Abstract
This retrospective cohort study examines the association between previous mode of delivery and subsequent live birth rate in women who become pregnant after in vitro fertilization (IVF) or intra cytoplasmic sperm injection (ICSI) after their first delivery. The study included 112 women with a previous caesarean section and 418 women with a previous vaginal delivery, and a total of 1588 embryo transfers between January 2005 and June 2016 (Leiden University Medical Centre, the Netherlands). The mean age was 35 years and mean number of embryos transferred per attempt, 1.18. The study population included a total of 429 pregnancies resulting in 296 live births. The crude odds ratio for a subsequent live birth per embryo transfer was 0.60 (CI; 0.44 to 0.83, p = 0.002) in women with a previous caesarean section compared to women with a previous vaginal delivery. After adjustment for age, fresh/frozen-thawed embryo transfer and quality of the embryo, the odds ratio was 0.64 (CI; 0.46 to 0.89, p = 0.01). It was concluded that in subfertile women trying to achieve a subsequent pregnancy with IVF or ICSI, a history of caesarean section was associated with a reduced live birth rate per embryo transfer compared to women with a history of one previous vaginal delivery.
Introduction
The caesarean section (CS) rate has been rising globally, mainly because of labour management choices (Boyle & Reddy, Citation2012). In the Netherlands, the CS-rate increased slightly from 14% in 2000 to nearly 17% in 2010 (Zhao et al., Citation2016). There are few studies on the effect of CS on live birth rate in subsequent pregnancies, with outcomes varying from a negative to no effect in the population (Evers, McDermott, Blomquist, & Handa, Citation2014; Fussing-Clausen et al., Citation2014; Gurol-Urganci, Cromwell, Mahmood, van der Meulen, & Templeton, Citation2014; O'Neill et al., Citation2014). The meta-analysis by Gurol-Urganci et al. (Citation2014) showed a reduced fertility (live birth rate) of 11% after a CS. However, the studies were heterogeneous in study design and quality.
Incomplete uterine healing after a CS, called a ‘niche’ (Roberge et al., Citation2016; Vervoort et al., Citation2015) can have effect on abnormal menstrual bleeding and subfertility. It is hypothesized that fluid from the caesarean scar is retained in the uterine cavity. This fluid may lead to a failure of embryo implantation due to the embryotoxic environment; a mechanism similar to that proposed for a hydrosalpinx (Tanimura et al., Citation2015).
Three studies on the effect of CS on pregnancies established by IVF or ICSI have recently been published. These indicated that embryo transfer (ET), a crucial part of IVF and ICSI, is more difficult in women with a caesarean scar (Patounakis et al., Citation2016). Of the three studies, two reported no significant decrease in fertility (Patounakis et al., Citation2016; Zhang et al., Citation2016), while one found a 14% decrease in pregnancy rate (Wang et al., Citation2017).
It is hypothesized that if a history of CS in fertile couples leads to a reduced live birth rate, the live birth rate will also be reduced in couples with a history of CS undergoing IVF or ICSI.
Study aim: to discover if previous mode of delivery influences subsequent IVF/ICSI live birth rates.
Materials and methods
This was a retrospective cohort study conducted in the Leiden University Medical Centre (LUMC), The Netherlands.
Women and data retrieval
The medical records between January 2005 and June 2016 were analysed and women with a previous live birth (after an IVF or ICSI induced pregnancy) who returned for a second child using IVF/ICSI and had at least one (fresh or frozen-thawed) embryo transfer were selected for the study. These women were divided into two groups: (i) previous CS or (ii) vaginal delivery (including vacuum extraction). All fresh ETs and/or frozen-thawed ETs of the included women after their first live birth were analysed until the end of treatment or second live birth.
Exclusion criteria were: history of multiple caesareans or vaginal deliveries, foetal death at first delivery (gestational age >16 weeks), missing mode of delivery of first live birth or history of spontaneous pregnancy (a history of spontaneous pregnancy indicates that such women are more fertile than those who never achieved a spontaneous pregnancy and were therefore excluded to reduce bias).
