Abstract
This study aims to systematically analyze the provision of information on Time-lapse Imaging (TLI) by UK fertility clinic websites. We conducted an analysis of 106 clinic websites that offer fertility treatment to self-funded patients. The analysis aimed to examine whether these clinics offer TLI, the associated cost for patients, and the clarity and quality of the provided information. Out of the 106 websites analysed, 71 (67%) claimed to offer TLI. Among these websites, 25 (35.2%) mentioned charging patients between £300 and £850, 25 (35.8%) claimed not to charge patients, and 21 (29.6%) did not provide any cost information for TLI. Furthermore, 64 (90.1%) websites made claims or implied that TLI leads to improved clinical outcomes by enhancing embryo selection. Notably, 34 (47.9%) websites did not mention or provide any links to the HFEA rating system. It is crucial to provide patients with clear and accurate information to enable them to make fully informed decisions about TLI, particularly when they are responsible for the associated costs. The findings of this study raise concerns about the reliability and accuracy of the information available on fertility clinic websites, which are typically the primary source of information for patients.
Introduction
In recent years, a variety of additional tests, treatments and technologies – usually known as add-ons – have been introduced and offered to fertility patients on top of standard IVF/ICSI cycles. These novel fertility interventions have sparked heated professional, public and media debates due to the lack of evidence supporting their efficacy (Harper et al., Citation2017; Heneghan et al., Citation2016), the poor quality of information available (Spencer et al, Citation2016; Van de Wiel et al., Citation2020) and their potential mis-selling (The Competition and Markets Authority (CMA), Citation2020).
In the United Kingdom, these concerns have prompted regulatory efforts by the Human Fertilisation and Embryology Authority (HFEA) and the Competition and Market Authority (CMA). The HFEA introduced a rating system in 2017 to assess the safety and effectiveness of treatment add-ons, which was revamped in October 2023 (see HFEA, Citation2023). This system is meant to help guide patients and healthcare providers in making informed decisions about fertility treatment options. In a similar manner, in June 2021 the CMA published guidelines which included recommendations to enhance the quality and accessibility of information given to patients and avoid potential mis-selling of add-on treatments (CMA, Citation2021a, Citation2021b).
Particular attention has been paid to fertility clinic websites, as these are often the first point of information for patients (CMA, Citation2020, Citation2022a; HFEA, Citation2019, Citation2022). The CMA carried out a review of add-on information available on clinics’ websites one year after the introduction of their clinic guidelines (CMA, Citation2022b), showing compliance issues in the information provided about some of the add-ons under examination. Concerns were raised about the lack of information on risks, insufficient clinical evidence, and misrepresentation of the HFEA rating system.
Notably, the CMA review did not cover time-lapse imaging (TLI), which according to a recent study (Van de Wiel et al., Citation2020) is the most common add-on offered by UK fertility clinics. The popularity of TLI is confirmed by two HFEA patient surveys, which indicate that this is the second most common add-on after acupuncture and its use in IVF cycles has increased from 19% in 2018 to 27% in 2021 (HFEA, Citation2019, Citation2022).
To address this gap, this study investigates the provision of information on TLI through a systematic analysis of UK fertility clinic websites. Incorporating a camera inside the incubator, TLI allows continuous monitoring and recording of the development of embryos, providing valuable insights to fertility professionals – insights meant to aid in the selection of embryos most likely to lead to a successful pregnancy. Despite the various advantages TLI offers to professionals (see Perrotta & Geampana, Citation2020), it was ranked amber in the previous version of the HFEA rating system. This indicated inconclusive evidence regarding its effectiveness in enhancing live birth rates (LBR). In the recent rating update (HFEA, Citation2023), TLI has been reclassified as black, signifying sufficient evidence that it does not improve the chances of having a baby for most fertility patients, irrespective of manual or automated embryo analyses. The recent guidelines issued by the European Society of Human Reproduction and Embryology (ESHRE Add-ons working group, Lundin et al., Citation2023) confirm that although TLI incubators have proven to be convenient and effective for monitoring the continuous development of embryos, there is insufficient evidence to support their role in improving LBR or reducing time-to-pregnancy, regardless of the use of embryo selection software.
Although TLI is not considered a risk to patient or embryo health, concerns have been raised regarding its high cost and whether it is acceptable to charge patients for using TLI without evidence that it increases their chances of having a baby (Armstrong et al., Citation2015, Citation2019; Kieslinger et al., Citation2023). These concerns hold greater significance considering recent evidence assessments (ESHRE Add-ons working group, HFEA, Citation2023; Lundin et al., Citation2023).
With 74% of the cycles privately funded in 2021 (HFEA, Citation2024), patients must be provided with clear and accurate information to make fully informed decisions about the fertility treatment they are paying for. According to the CMA guidelines (CMA, Citation2021a), this should include information about costs, the potential add-on treatment benefits to the patient and, if relevant, any risks.
To conduct our analysis, we collected data on how TLI is presented on clinic websites in June 2022 – one year following the introduction of the CMA guidelines on how to present information to fertility patients. Drawing on these guidelines, we systematically analysed all the websites of UK fertility clinics. After a detailed description of the materials and methods, we present our analysis of how TLI is presented on clinics websites, its cost for patients, benefits and risks, and adherence to the CMA guidelines.
