendment in turn proved unacceptable to the Council with the result that on 31 December 2003 the moratorium on human ESC research expired with no agreement on the principles that should guide Community funding of that research. By default, therefore, the criteria contained in the European Parliament and Council Decision of 27 June 2002 and the Council Decision of 30 September 2002 in respect of the Specific Research Programme remained in place. Human embryonic stem cell research using therapeutic cloning (, cells 13–16) could not be funded, that based on supernumary embryos (, cells 5–8) could, and the position of research using donated and aborted embryos (, cells 1–4 and 9–12) as the source remained unresolved.
The human tissues directive
The volatility of the continuing cultural politics of human embryonic stem cell research and FP6 is manifest both in the constantly shifting mosaic of ethical components in the policy discourse and in the absence of any pattern in the institutional struggles between Commission, Council and Parliament. A stable moral economy had yet to emerge. As different configurations of 's ethical components came to the fore at different times, so the institutions would change their positions. A further destabilizing influence was the engagement between the political chemistry, networks and forces at work in the policy domain of FP6 and that of the neighbouring policy field of human tissues where a directive was being considered. The proposal for a directive setting quality and safety standards in relation to human tissues and cells began its progress through the EU's legislative machinery on 19 June 2002, eight days before the approval of the FP6. It immediately became the focus of a conflict not about ethics as such (though this formed part of the debate) but, more importantly, about what ethics could legitimately be included in the discussion and what excluded. In this respect it became a test case for determining what role ethics should have in this policy making domain and thus whether it was legitimate for a moral economy to exist at all in certain policy fields.
The opponents of human embryo research saw the directive as the means for implementing a pre-emptive strike against the pro-ESC lobby. If the ethical components of could be inserted into the directive as a block on human embryonic stem cell research, Member States would be obliged to implement it at the national level. The activities of their scientists would thus be curtailed regardless of the outcome of the conflict in the FP6 policy domain. However, the idea that difficult ethical issues should be incorporated into the directive did not resonate well with the culture of the sponsoring Commission Directorate Health and Consumer Protection which saw the business of setting standards for the donation, procurement, testing, processing, storage and distribution of human tissues and cells as a largely technical exercise with ethics making a facilitative rather than a challenging contribution to the implementation of an existing policy agenda. The European Group on Ethics had earlier in 1998 produced its report Ethical Aspects of Human Tissue Banking dealing with ethical issues such as the protection of health, the integrity of the human body, informed consent and the protection of identity and these were happily incorporated into the first draft of the proposal for a directive (EGE, 1998; European Commission, Citation2002). In an aside, the proposal noted that ‘germ cells, foetal cells/tissues and embryonic stem cells pose particular ethical problems’, that ‘there is no consensus among Member States upon which basic harmonized decisions at EU level can be taken with regard to their use or prohibition’, and that ‘the proposal does not interfere with decisions made by Member States concerning the use or non-use of any specific type of human cells, including germ cells and embryonic stem cells’ (European Commission, Citation2002, pp. 5–6).
This hands-off approach was abruptly challenged by the Committee on the Environment, Public Health and Consumer Policy in its report to the Parliament as background to the First Reading of the proposal.Footnote5 Here it was proposed that Member States should at least prohibit research on human cloning for reproductive purposes or to supply stem cells, including by means of the transfer of somatic cell nuclei; that no tissues or cells derived from human embryos should be used for transplantation; and that cloned human embryos and human/animal hybrid embryos produced by cloning should be excluded as sources of material for transplant (European Parliament, Citation2003b: amendments 14, 30 and 51). The subsequent acceptance of these amendments by Parliament shifted the focus of the ethical debate from utilitarian values concerned with the details of directive implementation to fundamental values that questioned parts of the science on which the directive was, or might be, based. To counter this, in revising the proposal, the Commission used the interesting tactic of defining some ethics as appropriate to the directive and others not. Thus while it was able to accept ethical provisions related to the anonymity of donors and non-profit procurement, the Commission argued that other provisions (notably those concerned with human embryos) fell ‘outside the scope of Article 152 of the Treaty, which provides for public health protection and not for the implementation of ethical objectives’ (European Parliament, Citation2003c, p. 7, emphasis added). In other words, it argued that only a very restricted kind of moral economy was appropriate to this issue.
In the Second Reading by Parliament of the directive this selective approach to the role of ethics was sustained and the opponents of human embryonic stem cell research were obliged to accept a compromise amendment that protected the rights of Member States to ban or restrict the use of ESCs and stipulated that, where used, they should be subject to the directive's provisions for the protection of public health (European Parliament and European Council, 2004). The human tissues Directive 2003/23/EC was agreed and the attempt to use it as a vehicle for blocking HESC research was foiled.
