Abstract
A survey of 38 internet platforms of companies offering genetic testing services direct to consumers was carried out in summer 2008. The internet survey supported the notion held by many experts and discussed by policy advisory bodies that (1) many direct-to-consumer genetic testing (DCGT) offers do not meet a minimum set of quality criteria that can be regarded to be necessary for ensuring adequate information and protection of customers against misleading interpretation of the need for as well as the possible consequences of genetic testing; (2) most DCGT offers fail to provide proper information on the scientific evidence behind genetic testing services offered to customers (clinical validity and utility); (3) many of the companies offering genetic testing services via the internet do not include genetic counseling at all in their services. Only a few urge customers to involve an expert before purchasing a gene test, and “counseling” in most cases only is provided as written information via mail or via web-log.
Introduction
When genetic testing first entered medical practice during the mid-1980s, it was restricted to a few inherited diseases, such as cystic fibrosis. Genetic testing and counseling were only offered by experts working at university hospitals and institutes and by a limited number of doctors who specialized in human genetics. The limited number of persons seeking genetic testing and counseling, the quite complex and expensive technical procedure of testing as well as the limited number of well-educated experts who can offer genetic testing and counseling are all factors that have contained the problematic potential of genetic testing. Many of the negative expectations connected with genetic testing were based on the assumption of an uncontrolled growth of genetic testing for a great number of common diseases, which might open the door for misuse and clinically non-indicated applications of testing (Borry et al. Citation2007). Apart from the limited number of tests available, the fact that a small group of medical practitioners and genetic counselors has controlled the practice of testing has been regarded as guaranteeing a knowledgeable, cautious and responsible application of genetic testing, which contrasted with the negative scenarios of its widespread and clinically doubtful use (HGC Citation2003). In recent years, however, some of the barriers to a growth of genetic testing beyond the “protected” realm of genetic counseling carried out in hospitals for a restricted number of persons who might be carriers of rare inherited genetic diseases have vanished or are losing strength. New technological options are available that make it both technically easier and cheaper for a genetic test to be carried out. Connected with the lowering of the technical barriers to genetic testing is a tendency for new (private) suppliers to enter the market. And last but not least, genetic testing is being offered not only for some rare Mendelian diseases but increasing for susceptibilities to common diseases such as cancer, diabetes or cardiovascular diseases, indicating an above-average risk of a person developing a disease. However, doubtful the clinical validity and usefulness of these tests may be, such use has the potential of making genetic testing a part of everyday health care, as for instance is shown by ongoing discussions about “public health genetics” (see e.g. the European Public Health Genomics Network, www.phgen.eu).
A related phenomenon has been the transition to a new “business model” or “practical setting” for genetic testing since the late 1990s, namely genetic testing and counseling services offered directly to consumers. Some regard this way of bypassing the medical or health care setting (with specialized doctors and their clients) that previously controlled access to these services as providing free access to genetic testing, letting consumers decide on their own whether to make use of these testing options. Others consider direct-to-consumer genetic testing (DCGT) to be a possibly dangerous marketing ploy that will lead to genetic testing that is uncontrolled, scientifically unjustified, qualitatively doubtful and often intentionally misleading (GAO Citation2006, HGC Citation2007, Janssens et al. Citation2008).
In contrast to the established practice, medical benefits and ethical and social problems of genetic testing, which have been the subject of many studies and numerous inquiries by ethical committees and other non-governmental and governmental advisory boards during the past 10 to 15 years, in most European countries the debate on DCGT has just begun. DCGT is a rather new phenomenon that is apparently driven by the use of the internet. Although it is a growing market, it is still a niche market; new companies offering genetic testing via the internet currently are showing up constantly. It is however too early to tell whether they will succeed in establishing themselves in the long or even medium term. This makes it difficult to assess the actual relevance of DCGT, which might well develop into a serious competitor to the established forms of genetic counseling and require political or statutory regulation in order to protect consumers' rights and health.
The present paperFootnote1 intends to provide insight in the current use of DCGT by outlining the main results of an assessment of 38 internet pages offering genetic testing direct to consumers. The assessment is the result of a systematic scan of offers that can be found on the internet, which was carried out during June and July 2008. The results of the survey and their possible implications for policy intervention in the field were discussed with a group of experts at a meeting hosted by the Flemish Institute for Science and Technology Assessment (IST) in Brussels on 22 September 2008.Footnote2 The internet scan was part of a project that provided an in-depth analysis of available literature and policy documents on DCGT as well as a reflection on possible options for political intervention and regulation of the market for DCGT on the European level carried out by the European Technology Assessment Group (ETAG, www.itas.fzk.de/etag) on behalf of the European Parliament.Footnote3
Direct-to-consumer genetic testing: definition and problems
From the first appearance of offers for genetic testing via the internet (in the US and UK) some seven or eight years ago, DCGT has become the subject of discussion (so far among expert communities and advisory bodies only) since it appears that with DCGT genetic testing as a health care service may get out of control. In the existing setting of university institutes, public insurance systems, specialized genetic counselors, etc., it appears to be feasible to restrict the application of testing to the “useful,” to sort out what is sufficiently clinically valid to be used in medical practice and to provide for a high standard of support and counseling for clients according to established guidelines for good practice (see above). This quality of genetic testing is thought to be endangered when the system is circumvented by DCGT. Concerns are expressed mainly by doctors and experts in human genetics as well as by professional medical bodies and health authorities. Klaus Bartram, Director of the Institute for Human Genetics at the University of Heidelberg and former president of the German Human Genetic Society, said with regard to a growing and uncontrolled market for genetic testing: “We have to prevent the formation of a market of thousands of tests that do not come along with proper interpretation” (Lab Times Citation2007, p. 16). Bartram says: “The market for useless tests is steadily growing and operates according to the mantra: send us some saliva but don't forget the cheque” (p. 15). Whereas criticism is made of misinformation of customers, and bad quality of testing, companies offering genetic testing directly to consumers claim to support the consumers' right of free access to new developments in health care as a means of deliberate and self-determined prevention of disease.