In our study population, the age limit of women undergoing an oocyte pick up was 43 years, and for embryo transfer, 45 years. ‘Age’ was on the basis of the last ET. BMI (body mass index) and smoking characteristics of the women were extracted from the patient records mostly at the start of treatment. The cause of subfertility before first IVF treatment (before first delivery) was used to compare both groups. The quality of the embryos was graded using an adapted standard classification system. A class one (best quality) was defined as an embryo consisting of 8 cells (on day three) or 16, 32 or 64 cells (on day four to six). A class two was defined as an embryo of 7 or 9–15 cells (on day three) and a class three was defined as an embryo of fewer than 7 cells (on day three). This classification is based on that of Stylianou, Critchlow, Brison, and Roberts (Citation2012) and the results of the LUMC IVF clinic.
IVF and ICSI protocol
Ovarian stimulation protocols were individually adapted to a long (GnRH agonist) or a short protocol (agonist or antagonist and urinary or recombinant FSH). The most frequent was a short protocol with GnRH agonist. ET of frozen-thawed embryos took place in natural cycles, or artificial cycles with administration of oestradiol and progesterone. Information on ultrasound findings such as a niche or uterine lining before embryo transfer was not collected.
All embryo transfers were performed in dorsal lithotomy position. Single ET was the standard protocol, but depending on age and medical history two embryos were sometimes transferred. The twin pregnancy rate in this Centre varied between 2.8% and 6% of all IVF/ICSI pregnancies over 10 years. Fresh embryos were mainly transferred or frozen on the third day after oocyte pick up, based on patient characteristics, with only a minority (<20%) transferred or frozen on day two or four. Frozen embryos were thawed 4 days after the LH-surge (measured in blood or urine) or 5 days after hCG administration. Embryos were transferred on day zero or day one after the thawing procedure. All ETs were included in the study. Luteal support was started 2 days before fresh embryo transfer until 7 weeks of pregnancy.
In both groups, a Wallace® catheter was used for ET. In the ‘caesarean’ cohort, a Wallace Sureview® catheter (Smiths Medical, Dublin, OH, USA) was used from 2010. The catheter was used to place the embryo at a distance of 15 mm from the endometrial fundus in the sagittal plane under abdominal ultrasound guidance. In the ‘vaginal delivery’ cohort, ET was performed with the fixed distance method. The embryos were placed at a distance of 60 mm from the external cervical ostium with a Wallace® catheter. The Wallace® and Wallace Sureview® catheters are identical, except that the Sureview is echogenic, and both were used in >95% of all ETs. If ET failed with the Wallace® catheter, a TDT®-catheter (Irvine Scientific, Santa Ana, CA, USA) was used.
Outcomes
Primary outcome: a second live birth following IVF/ICSI in women with a history of CS compared with vaginal delivery (Harbin Consensus Conference Workshop Group, Citation2014).
Secondary outcome: biochemical pregnancy rate (βHCG >50U/L at 15 days or more after oocyte retrieval), miscarriage or ectopic pregnancy and ongoing pregnancy rate (as defined by more than 16 weeks gestational age) in women with a history of CS compared to a history of vaginal delivery.
Statistical analysis
Independent t-test analysis was used for equally distributed parameters of the continuous data. Chi-squared tests/Fisher’s exact tests were used for categorical data. Logistic regression with repeated measures (Generalized Estimated Equations, GEE) was used to assess the association corrected for number of embryo transfers on the main outcome measures: live birth rate, pregnancy, miscarriage. This GEE-model was also used to correct for confounding variables (age, fresh/frozen transfer, quality of embryos, cause of subfertility). Confounding variables with a p-value ≤0.05 were retained in the adjusted model. A p-value of ≤0.05 was considered significant. Statistical calculations were performed with IBM SPSS version 23.
Ethical approval
This retrospective cohort study was approved by the Medical Ethics Committee of the LUMC.