Materials and methods
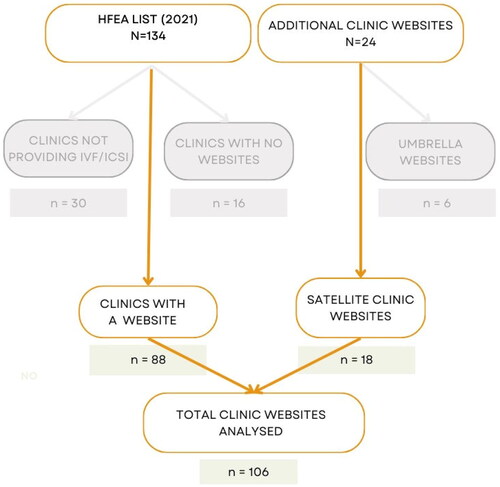
To identify all the fertility clinics in the UK offering TLI, we began by referring to the HFEA (Citation2021) list of licensed and active clinics for 2020/21, which consisted of 134 clinics (both NHS and private). We excluded 30 clinics that do not provide IVF/ICSI treatments (comprising of 14 clinics dedicated solely to storage and 16 clinics dedicated solely to research). We identified 104 clinics in total: 44 NHS and 60 private clinics. During the process of locating the clinics’ websites, we further excluded 16 clinics (10 NHS and 6 private) as their individual websites were not found. We identified an additional 24 websites, comprising six umbrella websites for groups of clinics and 18 additional websites of satellite clinics that are part of them. These satellite clinics were considered separate entries due to variations within the groups in terms of TLI availability, cost, and presentation of information. As a result, we analysed all 106 identified clinic websites (), comprising of 34 NHS clinics and 72 private clinics.
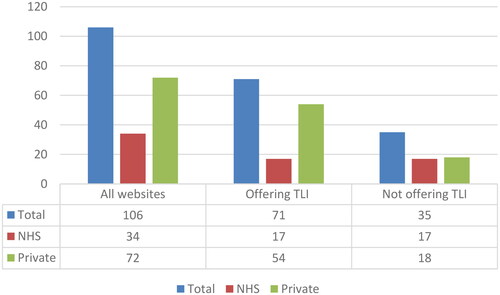
Our analysis of the 106 clinic websites revealed that 71 websites (67%) claimed to offer TLI as part of their treatment options. Among these clinics, 17 were NHS clinics that also provided treatments to self-funded patients, while 54 were private clinics. It is worth noting that while TLI appeared to be quite prevalent among the NHS clinics we analysed, with 50% (17 out of 34) offering it, it seemed to be even more commonplace among private clinics, with 75% (54 out of 72) providing this service (). It is important to mention that distinguishing between NHS and private clinics solely based on their websites was not always straightforward, and we had to rely on the official HFEA (Citation2021) data when available. This information may not be immediately accessible to patients seeking relevant information.
In June 2022, one of the authors (LZ) downloaded and saved as PDF files all webpages of these 71 websites containing information on TLI. These often included the main webpage if TLI was mentioned, a dedicated webpage to TLI, an additional webpage or separate PDF file with the pricelist, and any additional link or file related to TLI. Subsequently, all the information was anonymized and reported in a data matrix. In this matrix, a unique numeric code (from #1 to #71) was used instead of the name of the clinic. The data included in this matrix were organized under the following codes: 1. The description of TLI and any additional relevant information; 2. Information on the cost of TLI for patients; 3. Whether the website referred to the HFEA rating system webpage on TLI and the text introducing the link; 4. Statements on the (potential) benefits of TLI; 5. Statements on the (potential) risks of TLI; 6. Statements on the evidence supporting the effectiveness of TLI. This data matrix was then reviewed by two additional authors (MP and AG) to reach an agreement on codes, for instance whether a certain statement was presented as a clear benefit of TLI or a generic description of the intervention.
When consensus on the data matrix was reached among researchers, further rounds of analysis were performed, comprising analyses of the content, cost and adherence to CMA guidelines. For the latter, we draw on the guidelines included in the CMA consumer law compliance review of fertility clinics, which clarifies how consumer law applies to treatment add-ons:
Consumer law requires that existing and prospective patients are provided with material information at the time that they need it, and in a format that is clear and easy to understand. In our view this includes information about the risks, evidence base and the HFEA’s information about treatment add-ons, along with signposting to the HFEA’s website. This is so that the decisions patients make about whether to buy an add-on treatment are properly informed. (CMA Citation2022b, p. 64)
Results
In the following sections, prior to analysing adherence to CMA’s guidelines, we present a content analysis of how TLI is portrayed on clinic websites. It is worth noting that, according to CMA’s guidelines, the manner in which information on add-ons is presented may significantly influence patients’ decisions regarding their purchase.
We link any of the reported statements with the clinic websites, including information on the cost of TLI to patients (if available) or whether TLI is included in their standard package. As our interest is to analyse the provision of information on UK clinic websites as a whole rather than assessing how individual clinics provide information, we decided to anonymize the statements and refer to TLI or TLI-brand, when specific brands of TLI are mentioned.