Independent of the question of clinical validity of tests offered directly to consumers, the quality of testing and information forwarded to consumers (also in case of “lifestyle” testing) is unanimously regarded to be highly relevant to avoid false-positive or false-negative results or any other misleading or meaningless information. In the US a quality check of four selected web pages offering diet-related genetic testing has been conducted by the Government Accountability Office (GAO). Cheek swabs for 14 fictitious clients were sent to four companies offering DCGT via the internet. The test results and health recommendations received by the GAO provided strong evidence that a lack of quality control by professional or governmental bodies led to serious cases of misleading information or false results being forwarded to consumers (GAO Citation2006). A similar approach to test the quality of DCGT services offered has recently been chosen by some journalists (Harmon Citation2007, Fleming Citation2008). The findings revealed significant cause for concern about consumers being misled.
The UK Human Genetics Commission defines DCGT as “… any test to detect differences in DNA, genes or a chromosome that is not provided as part of a medical consultation” (HGC Citation2003, p. 7). This includes any genetic test available to the public outside the usual medical control system. The Belgian Advisory Committee for Bio-Ethics (Belgish Raadgevend Comité voor Bio-ethiek Citation2004, p. 6) uses the term “home-sampling test.” A sample of the material to be tested is taken at home and sent to a laboratory for analysis. The results from the laboratory tests are communicated to the user by telephone, mail, email or secured internet access. The definition includes a broad spectrum of tests, from ancestry testing, paternity determination and prenatal sex determination to heritable breast cancer testing. A related problem is direct-to-consumer promotion of gene tests via advertising campaigns as has been reported for Myriad genetics (Williams-Jones Citation2006).
In the present report as well as in most of the documents dealing with DCGT, the term “direct-to-consumer genetic testing” is used for testing services offered for health-related genetic variants and polymorphisms. This includes offers for so-called lifestyle-related genetic testing that provides recommendations regarding diet or everyday life (sports etc.). Consumers are the target of a growing number of offers on the internet for paternity testing and for ancestry testing. Paternity testing is associated with serious problems for privacy and data protection. In most European countries, such tests are not legal without the explicit consent of the child and the mother concerned or the explicit request of a court. Paternal and ancestry testing do, however, not address health-related questions or involve problems of interpreting results and consulting (since the “genetic fingerprinting” process applied for paternity testing is based on non-coding traits of the genome, which do not – at least to our current knowledge – imply information about the health status of a person). Paternity testing thus has to be regarded as a special field of genetic testing and is usually not explicitly dealt with in debates about DCGT (e.g. HGC Citation2003, p. 51).
It is in fact the health-related purpose of the test and the fact that the test is supplied outside the established system of health services (without costs being covered by a public health service or by health insurance, the referral by a doctor, or the consultation of a medical genetics expert) that give reason to discuss DCGT in the context of the probable detrimental effects on consumers and of a possible need for new or additional regulatory arrangements.
Assessment of websites of companies offering DCGT
Methodology
A systematic scan of the internet was carried out in order to gain a deeper insight into the scope and quality of information provided by companies offering DCGT at their websites, the main medium through which they interface with customers. The focus was on companies offering DCGT for health purposes and for purposes linked to diet and lifestyle. Companies exclusively performing paternity and ancestry testing were not included, since the related issues and concerns are different.
Selection of websites
The scan started from available listings of DCGT web pages (Hogarth et al. Citation2007) which were used as reference to check comprehensiveness. A Google search was conducted using the key words “home test” + “genetic,” “nutrigenetics,” “genetic test” + “diet,” “personalized nutrition” + “genetic,” “genetic test” + “cancer.” An initial list of 49 firms resulted, which was reduced to 38 () according to our criteria for exclusion. As we were mainly interested in what kind of offers the end-consumer can access directly on the internet, we also ruled out firms which just advertised but did not sell directly to the consumer.
Table 1. DCGT companies evaluated in the period 15 June 2008 to 15 July 2008 (if based outside the USA, country given in parentheses.
We assume these 38 websites to be a representative sample of English-language websites that the consumer would find on the internet when he/she is searching genetic tests for health- or diet-related purposes that can be ordered without contacting any medical personnel.
The 38 websites were checked in the period between 15 June 2008 and 15 July 2008, following an assessment form that covered general company data, to the type of offers and the testing procedure, and the quality of information available on the websites.
Quality criteria
An in-depth quality assessment of the 38 DCGT offers with respect to their scientific foundation, their clinical or other utility for the consumer and the ethical and legal status was beyond the scope of the project. In order to gain detailed and comprehensive data, one would have to purchase and perform real tests. Analyzing the content of the specific information or the usefulness for the consumer would have required on the one hand a comprehensive assessment of the possible medical value of the DCGT offers and, on the other hand, a detailed analysis of how the information on these websites is interpreted by consumers.