Results
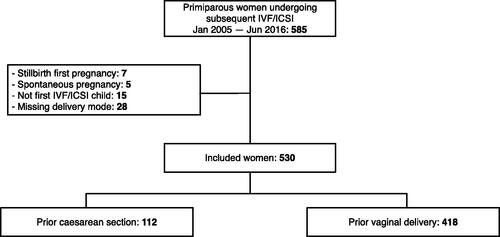
Inclusion criteria () were met by 530 women. A total of 112 women had a previous CS, and 418 women had a previous vaginal delivery (spontaneous or vacuum extraction).
The characteristics of both groups are represented in . BMI of the CS-group tended to be higher than the vaginal delivery group, however not significantly. For 244 women treated by LUMC, BMI was available (46%). Transfer ease was not tabulated in because of the low count of classes other than ‘easy’ (<10 women with class ‘medium’ or ‘hard’).
Table 1. Group characteristics.
In the study population 296 subsequent live births out of 1681 embryo transfers were documented (). Women underwent an average of three embryo transfers (minimum of one and maximum of 19 transfers).
Table 2. Outcome overview.
CS was associated with a significantly lower live birth rate per ET compared to vaginal delivery (; OR: 0.60, CI; 0.44 to 0.83). When adjusted for age, fresh or frozen-thawed ET and quality of the embryos, the analysis still showed a significantly lower live birth rate per ET (OR 0.64, CI; 0.46 to 0.89). The adjusting variables were not confounders (less than 10% influence on OR), but had a significant effect to remain in the model (p < 0.001). BMI, smoking and cause of subfertility had no significant influence on the live birth rate. Because there was a significant difference in the cause of subfertility in both groups (), a second adjusted OR for the primary outcome was included in (OR 0.65, CI; 0.46 to 0.93).
Table 3. Main outcomes per embryo transfer and miscarriage ratio per pregnancy; CS compared to vaginal delivery.
Ongoing pregnancy rate was not analysed separately from live birth rate, because only one single stillbirth occurred in the vaginal delivery group.
Discussion
Our study suggests that a previous live birth by CS following IVF/ICSI could lead to a reduced live birth rate among women embarking on subsequent IVF/ICSI. The miscarriage rate once pregnancy was achieved was significantly higher in the CS-group. Current literature also describes a slightly higher incidence of miscarriage after CS (O'Neill et al., Citation2013).
The main strength of our study is the higher number of women included than in previous studies. Our findings show a more distinct difference between both groups than reported by Gurol-Urganci et al., Citation2013; Patounakis et al., Citation2016; Wang et al., Citation2017 and Zhang et al., Citation2016). A literature search revealed three IVF/ICSI studies which only monitored outcomes of one (fresh) embryo transfer (Patounakis et al., Citation2016; Wang et al., Citation2017; Zhang et al., Citation2016) and two studies which included women with one or more CS’s or vaginal deliveries (Patounakis et al., Citation2016; Wang et al., Citation2017). The mode of conception of the first live birth was not mentioned and there was a difference in subfertility period in the study of Zhang (Zhang et al., Citation2016). Factors such as a previous spontaneous or IVF pregnancy, duration of subfertility, and number of IVF-cycles influence the live birth rate according to Templeton’s model (Templeton, Morris, & Parslow, Citation1996). It could be that all these factors reduced the association compared with our study in which only women with one previous delivery were included.
Our hospital has a fixed group of experienced individuals who carry out ETs. The analysis used repeated measures, and outcomes were measured per ET. This lowers the chance of bias when performing more ETs until success is achieved. There is also less bias towards couples who give up after a few attempts of IVF because of age or traumatic delivery. Traumatic delivery could be more frequent in the group of CS (which possibly were more often in an emergency setting), which could lead to less desire to have a second child. In population studies it is difficult to study decreased family size because the cause might focus on maternal choice, instead of a physiological effect of an impaired implantation (O'Neill et al., Citation2015). All women in our study tried to achieve a second pregnancy in contrast to population-based studies, so that maternal choice did not influence the outcomes.