Conclusions
The therapeutic promise of human embryonic stem cell research has generated a global competition for the control of its social, scientific and industrial future that is increasing in intensity. Countries are investing in the basic research necessary to develop the field, re-examining their regulatory arrangements, and seeking to attract transnational life sciences companies. But they do not operate in a cultural vacuum. Elements in their civil societies may draw upon a variety of cultural values to support or oppose what is officially regarded as being in the national scientific or industrial interest. To the extent that these cultural pressures are problematic, a cultural politics is generated characterized by a moral economy where the trading of values facilitates negotiation and compromise.
In the case of the EU, these pressures are localized through the interaction of Member State positions in the context of the EU's institutions and procedures. Member State cultures as revealed in legislative form are not static but themselves responsive to the international context. Thus, for example, in July 2004 the French Parliament banned reproductive human cloning as a ‘crime against the human species’ but postponed its ban on the use of supernumerary embryos for embryo research thus allowing certain types of HESC research to continue (Channelnewsasia, Citation2004). With less equivocation, in September 2004 the new Spanish socialist government announced that it would permit human embryonic stem cell research and viewed therapeutic cloning as ‘an open matter’ (Yahoo!news, Citation2004; The Scientist, Citation2004). As Member States change their positions so the matrix of forces at work in the Commission, Council and Parliament also shifts to create a continuing volatility in the balance between the ethical components of the moral economy.
However, some parts of that economy are more volatile than others. Whereas the public debates of the European Parliament on human ESCs and FP6 were usually characterized by the stark presentation of conflicting cultural positions, in the expert arena of bioethics the search for compromise ethical equations has generated a quite different political style characterized by reason, flexibility and adaptation. While in the former, the cultural politics were raw and challenging, in the latter the explicit search for political utility has necessitated the development of the rules and procedures that can contribute to a practical outcome. Cultural politics in the EU is therefore operating at two levels in order to accommodate the otherwise incompatible requirements of (a) the unchanging legitimacy of particular value positions; and (b) the need for those positions to be negotiable. As the application of the ethical components of to the policy discourse of both levels has illustrated, there now exists a range of finely graded value positions on human ESC research that constitute the currency for biopolitical trading. Although at the public level this trade would be denied, the evidence of the policy discourse is that such trading indeed occurs, though as yet inefficiently.
For the future, and as the therapeutic applications of human ESC research become more evident, the prospect is one of a continuing engagement between the policy domains of HESC science and human tissues. This will be overlaid with a continuing cultural struggle for control of the EU's emergent new methods for the transnational governance of science. There will then be an objective need for the clarification of the role of ethics in the policy discourse and of what Gottweis terms ‘the ethics infrastructure’ (Gottweis, Citation2003). National and transnational cultural groupings are becoming increasingly sophisticated in the formation and presentation of ethical arguments in this field and will require a parallel improvement in the way in which ethics is used as a form of political currency and exchange in the moral economy. Attempts to exclude ethical issues from the EU's politics as occurred in the case of the human tissue directive are likely to prove counter-productive because they ignore the established and growing cultural pressures on the policy making apparatus of the EU.
This analysis suggests that institutionalized modes of ethics engagement will become a political technology that constitutes a permanent feature of the new cultural politics as mechanisms are sought that will enable the refining, manipulating, resolving and legitimating of cultural differences through the trading of values in an authoritative language and setting. Such modes are likely to continue to operate in parallel to the formal procedures of Commission, Council and Parliament in an attempt to offset the ponderous limits of these institutions to deal with cultural politics. This paper has noted the politically functional contribution of the European Group on Ethics not only to the lubrication of the ethical interaction through its elaboration of fresh ethical distinctions and perspectives but also to the facilitation of decision making through the judicious use of its claim to impartiality. Bioethicists are emerging as a new epistemic power group capable of brokering difficult cultural deals at both the national and international levels and their inclusion in the transnational governance of the EU is part of a global process (Salter & Jones, Citation2005). As the EU case has shown, in the human embryonic stem cell field they can enable the interrogation of ethical options, and thus the refinement of the political currency of the moral economy, through the investment of ethical significance in such characteristics as the source, date of creation, age and research purpose of the embryo or ESC. Over time, and if functionally successful, we may find that the command of ethical as opposed to scientific expertise elevates bioethicists to the status of what may be termed ‘the new technocrats’ of transnational scientific governance.
Acknowledgements
The research for this paper formed part of the ‘Global politics of human embryonic stem cell science project’ funded by the Stem Cell Programme of the Economic and Social Research Council. Project number RES-340-25-0001.
Notes
1. The date of ESC line creation is, of course, the same criterion as that used by President Bush when he announced his decision to allow Federal funds to be used for research on existing—i.e. pre-9 August 2001—human ESC lines: an example, perhaps, of transnational policy learning.