Thus, the quality assessment of the 38 DCGT websites could only be performed in a quantitative (and thereby more “superficial” way). For this purpose, the presence or absence of the topic as such was counted on the websites. This approach has recently been used for assessing the quality of information accompanying online marketing of non-genetic home diagnostic tests in general (e.g. for allergies, hepatitis C, HIV or prostate cancer; no genetic testing) (Datta et al. Citation2008). To our knowledge, our analysis is the first of this kind for DCGT.
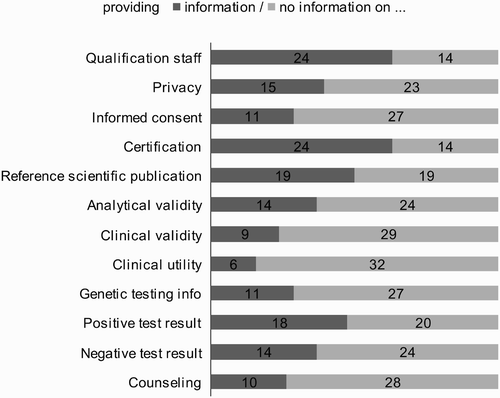
As a basis for the comparison of the 38 websites, 12 “information topics” drawn from established professional genetic counseling standards and from the available literature on DCGT (such as Borry et al. Citation2007, HGC Citation2007, COE Citation2008a) were defined, the presence of which was counted as a quality item or criterion (see ):
| • | Information on the qualifications of management team/scientific staff | ||||
| • | The company mentions guidelines on privacy and data protection | ||||
| • | The company mentions informed consent | ||||
| • | Certification | ||||
| • | Reference to scientific publication | ||||
| • | Information on analytical validity | ||||
| • | Information on clinical validity | ||||
| • | Information on clinical utility | ||||
| • | General information on genetic testing | ||||
| • | Information on consequences and actions to be taken in the case of a positive test result | ||||
| • | Information on consequences and actions to be taken in the case of a negative test result | ||||
| • | The company offers counseling | ||||
Companies and tests offered
Company characteristics
Of the 38 firms, 32 are located in the USA, three in the UK and one each in Germany, in Iceland, and in the United Arab Emirates (). The dominance of US-based firms probably reflects the actual situation, but because of the restriction on English-language offers, websites offered solely in other languages were not accessed in any case. So the results of the survey cannot be regarded as being comprehensive on a global scale but, since the companies often have international markets and thanks to the technological leadership and the specific openness of the US scientific and economic system to novel biomedical applications and enterprises, one can assume that the results show at least relevant trends and thus give important hints at recent developments.
For 14 of the companies, offering DCGT is the only field of activity. For the other 24 companies, offering DCGT is just one of several different services. Their other activities cover research in the field of human genetics, the performance of non-genetic tests, or the offer of dietary supplements. Other services offered include genome-related social networking, diet advice, different services for industry and academia. In some cases, there is a link to health and wellness institutions.
Nearly half of the companies (17 of 38) carry out the laboratory work themselves, while one-third of them explicitly outsource the laboratory work. The remaining 20% of companies do not offer unambiguous information on this topic.
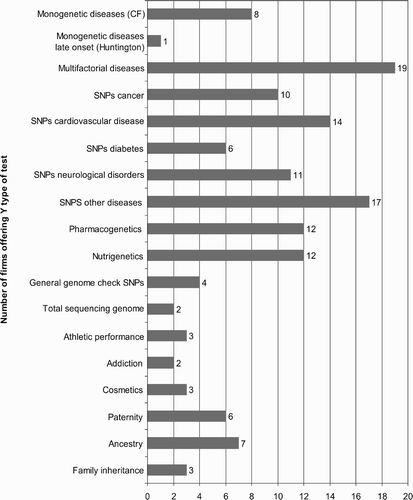
Type of genetic tests offered
According to the categories of the assessment form used, the numbers of companies offering the different kinds of tests are as shown in . Half of the firms offer testing for genetic variants (SNPs) for susceptibilities for multifactorial diseases (cancer, cardiovascular disease, diabetes, neurological disorders and others), while only 20% (eight companies) test for monogenetic Mendelian diseases (for example cystic fibrosis), and only one company tests for the fatal late-onset disease Chorea Huntington (Medi-Checks).
Twelve companies each explicitly offer pharmacogenetic testing (specific response to medical treatment) and “nutrigenetic” testing (SNP testing on “risk factors” for genetic factors related to personal diet).
An allegedly “complete” check for a variety of currently known SNPs (a genetic profile, as it were) was offered by four companies (23andMe, deCODE, Navigenics and SeqWright), while a total sequencing of the genome can be performed by GATC and Knome, the latter offering the service direct to consumers. Because of the high price (see below), these offers are aimed towards scientific institutions at the moment. But this is expected to change as soon as the announced $1000 or at least the $5000 genome (Hayden Citation2008) shows up.
Several companies offer genetic testing for other features, some of them only related in a very general sense to medical aspects, such as genetic factors related to addiction (23andMe and G-nostics), athletic performance (23andMe, CygeneDirect and Sciona), or cosmetics (Genelink Biosciences, Hair DX, Suracell).
Non-health-related paternity and ancestry testing is offered by six and seven companies, respectively, in three cases in the broader context of “family inheritance” (23andMe, Eastern Biotech and Lifesciences, SeqWright), which aims to discover inheritance patterns and relations between relatives without a specific question or goal.