Due to incomplete data on BMI, smoking, ultrasound guidance during transfer and cause of subfertility, the present study cannot rule out these factors as confounders. All other data for patients were complete and were used to adjust outcome measures. The database did not contain information about ultrasound findings such as a niche or uterine lining before embryo transfer, the type of scar (classical or transverse incision) or surgical techniques of uterine closure (associated with niches) (Roberge et al., Citation2016) and there was no information on whether women had a niche or complaints of bleeding problems after their CS. Visible endometrial fluid during IVF stimulation is known to impair outcomes in women with tubal factor (Akman, Erden, & Bahceci, Citation2005) and there are reports on endoscopic correction of the niche which resolve abnormal menstrual bleeding, and may have a (positive) effect on fertility problems (Gubbini et al., Citation2011; Tanimura et al., Citation2015).
The CS-group had a significantly higher age (). Higher age is correlated with a greater risk of CS and reduced ART success rates. Data were corrected for age, because it is significantly related to the outcome measure (Templeton et al., Citation1996). The CS group more often had non-male factors as a cause of subfertility, including endometriosis, which is linked to a higher risk of having a CS (Jacques, Freour, Barriere, & Ploteau, Citation2016). The cause of subfertility had no influence on the main outcome measure in the GEE model (). The CS group tended to have a higher BMI, which approached significance (p = 0.051). Data on BMI were only available in 48% of the patients and were mostly measured before the first pregnancy. Increased BMI is linked to a greater risk of CS (Barau et al., Citation2006). This difference in baseline characteristics could lead to confounding by indication. Data were not corrected for BMI or smoking in our study, because they had no significant effect on the model. However, this could be unreliable because of missing values. Moreover, BMI might not necessarily be correlated with live birth rate (Mutsaerts et al., Citation2016).
Ovarian stimulation protocols differed between women. We have no reason to believe that stimulation protocols were not distributed equally between groups, or would have an effect on outcomes. In the last few years of the study period, the CS-group had an echogenic catheter and ultrasound for the embryo transfer, as shown in . Currently there is little evidence that ultrasound-guided embryo transfer improves pregnancy outcomes (Brown, Buckingham, Abou-Setta, & Buckett, Citation2010; Brown, Buckingham, Buckett, & Abou-Setta, Citation2016). Separate analysis of live birth rate did not show a significant difference between the two periods (data not shown).
Counselling women carefully for a CS by obstetricians is an important element in reducing the number of caesarean sections worldwide. Women with a first ongoing pregnancy after IVF or ICSI are more likely to have a CS, even after matching for the most prominent confounders, notably, age (Helmerhorst, Perquin, Donker, & Keirse, Citation2004). The implication of our results is that a higher frequency of CS could reduce the chances of a second live birth through IVF or ICSI and could be more pronounced in certain groups of CS-women, for example those with a niche. We presume that this CS-effect is applicable to the rest of the population, i.e., to women without fertility problems though to a lesser extent. It is essential that further research on this subject be carried out. Preferably this would consist of prospective research with a larger number of women with more data available on BMI, smoking, endometrial cavity and the criteria for IVF. With an extended study size, it would be possible to distinguish more reliably between problems with implantation or with the conceptus reaching full-term.
Acknowledgements
The authors thank Dr. J.A. Vogelaar for data-analysis, Drs. R. Wolterbeek for statistical analysis and Dr. G.S.K. Pilgram, clinical embryologist, for helping create the dataset.
Disclosure statement
The authors have stated explicitly that they have no conflict of interest in connection with this article. No funding declared.
References
- Akman, M.A., Erden, H.F., & Bahceci, M. (2005). Endometrial fluid visualized through ultrasonography during ovarian stimulation in IVF cycles impairs the outcome in tubal factor, but not PCOS, patients. Human Reproduction, 20, 906–909. doi: https://doi.org/10.1093/humrep/deh737.
- Barau, G., Robillard, P.-Y., Hulsey, T.C., Dedecker, F., Laffite, A., Gérardin, P., & Kauffmann, E. (2006). Linear association between maternal pre-pregnancy body mass index and risk of caesarean section in term deliveries. BJOG: An International Journal of Obstetrics & Gynaecology, 113, 1173–1177. doi: https://doi.org/10.1111/j.1471-0528.2006.01038.x.