How TLI is presented on clinic websites
Overall, TLI it is presented as an advanced, cutting-edge incubator technology. TLI is portrayed as a ground-breaking piece of laboratory equipment on both NHS and private websites, independently of whether clinics charge patients for TLI or not:
This state-of-the-art equipment allows our specialist laboratory team to closely monitor your embryo development in undisturbed conditions. (NHS clinic #1, included)
We are proud to include TLI as standard for all embryology. TLI-brand is the world’s premier and highest-profile ‘time-lapse’ embryo incubation and camera monitoring system. It captures detailed images of embryo development from the one cell zygote stage, shortly after fertilisation, right through to the fully expanded blastocyst. (NHS clinic #3, included)
Designed to provide an individualized, undisturbed, optimal and stable environment, TLI gives each embryo the very best chance to develop, from fertilization through to embryo transfer. The incorporation of one of the most advanced time-lapse camera systems allows our embryologists to observe embryo development stage by stage. Whilst embryo safety is assured as each chamber is independently controlled and checked by a class leading monitoring system. (private clinic #4, price £475)
Standard in-vitro fertilization takes place within the embryology laboratory following an egg collection. The embryologist will carefully combine the eggs and sperm within an environment designed to mimic the conditions of the womb. Fertilization is allowed to take place naturally, with minimal intervention from the laboratory. Once the Embryologist has identified those eggs which have fertilized and become embryos, they will be moved into our TLI incubator to be monitored for 3-5 days. (NHS clinic #69, price unclear)
Many descriptions of TLI underscore the technology’s capacity for monitoring embryos, and with a unique ability to facilitate the detailed study of embryo morphology:
The introduction of TLI allows us to monitor the developing embryos throughout the full course of their development without removing them from a stable incubated environment. The integrated camera and microscope automatically take an image of your embryos every 10 minutes to produce a time-lapse video of the vital stages of development enabling enhanced assessment by our team. This detailed development information allows us to identify only the best quality embryos for a future transfer procedure. (private clinic #35, price £500)
Clinics tend to stress TLI’s ability to take pictures of embryos at regular intervals as a novel feature, especially when compared to traditional observation where it is only the embryologist that sees the embryo once a day under the microscope. Many, but not all clinics, also explain that the images taken can be put together to form a video where embryo development can be observed. Despite other research (Hamper & Perrotta, Citation2023) indicating that this is a relatively common practice among clinics, only eight websites mention that patients can be provided with a video of their implanted embryo. It is not clear based on the information provided when the video would become available.
Adherence to guidelines
As pointed out above, our analysis of the information provided by clinic websites follows the tenets of CMA’s guidelines on what information should be offered to patients. This includes information about the costs, benefits, the evidence base for these statements, including HFEA’s information about treatment add-ons, and their risks.
Cost analysis
We analysed the 71 websites identified to examine whether clinics include TLI in their standard package or charge patients who opt to have TLI in their treatment. It is worth noting that the range cost of basic treatment varies significantly across clinics, between £3,190 and £7,750, with a mean of £4,380. As stressed by the CMA review (CMA, Citation2022a), a strict comparison of treatment prices is not possible, as clinics use very different ways of presenting them and basic packages across clinics do not include the same elements. However, we signal that the average cost of treatment offered among the 17 NHS clinics that treat privately funded patients is £3,819, against an average of £4,547 among the 54 private clinic websites examined.
Of the 71 clinic websites analysed (), 25 (35.2%) claim to charge a cost for TLI ranging between £300 and £850, with a mean of £614. Of these 25 clinics, 21 are private ones and four are NHS clinics. The latter charge patients for TLI £450, £500 and £850 (two clinics), respectively. Adding complexity to the scenario, among the clinics that charge patients for TLI, nine offer special packages in which TLI is included. These packages include a combination of additional treatments, such as PGT-A, ICSI, or other various ‘advanced’ laboratory techniques (as claimed on these websites).
Of the 46 remaining websites, 25 (35.2%) clearly state that TLI is included in the treatment and patients will not be charged for it. These include 11 NHS and 14 private clinics, with one of the NHS clinics specifying that TLI is available and included in the treatment but not guaranteed to all patients.
Despite stating that the clinic is equipped with TLI, the remaining 21 (29.6%) websites do not offer any information (including in their price lists) about the cost of TLI and it is not possible to determine from the websites whether TLI is included in their standard packages or not. These include two NHS clinics and 19 private ones.
How the benefits of TLI are presented
In line with the aim of this article and CMA guidelines, in this section we focus specifically on the claims that, implicitly or explicitly, suggest that TLI can increase patients’ chances of having a baby.
Of the 71 websites analysed (), only seven (9.9%) clinic websites do not make any claim on TLI ability to increase chances of success. Three of these websites (one private and two NHS clinics) just mention they are equipped with TLI but do not have any information available on it. The other four websites (one private and three NHS clinics) offer information on TLI without claiming any benefit in terms of clinical outcome.
The remaining 64 (90.1%) websites claim or imply improvements in clinical outcome due to the use of TLI. In what follows, we summarize the most common statements including some examples, while in the next section we discuss how the evidence base for these claims is presented. The total of these different statements is more than 64, as some of these websites make multiple claims. These claims vary significantly among clinics.