2. Decisions on Framework Programmes have to be made by co-decision between the Council and Parliament.
3. The latter is of course elsewhere described as the ‘pre-embryo’, an important political category in the long-running UK embryo debate (Mulkay, Citation1997, pp. 30–2; Spallone, Citation1999).
4. In an interesting concession to the UK's pro-ESC research stance, the moratorium explicitly did not include ‘banked or isolated human embryonic stem cells in culture’.
5. It is no coincidence that the rapporteur for the Committee was Peter Liese: a Catholic Christian Democrat MEP who was also active in the HESC and FP6 arena.
References
- Asad, T., 1973. Asad, T., ed. Anthropology and the Colonial Encounter. London: Ithaca Press; 1973.
- Asad, T., 1979. Anthropology and the analysis of ideology, Man 14 (1979), pp. 607–27.
- Bosk, C. L., 1999. Professional ethicist available: logical, secular, friendly, Daedelus 128 (4) (1999), pp. 47–68.
- Channelnewsasia, 2004. French parliament bans human cloning, (2004), available at www.channelnewsasiacom/stories/afp_world/view/94729/1/.html. (accessed 21 June 2006).
- EGE, 1998a. Ethical Aspects of Human Tissue Banking (1998a), Opinion no. 11 to the European Commission, available at http://europa.eu.int/comm/european_group_ethics/docs/avis11_en.pdf (accessed 22 June 2006).
- EGE, 2000. Ethical Aspects of Human Stem Cell Research and Use (2000), Opinion no. 15 to the European Commission.
- EGE, 2002. Ethical Aspects of Patenting Inventions Involving Human Stem Cells (2002), Opinion no. 16 to the European Commission.
- European Commission, 2002. Proposal for a directive of the European Parliament and of the Council on setting standards of quality and safety for the donation, (2002), procurement, testing, processing, storage and distribution of human tissues and cells, COM(2002)319 final, 19/6/02.
- European Commission, 2003a. Life Sciences and Biotechnology—a Strategy for Europe. Progress Report and Future Orientations (2003a), COM(2003)96 final.
- European Commission, 2003b. Report on Human Embryonic Stem Cell Research (2003b), SEC(2003)441, 3 April.
- European Commission, 2003c. EU Funding and the Bio-ethics of Stem-cell Research, (2003c), Inter-institutional seminar, news report 25 April, available at http//:www2.europarl.eu.int/omk/sipade2?PUBREF = //EP//TE…/EN&LEVEL = 2&NAV= (accessed 7 June 2006).
- European Commission, 2004. Work programme for the specific programme for research, technological development and demonstration: ‘Integrating and strengthening the European Research Area’, (2004), Priority 1: Life Sciences, Genomics and Biotechnology for Health, Commission Decision C(2004)2002, 10 June.
- European Commission, 2005. Social Values, Science and Technology (2005), Special Eurobarometer, available at http://europa.eu.int/comm/public_opinion/archives/ebs/ebs_225_report_en.pdf (accessed 23 May 2006).
- European Council, 2002. Council Decision 2002/834/EC adopting a specific programme for research, technological development and demonstration: ‘Integrating and strengthening the European Research Area’ (2002–06), Official Journal (2002), pp. 1–43, L294, 29/10/02.
- European Parliament, 1989. Resolution on the ethical and legal problems of genetic engineering, Official Journal (1989), p. 171, C 096, 17/4/1989.
- Europea Parliament, 1993. Resolution on the cloning of the human embryo, Official Journal (1993), p. 224, C 315, 22/11/1993.
- European Parliament, 1997. Resolution on the cloning of human beings, Official Journal (1997), p. 92, C 115, 14/4/1997.
- European Parliament, 1998. Resolution on the cloning of human beings, Official Journal (1998), p. 164, C 034, 2/2/1998.
- European Parliament, 2000. Resolution on human cloning, (2000), PE T5-0375/2000.
- European Parliament, 2001a. Proposal for a European Parliament and Council decision concerning the multiannual framework programme 2002–06 of the European Community for research, (2001a), technological development and demonstration activities aimed at contributing towards the creation of the European Research Area, COM(2001)94, 14 November.
- European Parliament, 2001b. "Debates of the European Parliament, sitting of 29th November 2001". Human genetics; 2001b.
- European Parliament, 2003a. European Parliament Resolution on the proposal for a Council Decision amending Decision 2002/834/EC on the specific programme for research, (2003a), technological development and demonstration: ‘Integrating and strengthening the European research area’ (2002–06), COM(2003)390, 19 November.
- European Parliament, 2003b. Report on the Proposal for a European Parliament and Council Directive on Setting Standards of Quality and Safety for the Donation, Procurement, Testing, Processing, Storage, and Distribution of Human Tissues and Cells (2003b), A5-0103/2003, Final.