- Boyle, A., & Reddy, U.M. (2012). Epidemiology of cesarean delivery: the scope of the problem. Seminars in Perinatology, 36, 308–314. doi: https://doi.org/10.1053/j.semperi.2012.04.012.
- Brown, J., Buckingham, K., Abou-Setta, A.M., & Buckett, W. (2010). Ultrasound versus “clinical touch” for catheter guidance during embryo transfer in women. The Cochrane Database of Systematic Reviews, 2010, CD006107. doi: https://doi.org/10.1002/14651858.CD006107.pub3.
- Brown, J., Buckingham, K., Buckett, W., & Abou-Setta, A.M. (2016). Ultrasound versus ‘clinical touch’ for catheter guidance during embryo transfer in women. Cochrane Database of Systematic Reviews, 17, CD006107. doi: https://doi.org/10.1002/14651858.CD006107.pub4.
- Evers, E.C., McDermott, K.C., Blomquist, J.L., & Handa, V.L. (2014). Mode of delivery and subsequent fertility. Human Reproduction, 29, 2569–2574. doi: https://doi.org/10.1093/humrep/deu197.
- Fussing-Clausen, C., Geirsson, R.T., Hansen, T., Rasmussen, S., Lidegaard, Ø., & Hedegaard, M. (2014). Mode of delivery and subsequent reproductive patterns. A national follow-up study. Acta Obstetricia et Gynecologica Scandinavica, 93, 1034–1041. doi: https://doi.org/10.1111/aogs.12469.
- Gubbini, G., Centini, G., Nascetti, D., Marra, E., Moncini, I., Bruni, L., … Florio, P. (2011). Surgical hysteroscopic treatment of cesarean-induced isthmocele in restoring fertility: Prospective Study. Journal of Minimally Invasive Gynecology, 18, 234–237. doi: https://doi.org/10.1016/j.jmig.2010.10.011.
- Gurol-Urganci, I., Bou-Antoun, S., Lim, C.P., Cromwell, D.A., Mahmood, T.A., Templeton, A., & van der Meulen, J.H. (2013). Impact of Caesarean section on subsequent fertility: A systematic review and meta-analysis. Human Reproduction, 28, 1943–1952. doi: https://doi.org/10.1093/humrep/det130.
- Gurol-Urganci, I., Cromwell, D.A., Mahmood, T.A., van der Meulen, J.H., & Templeton, A. (2014). A population-based cohort study of the effect of Caesarean section on subsequent fertility. Human Reproduction, 29, 1320–1326. doi: https://doi.org/10.1093/humrep/deu057.
- Harbin Consensus Conference Workshop Group. (2014). Improving the Reporting of Clinical Trials of Infertility Treatments (IMPRINT): modifying the CONSORT statement. Fertility and Sterility, 102, 952–959.e15. doi: https://doi.org/10.1016/j.fertnstert.2014.08.002.
- Helmerhorst, F.M., Perquin, D.A.M., Donker, D., & Keirse, M.J.N.C. (2004). Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. British Medical Journal, 328, 261. doi: https://doi.org/10.1136/bmj.37957.560278.EE.
- Jacques, M., Freour, T., Barriere, P., & Ploteau, S. (2016). Adverse pregnancy and neo-natal outcomes after assisted reproductive treatment in patients with pelvic endometriosis: a case-control study. Reproductive Biomedicine Online, 32, 626–634. doi: https://doi.org/10.1016/j.rbmo.2016.03.005.
- Mutsaerts, M.A.Q., van Oers, A.M., Groen, H., Burggraaff, J.M., Kuchenbecker, W.K.H., Perquin, D.A.M., … Hoek, A. (2016). Randomized trial of a lifestyle program in obese infertile women. New England Journal of Medicine, 374, 1942–1953. doi: https://doi.org/10.1056/NEJMoa1505297.
- O'Neill, S.M., Kearney, P.M., Kenny, L.C., Khashan, A.S., Henriksen, T.B., Lutomski, J.E., & Greene, R.A. (2013). Caesarean delivery and subsequent stillbirth or miscarriage: Systematic review and meta-analysis. PLoS One, 8, e54588. doi: https://doi.org/10.1371/journal.pone.0054588.