Three websites refer broadly to improvements in clinical outcome. For instance, among other advantages of TLI such as undisturbed culture or its ability to detect any abnormalities in cell division times and developmental behaviour, one of the websites claims that:
TLI-brand is the most widely adopted time-lapse system worldwide with documented improvements in clinical outcome. (NHS clinic #12, price £500)
Enhanced information available to help identify the embryos with the highest potential for pregnancy. (private clinic #28, included)
In IVF, TLI is used to help select the embryos most likely to successfully develop into a baby. (NHS clinic #38, included)
With the increased information from TLI of embryo development and subsequent detailed analysis the embryologists can more confidently select the best embryos for transfer, significantly improving the chance of a successful single-embryo transfer. […] TLI can aid the IVF expert when assessing your developing embryos and help in selecting the best embryo(s) for transfer or freezing to optimize the chances of a successful pregnancy. (private clinic #4, price £475)
Indeed, being undisturbed while they grow may improve the quality of the embryos. (private clinic #71, price unclear)
It records the embryo development and allows us to analyse and compare the growth of each embryo, enabling us to select or exclude embryos for ET based on key information that is not available or apparent using traditional methods of embryo grading. Use of this technology allows us to avoid using embryos that have undergone abnormal development and would not be expected to implant or have a low chance of success. (Private clinic #50, price unclear)
We have found that using TLI has also increased the proportion of patients who develop blastocysts and have embryos to freeze. (NHS clinic #11, price £450)
Although in theory this technology can be applied to any type of patient undergoing IVF treatment, the chances of an improvement in the results are greatest among patients who generate more embryos because there is a better potential for selection. TLI is an embryo-selection tool that helps us more when we have a lot of embryos to choose from. It can be used in cases where more information about the embryo is desired in situations where there is repeated implantation failure, advanced maternal age and history of recurrent miscarriage. It will help couples and women to make an informed decision about future treatment plan or closure as appropriate. TLI will not be suitable for all patient groups. Please discuss this with your consultant if this technology can be useful to you. Until good quality RCT evidence is available, this technology is offered only in certain circumstances such as repeated implantation failure, after counselling as to its cost effectiveness. (private clinic #29, price unclear)
Evidence base and signposting to the HFEA’s website
In the previous section, we presented the claims made about TLI benefits in relation to improving clinical outcomes. Most of the statements reported above did not report specific supporting evidence in the section of the websites where they were presented. A few referred to generic studies (without references) and some suggested the information was based on the clinical experience of the team.
In this section, we discuss the evidence presented to justify the ability of TLI to increase success rates, focusing on whether these statements align with the HFEA assessment and whether the clinic websites signpost to the HFEA website. As data collection occurred in 2022, in this paper we refer to the prior assessment of TLI in the rating classification, where it was ranked amber.
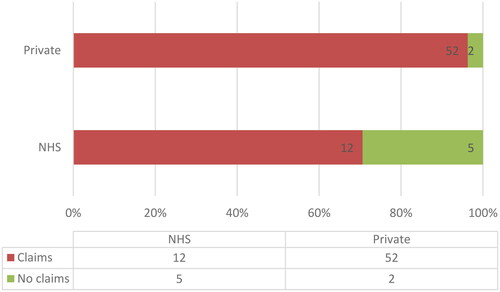
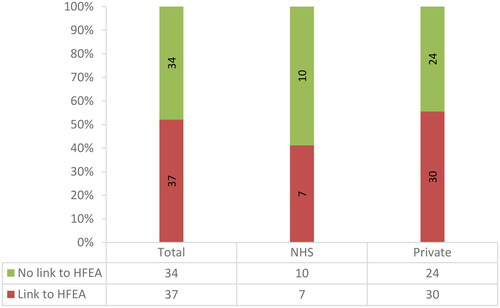
34 (47.9%) of the 71 websites analysed do not have any signpost to the HFEA rating system (). Of these 34, 10 are NHS clinics and 24 are private ones. Notably, none of the 7 clinics that do not make claims regarding the benefits of TLI in terms of clinical outcome include a signpost to the HFEA websites. 16 (22.5%) websites among those that include claims of TLI effectiveness do not present any alternative evidence supporting these claims. Among the remaining 11 (15.5%) websites, 10 private clinic websites mention non-specified studies suggesting that TLI is beneficial for some groups of patients. For example:
Studies suggest that embryos selected with the help of TLI have a high chance of forming a healthy pregnancy, so the technology will be especially welcome for patients with a poor reproductive record - that is, women who have already been unsuccessful in IVF and/or those of older reproductive age. (private clinic #39, price £850).