Other individual types of offers are tests for sex testing of fetuses (Acu-Gen Biolab), infertility testing (DNA Direct), premarital screening (Eastern Biotech and Lifesciences), or tests for mutations influencing HIV resistance (HIVGene and HIV Mirror).
Testing procedure and role of health care professionals
Most of the companies (34 or 86%) offer a test kit for home use with the DNA probe (cheek swab or saliva/blood) to be sent to the provider for analysis. A total of 33% of the companies offer test kits to be used under the supervision of a doctor. Of these 12 companies, seven advise the patient to consult his/her doctor, and five advise the patient to contact the company's doctor.
Results are never obtained directly at home. They are submitted to the client by letter (33%), online/by email (76%), by telephone (12%) and/or to the doctor stated (19%).
There is a wide variation in the mandatory or suggested consultation of health care professionals:
| • | In most cases, the results are submitted to the client without any option of consulting an expertFootnote4 (41%). Some companies, such as Health Check USA, urge the consumer to discuss the result with his/her physician. | ||||
| • | Twenty-seven percent of the companies submit the results to the client with the option of consulting an expert. | ||||
| • | Nineteen percent of the companies submit the results to the client with consultation as a mandatory part of the process. For the company Psynomics for example, which specializes in testing for neurological and related disorders, the consumer needs to provide the licensing number of his/her psychiatrist, since the result must be interpreted by a psychiatrist. For the company Kimball Genetics “the name, address, phone and fax number of a physician are required in order to report your test results, samples submitted without a physician indicated on the Test Request form cannot be processed.” (Kimballgenetics.com) | ||||
| • | For the other 14% of the companies, the website gives no clear information on whether the submission of results is connected with consultation of an expert. | ||||
On the UK website of the company Genova Diagnostics, the following information is provided:
The majority of our test kits can be used in your own home, but some kits requiring a blood sample will need the assistance of your GP/practice nurse, or could be taken from one of our Phlebotomy centres. Please note that in accordance with UK Laboratory regulations, results will be released to your referring practitioner where applicable. If you are not currently under the guidance of a practitioner, we are able to release the results to you; however these should be taken to a practitioner for interpretation and support.
At the company DNA Direct, all tests are first authorized by a medical doctor on the basis of a pre-test questionnaire and consultation. For genetic tests for breast and ovarian cancer, infertility and recurrent pregnancy loss, a pre-test consultation is a mandatory part of the process. If the genetic testing is performed by DNA Direct's clinical services, post-test consultation is included in the service fees.
Scope and kind of information available on the websites
Information on qualification of institute and personnel
Apart from a general assurance of good quality of the company's service (which was highlighted by 71% of the websites), more detailed information about the qualification (CVs) of the management team and the scientific staff was presented on 63% of the websites. Only two of the 38 companies' websites mention a membership of professional bodies (Smart Genetics/ALZ Mirror and Health Check USA) and only three mention that they are subject to control by public authorities (23andMe, SaluGen and SeqWright).
Two-thirds (26/38) of the companies highlight their scientific advisory board, while only seven (less than a fifth) mention an ethical advisory board as well on their website. A total of 39% of the companies mention privacy guidelines (data protection), 29% refer to the topic of informed consent,Footnote5 and 18% indicate other ethical guidelines.
Information on the accuracy of test data
Overall, 63% of the companies mention that they are certified by the US Food and Drug Administration (FDA) according to CLIA (Clinical Laboratory Improvement Amendments) which defines quality standards for all laboratory testing to ensure the accuracy, reliability and timeliness of patient test results (see http://www.fda.gov/CDRH/clia/).
Thirty-seven percent of the companies' websites give specific information on the analytical validity of the genetic tests offered (accuracy of the test identifying the biomarker), 24% give information on the clinical validity (relationship between the biomarker and the clinical status), and 16% give information on the clinical utility (likelihood that the test will lead to an improved outcome). In 47% of the scanned websites, reference is made to expert knowledge and/or scientific evidence.
Information on genetic testing in general and test-specific information
On 61% of the assessed websites, information for lay people is given on the scientific basis of the genetic tests offered. Thirty-two percent of the companies' websites contain information on the subgroups of population suitable for testing or information on the question of when a genetic test can be useful and when not. Fifty percent of the companies make reference to one or more scientific publications to support the validity of tests offered.
Of the 12 companies offering pharmacogenetic tests, four present general information on the topic of pharmacogenetics, the other eight do not explain what pharmacogenetics is. Of the 12 companies offering nutrigenetic tests, seven websites give general information on nutrigenetics.
A total of 53% of the websites give information on which SNPs are tested. Three of the four companies that offer genome-wide SNP testing (23andMe, deCODE, Navigenics) deliver information on the methods used to calculate a composite risk from different SNPs which influence the same condition.
Information on the necessity and possible methods of counseling
Ten of the 38 companies mention on their websites that they offer counseling (Carolyn Katzin's The DNA Diet, DNA Direct, Eastern Biotech Lifesciences, Genelex, Genetic Health UK, Health Check USA, Kimball Genetics, Navigenics, Smart Genetics/HIV Mirror, Smart Genetics/ALZ Mirror), but in completely different ways. Kimball Genetics, for example, delivers information on consequences in the form of a detailed report with genetic interpretation, recommendations and education, which is prepared by certified genetic counselors and geneticists. At Genelex, counseling is offered for physicians and patients. DNA Direct offers separate counseling for customers of 23andMe, before and after a genome-wide SNP scan, which is normally accompanied only by written information via internet access (a service which 23andMe does not provide itself).