- O'Neill, S.M., Khashan, A.S., Henriksen, T.B., Kenny, L.C., Kearney, P.M., Mortensen, P.B., … Agerbo, E. (2014). Does a Caesarean section increase the time to a second live birth? A register-based cohort study. Human Reproduction, 29, 2560–2568. doi: https://doi.org/10.1093/humrep/deu217.
- O'Neill, S.M., Khashan, A.S., Kenny, L.C., Kearney, P.M., Mortensen, P.B., Greene, R.A., … Henriksen, T.B. (2015). Time to subsequent live birth according to mode of delivery in the first birth. BJOG: An International Journal of Obstetrics & Gynaecology, 122, 1207–1215. doi: https://doi.org/10.1111/1471-0528.13359.
- Patounakis, G., Ozcan, M.C., Chason, R.J., Norian, J.M., Payson, M., DeCherney, A.H., & Yauger, B.J. (2016). Impact of a prior cesarean delivery on embryo transfer: a prospective study. Fertility and Sterility, 106, 311–316. doi: https://doi.org/10.1016/j.fertnstert.2016.03.045.
- Roberge, S., Demers, S., Girard, M., Vikhareva, O., Markey, S., Chaillet, N., … Bujold, E. (2016). Impact of uterine closure on residual myometrial thickness after cesarean: a randomized controlled trial. American Journal of Obstetrics and Gynecology, 214, 507.e1–507.e6. doi: https://doi.org/10.1016/j.ajog.2015.10.916.
- Stylianou, C., Critchlow, D., Brison, D.R., & Roberts, S.A. (2012). Embryo morphology as a predictor of IVF success: an evaluation of the proposed UK ACE grading scheme for cleavage stage embryos. Human Fertility, 15, 11–17. doi: https://doi.org/10.3109/14647273.2011.652251.
- Tanimura, S., Funamoto, H., Hosono, T., Shitano, Y., Nakashima, M., Ametani, Y., & Nakano, T. (2015). New diagnostic criteria and operative strategy for cesarean scar syndrome: Endoscopic repair for secondary infertility caused by cesarean scar defect. Journal of Obstetrics and Gynaecology Research, 41, 1363–1369. doi: https://doi.org/10.1111/jog.12738.
- Templeton, A., Morris, J.K., & Parslow, W. (1996). Factors that affect outcome of in-vitro fertilisation treatment. The Lancet, 348, 1402–1406. doi: https://doi.org/10.1016/S0140-6736(96)05291-9.
- Vervoort, A.J.M.W., Uittenbogaard, L.B., Hehenkamp, W.J.K., Brölmann, H.A.M., Mol, B.W.J., & Huirne, J.A.F. (2015). Why do niches develop in Caesarean uterine scars? Hypotheses on the aetiology of niche development. Human Reproduction, 30, 2695–2702. doi: https://doi.org/10.1093/humrep/dev240.
- Wang, Y.-Q., Yin, T.-L., Xu, W.-M., Qi, Q.-R., Wang, X.-C., & Yang, J. (2017). Reproductive outcomes in women with prior cesarean section undergoing in vitro fertilization: A retrospective case-control study. Current Medical Science, 37, 922–927. doi: https://doi.org/10.1007/s11596-017-1828-3.
- Zhang, N., Chen, H., Xu, Z., Wang, B., Sun, H., & Hu, Y. (2016). Pregnancy, delivery, and neonatal outcomes of in vitro fertilization-embryo transfer in patient with previous cesarean scar. Medical Science Monitor, 22, 3288–3295. doi: https://doi.org/10.12659/MSM.900581.
- Zhao, Y., Zhang, J., Hukkelhoven, C., Offerhaus, P., Zwart, J., Jonge, A. D., & Geerts, C. (2016). Modest rise in caesarean section from 2000-2010: The Dutch experience. PLoS One, 11, e0155565. doi: https://doi.org/10.1371/journal.pone.0155565.