In addition, one NHS clinic refers to a study (including a link to the company webpage where this study is available) of one of the companies producing and marketing a specific brand of TLI, which suggests that centres who use their product have better implantation rates:
Newly released data has compared UK centres with at least one (specific brand of) TLI to centres without a TLI. The data reveals an implantation rate (IR) in UK centres with a TLI as 38.3% compared to 30.5% in centres without a TLI. Implantation rate is defined as the number of gestational sacs observed at the 6 weeks pregnancy scan divided by the number of embryos that were transferred. The ‘uplift’ in IR of around 8% is similar to that reported in many of the published papers on the subject. (NHS clinic #26, price £300)
For information from the HFEA on the risks and benefits of time lapse imaging click here. (private clinic #28, included)
For more information on supplementary treatments, please visit the HFEA rating system on the HFEA website. The rating system gives further details on the most common treatment add-ons and how effective they are. (NHS clinic #33, included)
TLI is an optional additional treatment to routine IVF treatment, to ensure our patients make an informed decision about whether using TLI as part of their treatment the HFEA provide further information which can be found on their website here. (private clinic #30, unclear)
Read further details below and for the latest on the effectiveness and safety of add-ons or adjuvants we recommend that you visit the HFEA website where our regulator has summarized the consensus of UK medical and scientific opinion. (private clinic #5, price £300)
We support HFEA’s view on add-ons, please visit their webpage for more information. (private clinic #66, price unclear)
It is important to remember that there are still relatively few robust research studies which show that TLI will increase the chances of success. Please visit Treatment add-ons with limited evidence | Human Fertilization and Embryology Authority for more information on treatment Add Ons. The Human Fertilization and Embryology Authority (HFEA) are a government regulator, who ensure that fertility clinics and research centres comply with the law. The HFEA have provided TLI an amber rating. An amber rating means there is a conflicting amount of evidence on the effectiveness of this add-on treatment for improving your chances of having a baby. As a result, further research is still needed for this treatment. The HFEA reveals research into time-lapse imaging shows ‘promise’ but is still too early to determine the effectiveness of this treatment. Initial data from studies support the idea that embryo selection or de-selection can be improved using TLI, and that embryo culture can be improved in an undisturbed environment. Both of these factors are important in improving the chance of success in IVF procedures. (private clinic #4, price £475)
Some retrospective studies have shown that embryos with specific division times and certain development patterns can have up to 15 – 20% better chance of pregnancy. The optimum times for cell division can be checked more easily and the chances of implantation improved in cases in which selection using TLI technology is possible. (private clinic #29, price unclear)
Our own data from a study published in 2017 of more than 23,000 treatment cycles showed a highly significant increase in births when TLI-algorithm was used to select embryos for patients aged younger than 38 using their own eggs. A paper published by us in 2019 showed that TLI-algorithm is superior for selecting embryos most likely to result in a birth than standard selection methods. (private clinic #13, price £850).
Early studies have shown an improvement in the chance of live birth by 56% over conventional methods of embryo selection. (private clinic #50, price unclear)
A recent study showed a correlation between assessing the embryos via TLI and chromosomal integrity of the embryos. Choosing the embryos of low risk chromosomal abnormality improved the pregnancy rate by 56%. However, this is a preliminary small study and the conclusion needs to be confirmed in bigger prospective studies. (private clinics #68, price £500).
Since TLI were introduced over eight years ago, over a million embryos have been cultured and there is now evidence that embryos cultured in a TLI will have a higher chance of implantation and a lower chance of early pregnancy loss. (private clinics #52, price £450).
How the risks of TLI are presented
According to the information available from the current HFEA website (2023) and prior versions, ‘time-lapse imaging and incubation do not carry any additional known risks for the person undergoing fertility treatment or any child born as a result of fertility treatment’. Although the relevance of the information on risk might be less concerning than other add-ons, this remains important in terms of adherence to CMA’s guidelines.
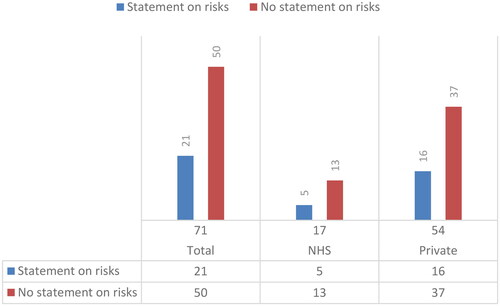
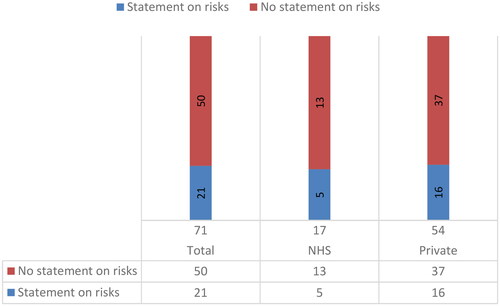
Of the 71 websites analysed (), only 21 (29.6%) offer information on the risks of TLI, including 5 NHS and 16 private clinics. The statements on the risks of TLI can be divided into two groups. 8 clinic websites report generic statements similar to the information of the HFEA: ‘there are no known risks to the woman or her embryos from TLI (NHS clinic #38, included; private clinic #71, unclear)’. The remaining 13 clinic websites report a more detailed statement. For instance, one of these clinic websites states:
There are no risks that have been identified from the use of TLI. It is possible, however, that TLI might identify that none of your embryos are suitable for transfer and if that happens your doctor will work with you to identify the best way forward, taking account of all of the information from your treatment cycle, the embryo monitoring and your medical history. (private clinic #25, price £850)
Discussion
This study aimed to investigate the provision of information on TLI through a systematic analysis of UK fertility clinic websites. The findings shed light on several important aspects related to the availability and transparency of information regarding TLI on these websites, and their adherence to regulatory guidelines.