Of the 10 companies that offer counseling, seven organize the genetic counseling within the company, and one explicitly outsources the counseling to another DCGT firm (HealthCheckUSA to Kimball Genetics). Two websites are not clear on how they organize the counseling (Eastern Biotech Lifesciences and Genetic Health UK).
Six of the 10 companies offer counseling before testing, and eight after testing. The counseling is performed via telephone in nine cases, and two companies offer it in an internet-based form.
Seven companies give information on the qualification of the counseling staff. Often it is not clear what is understood by the term “counseling.” Terms such as “board certified counselor,” “genetic representative” and “genetic consultation expert” are used.
Two companies make reference to a professional code of practice (Smart Genetics/ALZ Mirror and DNA Direct). DNADirect and Smart Genetics/ALZ Mirror make reference to the US National Society of Genetic Counselors' direct-to-consumer guidelines (which include informed consent, privacy guidelines, laboratory certification, etc.), and DNADirect also refers to the American College of Medical Genetics statement on direct-to-consumer-genetics (with information on the scientific evidence).
Nineteen companies state explicitly that they do not offer counseling, five websites give no information on this topic, and four websites are not clear. The company Mygenome for example says: “Mygenome information services will provide a simple interpretation of the test results and guidance on how to use these results. We can also refer you to doctors who can provide appropriate care” (http://www.mygenome.com).
Information on consequences and actions to be taken
Forty-seven percent of the companies' websites present information on consequences and actions to be taken if the test result is positive, and 37% give information on the consequences and the actions to be taken if it is negative.
Some firms offer “specific” products related to the test results, especially dietary supplements. Suracell for example promotes an “age-management program” which consists of taking one or more of their proprietary nutriceuticals and follow-up urine testing.
Information on the price of genetic testing
Seventy-one percent of the websites give clear information on the price of the genetic tests, but the heterogeneity in price levels is difficult to interpret, as it is not always clear which services are included in a particular testing offer. Prices for a genetic test for monogenetic diseases range from US$70 to US$4200, and for multifactorial diseases from US$199 to US$3456. General SNP risk factor testing costs between US$199 and US$3456, pharmacogenetic tests cost between US$175 and US$630, and nutrigenomic tests between US$99 and US$625. The price for a total sequencing of the genome was US$156,900 (Knome) or US$350,000 (GATC).Footnote6
Other companies are not very clear on the total price of the service. The company SaluGen for example asks customers to agree to a contract for a monthly supply of GenoTrim (US$99), with a fee for early termination. Consumers who do not read this carefully will have to pay US$99 every month.
Quality assessment of the information available on the websites
The general quality assessment of the information offered by the 38 web pages on the basis of our set of 12 quality criteria revealed that none of the websites complied with all of the 12 quality criteria, and only one presented information on 11 items (only the information on analytical validity was missing). Six websites (18%) complied with eight criteria, and two with seven. Thus, only a quarter (9/38) complied with seven and more of the 12 quality criteria.
In turn, this means that three-quarters of the websites present information only on six items or fewer. More than half of the websites (21/38, 55%) complied with four or fewer of the 12 quality criteria, and still one-fifth of the websites (8/38, 21%) complied with only two or fewer of the 12 quality criteria.
Thus, in general, the quality assessment shows that the majority of websites checked display fundamental information deficits. In the light of the possibly far-reaching consequences for consumers purchasing genetic tests via the internet, this seems to be a serious problem, which should be analyzed and probably continuously monitored in the future. To be able to understand how the information on these websites is interpreted by consumers, research could be conducted using focus groups with lay people.
Conclusions
The findings presented above are based on a scan of a non-random sample of websites of companies offering DCGT for health, diet and lifestyle purposes. This approach was based on the assumption that the website is an important information source for consumers and often the basis on which the consumer decides to order a test or not. From the results, we can conclude that the quality of the information posted on websites is unsatisfactory for consumers to make a well-based decision to make use of the services of the company. The transparency of the websites is usually very low, especially for information on analytical validity, clinical validity and clinical utility. The lack of information on the website is not compensated for by the offer of counseling. For the majority of the companies in this assessment, no genetic counseling was offered at all.