- European Parliament, 2003c. Amended proposal for a directive of the European Parliament and of the council on setting standards of quality and safety for the donation, procurement, testing, processing, storage, and distribution of human tissues and cells (presented by the Commission pursuant to Article 250 (2) of the EC Treaty)/COM/2003/0340final-COD 2002/0128, (2003c), available at http://eur-lex.europa.eu/lexUriServ/LexUriSer.do?uri = CELEX:52003PC0340: EN:HTML (accessed 8 November 2007).
- European Parliament and the European Council, 2002. Decision 1513/2002/EC of the European Parliament and Council concerning the Sixth Framework Programme of the European Community for research, technological development and demonstration activities, contributing to the creation of the European Research Area and to innovation (2002–06), Official Journal (2002), L232, 29/8/02.
- European Parliament and the European Council, 2004. Directive 2003/23/EC of the European Parliament and of the Council on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells, Official Journal (2004), L102, 31/03/04.
- Fink, S., 2005. Live and let…? Measuring and explaining the strictness of embryo research laws in 21 countries. Presented at Paper presented at the Third European Consortium for Political Research Conference. Budapest, 8–10, September, Panel 3–7: The Regulation of the Life Sciences.
- Gearhart, J. D., 1998. New potential for human embryonic stem cells, Science 282 (1998), pp. 1061–2.
- Githens, M., 1996. "Reproductive rights and the struggle with change in Eastern Europe". In: Githens, M., and McBride, Stetson D., eds. Abortion Politics: Public Policy in Cross-Cultural Perspective. London: Routledge; 1996. pp. 55–68.
- Gottweis, H., 2003. Human embryonic stem cells, cloning, and the transformation of biopolitics, Biotechnology Between Commerce and Civil Society (2003), pp. 239–65, (Transaction Books).
- Gough, K., 1968. New proposals for anthropologists, Current Anthropology 9 (1968), pp. 403–7.
- Hoffman, J. P., and Johnson, S. M., 2005. Attitudes toward abortion among religious traditions in the United States: change or continuity?, Sociology of Religion (2005), available at www.findarticles.com/p/articles/mi_m0SOR/is_2_66/ai_n14817990 (accessed 28 May 2006).
- Hunter, J. D., 1994. Before the Shooting Begins: Searching for Democracy in America's Culture War. New York: The Free Press; 1994.
- Knoppers, B. M., and Chadwick, R., 1994. The Human Genome Project: under an international ethical microscope, Science 265 (1994), pp. 2035–6.
- Knoppers, B. M., and Chadwick, R., 2005. Human genetics research: emerging trends in ethics, Nature Reviews Genetics 6 (2005), pp. 75–9.
- Lopez, J., 2004. How sociology can save bioethics … maybe, Sociology of Health and Illness 25 (7) (2004), pp. 875–96.
- McConkey, D., 2001. Whither Hunter's culture war? Shifts in evangelical morality 1988–1998, Sociology of Religion 62 (2001), pp. 149–74.
- Minkenberg, M., 2002. Religion and public policy: institutional, cultural and political impact on the shaping of abortion policies in western democracies, Comparative Political Studies 35 (2) (2002), pp. 221–47.
- Mulkay, M., 1997. The Embryo Research Debate: Science and the Politics of Reproduction. Cambridge: Cambridge University Press; 1997.
- Research Europe, 2003. EU stem cell guidelines raise supply issues, Research Europe (2003), p. 4, 31 July.
- Salter, B., and Jones, M., 2005. Biobanks and bioethics: the politics of legitimation, Journal of European Public Policy 12 (4) (2005), pp. 710–32.
- Spallone, P., 1999. How the pre-embryo got its spots. Presented at Paper presented at Einstein Forum's International Conference on Genetics and Genealogy. Potsdam, 4–6, July, 1999.
- Street, B., 1993. "Culture is a verb: anthropological aspects of language and cultural process". In: Graddol, D., et al., eds. Language and Culture. Clevedon, Avon: British Association for Applied Linguistics in association with Multilingual Matters; 1993. pp. 23–45.
- The Scientist, 2004. Big changes afoot in Spain, The Scientist (2004), 29 March, available at www.biomedcentral.com/news/20040329/02/ (accessed, 23 May 2006).
- Thomson, J. A., et al., 1998. Embryonic stem cell lines derived from human blastocysts, Science 282 (1998), pp. 1145–47.
- United Nations, 2002. Abortion Policies: A Global Review (2002), available at www.un.org/esa/population/publications/abortion/ (accessed 8 September 2005).
- Wright, S., 1998. The politicisation of culture, Anthropology Today 14 (1) (1998), pp. 7–15.
- Yahoo!news, 2004. Spain to authorise stem cell research, (2004), available at http://story.news.yahoo.com/news?tmpl = story&cid = …/spain_health_biotech_04092718073 (accessed 7 June 2006).