The findings reported in this article are consistent with the existing body of literature examining the quality of information provided on fertility clinic websites globally (Abusief et al., Citation2007; Sauerbrun-Cutler et al., Citation2021). This alignment is observed across both earlier studies (Spencer et al., Citation2016) and more recent investigations (Galiano et al., Citation2021; Lensen et al., Citation2021; Van de Wiel et al., Citation2020), which have specifically explored the provision of information concerning add-on treatments.
One of the key findings of this study is that a significant number of clinics (71 out of 106) claim to offer TLI on their websites. TLI has gained recognition and acceptance within NHS settings, where a considerable proportion (50%) of clinics offered TLI as part of their services. Although TLI is quite prevalent in both NHS and private settings, it appears to have become an integral part of the repertoire of services provided by private clinics, with a 75% offering this technology.
The provision of cost information for TLI on fertility clinic websites is an important aspect to consider when patients make decisions about their treatments. However, the varying cost structures observed among the clinics raise concerns about financial transparency. Only over a third (35.2%) of the websites analysed clearly state that TLI is included in the treatment and patients would not be charged for it. Conversely, the same proportion of clinics (35.2%) charged patients a considerable fee to use TLI (between £300 and £850), indicating that the financial burden of TLI falls still largely on patients seeking treatment. Furthermore, while the vast majority of websites (70.4%) provided information on the cost of TLI, a significant portion (29.6%) either did not disclose the cost or omitted this crucial information. This lack of transparency can significantly impact patients’ decision-making processes, particularly when they are self-funded and need to consider the financial implications of TLI.
A concerning finding of this study is the lack of mention or signpost to the HFEA rating system on a considerable number of websites (47.9%). Despite its limitations (Lensen et al., Citation2023), the previous rating system provided patients with an easily understandable assessment of the most common fertility treatment add-ons, including TLI. The absence of this information may hinder patients’ ability to make informed decisions. In addition, CMA guidelines make it imperative for clinics to include a clear reference and link to the HFEA rating system to ensure transparency and facilitate patients’ access to crucial information.
More worryingly, most websites (90.1%) claimed or implied that TLI improves clinical outcomes by enhancing embryo selection. It is important to note that these claims are neither supported by the prior or current assessments conducted by the HFEA, the two available Cochrane reviews on TLI (Armstrong et al., Citation2015, Citation2019), or the ESHRE guidelines (ESHRE Add-ons working group, Lundin, et al., Citation2023). A significant percentage of websites (42.2%) claiming an increase on clinical outcome referenced early, unspecified studies that conflict with the HFEA’s evaluations. Only on a few occasions links to these studies or references are offered, but there is no clear discussion of what type of studies these are (mostly retrospective studies conducted by private companies). The discrepancy between the HFEA’s assessments and the conflicting evidence reported highlights the need for consistency and evidence-based claims on clinic websites. Patients rely on these websites as a primary source of information (CMA, Citation2020, Citation2022a; HFEA, Citation2019, Citation2022), and it is crucial that the information presented aligns with the most up-to-date and reliable scientific evidence.
Finally, the study indicates that fewer than a third (29.6%) of websites offer information regarding the potential risks associated with TLI. While this finding is less concerning given that TLI is generally considered safe for both patients and embryos, it confirmed an overall poor adherence to regulatory guidelines on information provision.
Limitations of the study
This study has three key limitations. The first and most significant limitation pertains to the nature of the data collected. It should be noted that websites are dynamic entities, constantly subject to changes in content and pricing. Therefore, the analysis presented here reflects the information and prices available on clinic websites at a specific point in time (June 2022). This timeframe was chosen because it marked one year after the introduction of guidelines by the CMA, which clearly outlined the expected information regarding add-ons. Given the dynamic nature of websites, it is possible that some of the data discussed in this study may already be out of date. We hope that, if they have not done already, these findings will prompt clinics to promptly update and improve the clarity and quality of information on TLI available on their websites, adhering to the CMA guidelines.
The second limitation of this study is that only the information appearing on clinic websites was analysed. This means that information could be shared through additional advertising materials (such as leaflets or information sent to patients), open events hosted by clinics, and in consultations. While we acknowledge that these sources are also relevant for patients seeking information, it is worth noting that both the HFEA patient surveys (2019, 2022) and the CMA research (Citation2020, Citation2022a) highlighted that clinic websites are a primary source of information for patients in the context of fertility treatment.
The third limitation pertains to the unavailability of data, either from the HFEA or other sources, regarding the usage of TLI by specific clinics and the number of cycles in which it is used. This limitation implies that we are unable to verify whether clinics claiming to offer TLI actually provide this service to their patients, or whether clinics that do not mention TLI on their websites may still offer it. The lack of data on TLI usage and availability poses a challenge in accurately assessing the actual provision of this technology by clinics.
These limitations should be taken into account when interpreting the findings of this study. Despite these limitations, the analysis of clinic websites provides valuable insights into the provision, transparency, and adherence to regulatory guidelines of information regarding TLI. Further research is warranted to address these limitations and obtain a more comprehensive understanding of the availability and usage of TLI across clinics.