The findings of our survey are very much in line with and support the appraisal of DCGT offers by public authorities, policy advisory bodies and expert groups that dealt with the issue in recent years. There is no doubt that the increasing numbers of DCGT offers showing up on the internet cause concern to experts, medical authorities and governmental bodies in Europe and in the US. In the US the American College of Medical Genetics has advised the public to avoid “home DNA tests” as they could be potentially harmful because of inappropriate test utilization, misinterpretation of results and the absence of follow-up counseling (ACMG Citation2004). The Federal Trade Commission together with the Food and Drug Administration and the Centers for Disease Control in July 2006 released a consumer alert because of the lack of scientific validity in some gene tests offered (FTC Citation2006). Among US authorities there seems to be serious concern that DCGT may escape from proper quality control and oversight (NHGRI Citation2004, Javitt and Hudson Citation2006, Smith Citation2006, SACGHS Citation2007). In Europe, DCGT has so far been constantly observed and discussed avidly in the UK, thanks to the initiative taken by the Human Genetics Commission (HGC Citation2003, Citation2007). DCGT is closely watched by the community of medical genetics and counselors, and the EU funded Eurogentest Network of Excellence (www.eurogentest.org). In 2008, the German Society of Human Genetics (GfH) in an official opinion judged DCGT offers for SNP testing as scientifically unsound and highlighted that genetic diagnostics in each case should be based on a profound medical consultation (GfH Citation2008). The Council of Europe has also taken up the issue (COE Citation2008a, Citation2008b).Footnote7
As for instance has been shown by statements of representatives of companies offering DCGT (Sciona, Suracell, Genox, Genelex) at the US Senate Hearing on DCGT in 2006, the suppliers of DCGT understand their offers as a means to give consumers access to the newest achievements of human genome research; by this they claim to support progress in health care supply and to foster consumer autonomy by helping them make the long-term behavioral changes required for optimizing health care (US Senate Citation2006).Footnote8
However, as the internet survey reveals, only the minority of DCGT offers meet a minimum set of quality criteria that can be regarded as necessary for ensuring adequate information and protection of customers against misleading interpretation of the need for and possible consequences of genetic testing. The majority of observers are concerned about:
| 1. | the often poor scientific evidence of the clinical validity and usefulness of the testing offered (particularly for common diseases and lifestyle purposes); | ||||
| 2. | the poor quality of information offered to consumers and the problems of providing proper genetic counseling via the internet. | ||||
Poor scientific evidence for the clinical validity of tests
As is supported by our internet survey the majority of DCGT offers appear to be for susceptibilities to common diseases (based merely on SNPs). This is plausible from an economic perspective, since the market potential for common diseases and lifestyle testing massively exceeds that for rare hereditary diseases and carrier testing.
Many experts regard most offers of testing for susceptibilities based on SNPs to be pointless from a scientific point of view, since the clinical validity of most of the tests has not (yet) been sufficiently proven (Frayling et al. Citation2007, Hogarth and Melzer Citation2007, Kroese et al. Citation2007, Morgan et al. Citation2007, Ropers and Ullmann Citation2007). However, since recommendations that can be drawn (and are drawn by providers) from positive test results usually do not go beyond what a doctor would recommend to any patient as being good for his/her health (e.g. practice sports, avoid fatty foods), some consider offering this directly to consumers to be harmless. Others, however, opine that even this kind of testing may harm clients. If results are negative, the client may gain the false impression of being safe with regard to developing a certain disease and might not see the need for adopting a healthy lifestyle; this would be totally misleading, as the absence of “negative” SNPs tested does not imply an absence of the risk of developing e.g. high blood pressure from bad dietary habits, other behavioral and environmental factors or other (so far unknown) genetic traits.
There is obviously a problem with interpreting the results of susceptibility tests correctly. It has been argued that problems with handling the interpretation of results are also reported from medical tests that are already offered for private (home) use, such as a test for osteoporosis. Also, in such cases the use of tests might lead to false-positive or false-negative results, with adverse effects on the consumer's health or psychological condition (e.g. causing serious concerns without reason). On the other hand, it can be argued that there are reasons to treat genetic testing with special consideration and caution. Particularly in the case of susceptibility testing the relationship between a detected genetic trait and the onset of disease is complex (due to the interrelation of several genes and the environment), and thus the connection between the result of the test and the consequences for the person tested is not straightforward. In addition, the results of genetic testing may be relevant and have an impact not only on the individual tested but also on other family members (HGC Citation2003, p. 23).
The results of our internet survey provide the impression that most DCGT offers fail to provide proper information on the scientific evidence behind genetic testing services offered to customers (clinical validity and utility). A recently published study on the scientific evidence available for offers of predictive testing for health risks and personalized health interventions from seven companies (Genelex, Genovations, Genosolutions, Integrative Genomics, Salugen, Sciona and Suracell) supports the notion of doubtful or even intentionally misleading information being forwarded to consumers on the basis of genetic testing of susceptibilities to common diseases and dietary related health problems (Janssens et al. Citation2008). In examining scientific meta-studies on the markers used by the seven companies, the study found no or only poor evidence for the clinical validity of tests. The study found the companies' practice of combining tests for a large number of genetic variants into so-called “profiles” to be “… worrisome given the limited predictive value of results from testing single susceptibility genes with small effects” (Janssens et al. Citation2008, p. 597). The study also found the companies' practice of using these profiles to tailor individualized nutrition supplements and lifestyle recommendations to be “another intriguing puzzle” (p. 16), since trials to test gene–diet interactions had thus far only yielded mainly inconclusive results. Moreover, for several genes tested it is known that they increase the risk for some diseases and decrease it for others, thus the health effects of preventive interventions on the basis of a related test may not be entirely beneficial (Janssens et al. Citation2008, p. 598; see also results of a recent test of services offered by 23andMe and Navigenics: Ng et al. Citation2009).
Problems of providing proper genetic counseling
The salience of medical consultation and genetic counseling in the context of genetic testing and the sensitive nature of genetic testing from the perspective of the general public can be gleaned from the fact that two-thirds of respondents to an opinion poll carried out on behalf of HGC in 2002 would also prefer to consult a doctor for genetic testing that is not related to possible severe diseases but only to lifestyle aspects and paternity (HGC Citation2003, p. 24). Similar results were found by an online survey of social networkers in the US recently (McGuire et al. Citation2009). The main concern regarding DCGT is obviously that the services offered cannot live up to the high professional standards of medical and genetic consultation required (by statutory regulations or professional guidelines) for normal genetic testing in the context of genetic counseling. It can of course be argued that DCGT offers support free access and free choice for consumers by broadening the scope of options for genetic testing. However, at the core of “free choice” is good information to provide informed consent from the customer. This seems to be far from being guaranteed when there is an economic interest in “convincing” a customer that he or she will benefit from testing. According to our internet survey, most companies offering genetic testing services via the internet do not include genetic counseling at all in their services. Only a few urge customers to involve an expert before purchasing a gene test, and “counseling” in most cases only is provided as written information via mail or via web-log.
The information offered via the web is often very poor and does not comply with professional standards of counseling as the results from our quality criteria assessment reveal. Only one company complied with 11 of our 12 information quality criteria. Moreover, during the experts' workshop, it was emphasized that irrespective of the quality of information on single topics, a DCGT offer can be senseless or even harmful if only one or two relevant points are missing (e.g. on clinical validity and clinical utility). Thus, the presence of information on six, seven or eight topics is hard to interpret in “positive” terms – but the absence of seven, nine or even 11 “quality criteria” must certainly be interpreted “negatively.”
When communication and “counseling” are only provided via mail or web-exchange, it is almost impossible to make sure that the information given has been properly understood by the customer. In testing for complex and serious diseases, personal communication is needed about the individual's situation, relatives that may have to be informed about the test result, and information on possible treatment or preventive measures. The confidentiality and empathy required would probably not be possible via written information and communication (HGC Citation2003, p. 28f.). This, according to HGC, does not necessarily imply that the involvement of a doctor is crucial. What is important, however, is the extent to which the setting in which the service is offered and applied allows (or suits) consideration of high-level professional standards. Offers via the internet can thus be criticized for taking place not in a context defined by medical consultation in the best interests of the patient/client, but according to a commercial principle, “where the health care professional was simply facilitating a transaction for a kit or self-testing mail order service” (HGC Citation2003, p. 25).
Notes
The comments of two anonymous referees have been very helpful to improve the quality of the paper. However, the authors regret that they were not able to follow all advice given by the referees.
It was impossible to discern between information given for different types of tests, as requested by one referee. Information provided by the websites before carrying out a test, which was the object of our survey, usually is not differentiated for different test types. | |||||
Despite the fact that tests for monogenetic diseases are offered by a few websites and many websites offer a mix of different test types to consumers, susceptibility testing based on SNPs makes up the center of the DCGT business model. Our arguments regarding bad quality of consumer information offered at the websites and problems of DCGT thus apply mainly for susceptibility testing. We are confident that this is made clear enough in our concluding discussion. | |||||
We could not bring ourselves to exclude those companies that (meanwhile) claim not to offer services for health purposes from our analysis. The fact that several companies in their terms of service claim not to offer services for health purposes confirms our findings because the appearance of their web offers tells the opposite story to the potential customer. We regard these claims to be a central part of the problem as a strategy to move out of the focus of governmental oversight. | |||||
The following experts participated in the meeting: Pascal Borry, University of Leuven; Stuart Hogarth, University of Loughborough; Heidi Howard, McGill University Montréal; Alastair Kent, Genetic Interest Group; Ulf Kristoffersson, Lund University Hospital; Peter Pohl, GATC Biotech; Helen Wallace, Gene Watch UK.
The full report of the project is available at the website of the European Parliament's Science and Technology Options Assessment Panel (STOA Citation2008): http://www.europarl.europa.eu/stoa/default_en.htm.
An expert is interpreted as a health care professional and not necessarily as a genetic counselor.
Within the scope of this study, we did not examine to what exactly the consumer gives his/her informed consent.
At the experts' workshop held in September 2008 it was doubted that Knome really can perform the total sequencing at that price (or that the company can earn money by doing this), because the chemical reagents needed alone cost more than the offered price.
Other official bodies that have discussed the issue of DCGT are the American Medical Association, the European Group on Ethics, the Belgian National Consultative Committee on Bioethics and the French National Consultative Committee on Bioethics.
Obviously as a reaction to criticism and intervention by public authorities 23andMe now argues – and states in a disclaimer on its webpage – that it is providing genetic information for research and educational use only but not medical advice (“not intended to be used for any diagnostic purpose and is not a substitute for professional medical advice”: www.23andMe.com, “terms of service” as of 3 November 2008). It is however not visible that 23andMe has excluded tests for SNPs indicating health-related risks from its services.
References
- ACMG (American College of Medicine Genetics), 2004. ACMG statement on direct-to-consumer genetic testing, (2004), Available from: http://www.acmg.net/StaticContent/StaticPages/Direct_Consumer.pdf [Accessed 29 April 2010].
- Belgisch Raadgevend Comité voor Bio-ethiek, 2004. Advies nr. 32 van 5 juli 2004 betrffende de vrije beschikbaarheid van genetische tests. Brussels. Available from: https://portal.health.fgov.be/pls/portal/docs [Accessed 29 April 2010].
- Borry, P., et al., 2007. Genetic testing and counselling, (2007), European Guidance, European Ethical-Legal Papers No. 3, Leuven, 2007. http://www.eurogentest.org/web/files/public/unit4/EELP_3_voor_site.pdf [Accessed 29 April 2010].
- COE (Council of Europe), 2008a. Additional Protocol to the Convention on Human Rights and Biomedicine concerning Genetic Testing for Health Purposes, (2008a), 7 May 2008 [online]. Available from: http://conventions.coe.int/Treaty/EN/Treaties/Html/203.htm [Accessed 29 April 2010].
- COE, 2008b. "Explanatory report to the Additional Protocol to the Convention on Human Rights and Biomedicine concerning Genetic Testing for Health Purposes [online]". Strasbourg. 2008b, Available from: http://conventions.coe.int/Treaty/EN/Reports/Html/203.htm [Accessed 29 April 2010].