Conclusion
The case of TLI raises important considerations regarding the innovation model of fertility care. Challenges in generating reliable data on the effectiveness of add-on treatments (see Perrotta & Geampana, Citation2021) are often used as a justification to introduce interventions before robust evidence of their efficacy is available. While this practice raises concerns regarding the allocation of public funds for health services, ensuring transparency of information becomes paramount in a sector where patients bear the financial burden of their treatment, essentially subsidizing research into novel interventions. Previous research (Perrotta & Geampana, Citation2020) has highlighted various perceived advantages of TLI, including its utility as a laboratory tool, its potential for knowledge generation in embryology, and its role in managing patient expectations and treatment processes. In addition, ESHRE’s guidelines (ESHRE Add-ons working group, Lundin, et al., Citation2023) confirm that TLI is a convenient and effective tool for observing the continuous development of embryos. Nevertheless, the justification for directly charging patients for an advanced technology that does not increases their chances of success (ESHRE Add-ons working group, Lundin, et al., Citation2023; HFEA, Citation2023) remains unclear, particularly when clinic websites predominantly emphasize an alleged improvement in clinical outcomes as the primary benefit. This issue gains significance in light of recent research demonstrating that individuals can easily be misled when multiple outcomes are reported (Wilkinson & Stocking, Citation2021), mistakenly equating any rise in clinical outcomes with an actual increase in their chances of achieving pregnancy (Carrick et al., Citation2023).
Previous studies (Perrotta & Hamper, Citation2021, Citation2023) have shown that patients often feel compelled to explore any available interventions that could enhance their chances of success in order to avoid future regret. Additionally, research has revealed that many patients rely on clinic websites as trusted sources of information (CMA, Citation2020, Citation2022a; HFEA, Citation2019, Citation2022). Therefore, the information provided on clinic websites is crucial not only for individuals considering treatment at specific clinics but also for prospective and current patients seeking reliable information. The observed discrepancies in cost transparency, unsupported claims on clinical outcomes, and lack of adherence to regulatory guidelines raise concerns about the reliability and accuracy of the information provided on these websites.
While the article highlights whether clinics charge for TLI or not, it is important to note that inaccurate information on clinic websites can be harmful to all prospective and current patients who rely heavily on the information provided by clinics – information that they expect to be trustworthy. Overall, the increasing prevalence of TLI among clinics, particularly within the standard offerings of private clinics, may lead patients to assume that it significantly enhances their chances of achieving successful outcomes.
Without clear and accurate information, patients are left without the necessary tools to make well-informed choices about their treatment. Therefore, fertility clinics should prioritize the enhancement of their websites to ensure the provision of accurate and evidence-based information, thereby empowering patients to make informed decisions regarding their fertility treatment.
In conclusion, the findings presented in this article underscore several policy implications in the UK. Despite the establishment of information standards in the sector and clinics’ obligations under consumer law, compliance with these standards remains inadequate. Therefore, it is imperative to implement additional measures to ensure fertility clinics adhere to existing guidelines provided by the CMA for website information disclosure. For instance, the HFEA has advocated for reforming the Human Fertilisation and Embryology Act to monitor and enforce compliance with information standards, potentially including the imposition of financial fines. Moreover, introducing clear roles of responsibility for website maintenance and information provision beyond the legal requirements is crucial to prevent further erosion of trust in the sector. Clarifying these responsibilities will enhance transparency and accountability, ultimately benefiting patients seeking fertility treatment.
Author contribution statement
MP conceived the idea for the study and obtained a small grant from her institution to support data collection. LZ, AG, and PB were involved in the design of the study. LZ undertook data collection in June 2022. Analysis was undertaken by LZ, MP and AG. MP wrote the initial draft with the support of AG and LZ. All authors edited the first draft and approve the final version for submission.
Acknowledgements
The authors thank the School of Business and Management at Queen Mary University of London for their support in data collection through a small grant.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, MP, upon reasonable request.