- Datta, A. K., et al., 2008. Quality of information accompanying on-line marketing of home diagnostic tests, Journal of the Royal Society of Medicine 101 (2008), pp. 34–38.
- Fleming, N., 2008. Rival genetic tests leave buyers confused, The Sunday Times (2008), 7 September [online]. Available from: http://www.timesonline.co.uk/tol/news/uk/science/article4692891.ece [Accessed 29 April 2010].
- Frayling, T., et al., 2007. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity, Science 316 (2007), pp. 889–894.
- FTC (Federal Trade Commission), 2006. At-home genetic tests: a healthy dose of skepticism may be the best prescription, (2006), FTC – Facts for Consumers, July.
- GAO (US Government Accountability Office), 2006. Nutrigenetic testing, (2006), Tests purchased from four web sites mislead consumers. Testimony before the Special Committee on Aging, US Senate [online]. Available from: http://www.gao.gov/new.items/d06977t.pdf [Accessed 29 April 2010].
- GfH (Deutsche Gesellschaft für Humangenetik), 2008. Stellungnahme der Deutschen Gesellschaft für Humangenetik zum Thema: “Kommerzielle Internet-Angebote zur Analyse genetischer SNP-Varianten.”, Medizinische Genetik 20 (2008), p. 237.
- Harmon, A., 2007. The DNA age – my genome, myself: seeking clues in DNA, The New York Times (2007), 17 November [online]. Available from: http://www.nytimes.com/2007/11/17/us/17dna.html [Accessed 29 April 2010].
- Hayden, E. C., 2008. $5,000 genome next year, company promises, Nature Online (2008), 6 October [online]. Available from: http://www.nature.com/news/2008/081006/full/news.2008.1151.html [Accessed 29 April 2010].
- HGC (Human Genetics Commission), 2003. Genes direct: ensuring the effective oversight of genetic tests supplied directly to the public. London: Department of Health; 2003.
- HGC, 2007. More genes direct: a report on developments in the availability, marketing and regulation of genetic tests supplied directly to the public. London: Department of Health; 2007.
- Hogarth, S., and Melzer, D., 2007. The IVD Directive and genetic testing: problems and proposals [online]. Presented at A briefing presented to the 20th meeting of Competent Authorities. Lisbon, July, Available from: http://www.kcl.ac.uk/schools/sspp/interdisciplinary/cbas/staff/acad/sh.html [Accessed 29 April 2010].
- Hogarth, S., et al., 2007. Closing the gaps: enhancing the regulation of genetic tests using responsive regulation, Food and Drug Law Journal 62 (2007), pp. 831–848.
- Janssens, A. C.J.W., et al., 2008. A critical appraisal of the scientific basis of commercial genomic profiles used to assess health risks and personalize health interventions, American Journal of Human Genetics 82 (2008), pp. 593–599.
- Javitt, G. J., and Hudson, K., 2006. Federal neglect: regulation of genetic testing, Issues in Science and Technology (2006), Spring [online]. Available from: http://www.issues.org22.3/javitt.html [Accessed 29 April 2010].
- Kroese, M., Elles, R., and Zimmern, R. L., 2007. The evaluation of clinical validity and clinical utility of genetic testing. Summary of an expert workshop, 26 and 27 June 2006. Manchester: National Genetics Reference Laboratory; 2007.
- Lab Times, 2007. Genetically yours, Lab Times 1-2007 (2007), pp. 14–18.
- McGuire, A., et al., 2009. Social networkers' attitudes toward direct-to-consumer personal genome testing, American Journal of Bioethics 9 (7) (2009), pp. 3–10.
- Morgan, T. M., et al., 2007. Non-validation of reported genetic risk factors for acute coronary syndrome in a large-scale replication study, Journal of the American Medical Association 297 (2007), pp. 1551–1561.
- Ng, P. C., et al., 2009. An agenda for personalized medicine, Nature 461 (2009), pp. 724–726.
- NHGRI (National Human Genome Research Institute), 2004. Summary of the Workshop “Direct to consumer marketing of genetic tests” (2004), 23 March. Available from: http://www.genome.gov/12010660 [Accessed 29 April 2010].
- Ropers, H. H., and Ullmann, R., 2007. "Neue Technologien für Genomforschung und Diagnostik". In: Schmidtke, J., et al., eds. Gendiagnostik in Deutschland. Status quo und Problemerkundung. Berlin: Berlin Brandenburgische Akademie der Wissenschaften; 2007. pp. 21–32.
- SACGHS (Secretary's Advisory Committee on Genetics, Health, and Society), 2007. Coverage and reimbursement of genetic tests and services. Washington: Department of Health and Human Services; 2007.
- Smith, G. H., 2006. Statement of Chairman Gordon H. Smith. Presented at Proceedings of the Hearing of the Special Committee on Aging. 27, July.
- STOA (Science and Technology Options Assessment), 2008. Direct to Consumer Genetic Testing. Study, European Parliament, Brussels. Available from: http://www.europarl.europa.eu/stoa/publications/studies/stoa32and39en.pdf [Accessed 29 April 2010].
- US Senate, 2006. Presented at Proceedings of the Hearing of the Special Committee on Aging. 27, July.
- Williams-Jones, B., 2006. “Be ready against cancer now”: direct to consumer advertising for genetic testing, New Genetics and Society 25 (2006), pp. 89–107.