References
- Abusief, M. E., Hornstein, M. D., & Jain, T, American Society for Reproductive Medicine, & Society for Assisted Reproductive Technology. (2007). Assessment of United States fertility clinic websites according to the American Society for Reproductive Medicine (ASRM)/Society for Assisted Reproductive Technology (SART) guidelines. Fertility and Sterility, 87(1), 88–92. https://doi.org/10.1016/j.fertnstert.2006.05.073
- Armstrong, S., Arroll, N., Cree, L. M., Jordan, V., & Farquhar, C. (2015). Time-lapse systems for embryo incubation and assessment in assisted reproduction. The Cochrane Database of Systematic Reviews, 2015(2), CD011320. https://doi.org/10.1002/14651858.CD011320.pub2
- Armstrong, S., Bhide, P., Jordan, V., Pacey, A., Marjoribanks, J., & Farquhar, C. (2019). Time-lapse systems for embryo incubation and assessment in assisted reproduction. The Cochrane Database of Systematic Reviews, 5(5), CD011320. https://doi.org/10.1002/14651858.CD011320.pub4
- Carrick, M., Wilkinson, J., Polyakov, A., Kirkham, J., & Lensen, S. (2023). How do IVF patients interpret claims about fertility treatments? A randomised survey experiment. Human Fertility (Cambridge, England), 26(2), 347–354. https://doi.org/10.1080/14647273.2023.2191222
- Galiano, V., Orvieto, R., Machtinger, R., Nahum, R., Garzia, E., Sulpizio, P., Marconi, A. M., & Seidman, D. (2021). Add-Ons" for assisted reproductive technology: Do patients get honest information from fertility clinics’ websites? Reproductive Sciences (Thousand Oaks, Calif.), 28(12), 3466–3472. https://doi.org/10.1007/s43032-021-00601-7
- Hamper, J., & Perrotta, M. (2023). Watching embryos: Exploring the geographies of assisted reproduction through encounters with embryo imaging technologies. Social & Cultural Geography, 24(9), 1557–1575. https://doi.org/10.1080/14649365.2022.2073467
- Harper, J., Jackson, E., Sermon, K., Aitken, R. J., Harbottle, S., Mocanu, E., Hardarson, T., Mathur, R., Viville, S., Vail, A., & Lundin, K. (2017). Adjuncts in the IVF laboratory: where is the evidence for 'add-on’ interventions? Human Reproduction (Oxford, England), 32(3), 485–491. https://doi.org/10.1093/humrep/dex004
- Heneghan, C., Spencer, E. A., Bobrovitz, N., Collins, D. R., Nunan, D., Plüddemann, A., Gbinigie, O. A., Onakpoya, I., O'Sullivan, J., Rollinson, A., Tompson, A., Goldacre, B., & Mahtani, K. R. (2016). Lack of evidence for interventions offered in UK fertility centres. BMJ (Clinical Research ed.), 355, i6295. https://doi.org/10.1136/bmj.i6295
- Human Fertilisation and Embryology Authority (HFEA). (2019). Our national patient survey results. https://www.hfea.gov.uk/about-us/news-and-press-releases/2018-news-and-press-releases/our-national-patient-survey-results/
- Human Fertilisation and Embryology Authority (HFEA). (2021). State of the fertility sector 2020/21. https://www.hfea.gov.uk/about-us/publications/research-and-data/state-of-the-fertility-sector-2020-2021/
- Human Fertilisation and Embryology Authority (HFEA). (2022). National Patient Survey 2021. https://www.hfea.gov.uk/about-us/publications/research-and-data/national-patient-survey-2021
- Human Fertilisation and Embryology Authority (HFEA). (2023). Treatment add-ons with limited evidence. https://www.hfea.gov.uk/treatments/treatment-add-ons/
- Human Fertilisation and Embryology Authority (HFEA). (2024). HFEA dashboard. https://www.hfea.gov.uk/about-us/hfea-dashboard/
- Kieslinger, D. C., Vergouw, C. G., Ramos, L., Arends, B., Curfs, M. H. J. M., Slappendel, E., Kostelijk, E. H., Pieters, M. H. E. C., Consten, D., Verhoeven, M. O., Besselink, D. E., Broekmans, F., Cohlen, B. J., Smeenk, J. M. J., Mastenbroek, S., de Koning, C. H., van Kasteren, Y. M., Moll, E., van Disseldorp, J., … Lambalk, C. B. (2023). Clinical outcomes of uninterrupted embryo culture with or without time-lapse-based embryo selection versus interrupted standard culture (SelecTIMO): a three-armed, multicentre, double-blind, randomised controlled trial. Lancet (London, England), 401(10386), 1438–1446. https://doi.org/10.1016/S0140-6736(23)00168-X
- Lensen, S., Armstrong, S., Vaughan, E., Caughey, L., Peate, M., Farquhar, C., Pacey, A., Balen, A. H., & Wainwright, E. (2023). It all depends on why it’s red": qualitative interviews exploring patient and professional views of a traffic light system for IVF add-ons. Reproduction and Fertility, 4(2), e220136. Advance online publication. https://doi.org/10.1530/RAF-22-0136
- Lensen, S., Chen, S., Goodman, L., Rombauts, L., Farquhar, C., & Hammarberg, K. (2021). IVF add-ons in Australia and New Zealand: A systematic assessment of IVF clinic websites. The Australian and New Zealand Journal of Obstetrics and Gynaecology, 61(3), 430–438. https://doi.org/10.1111/ajo.13321
- Lundin, K., Bentzen, J. G., Bozdag, G., Ebner, T., Harper, J., Le Clef, N., Moffett, A., Norcross, S., Polyzos, N. P., Rautakallio-Hokkanen, S., Sfontouris, I., Sermon, K., Vermeulen, N., & Pinborg, A, ESHRE Add-ons working group. (2023). Good practice recommendations on add-ons in reproductive medicine†. Human Reproduction (Oxford, England), 38(11), 2062–2104. https://doi.org/10.1093/humrep/dead184
- Perrotta, M., & Geampana, A. (2020). The trouble with IVF and randomised control trials: Professional legitimation narratives on time-lapse imaging and evidence-informed care. Social Science & Medicine (1982), 258, 113115. https://doi.org/10.1016/j.socscimed.2020.113115
- Perrotta, M., & Geampana, A. (2021). Enacting evidence-based medicine in fertility care: Tensions between commercialisation and knowledge standardisation. Sociology of Health and Illness, 43(9), 2015–2030. https://doi.org/10.1111/1467-9566.13381
- Perrotta, M., & Hamper, J. (2021). The crafting of hope: Contextualising add-ons in the treatment trajectories of IVF patients. Social Science and Medicine (1982), 287, 114317. https://doi.org/10.1016/j.socscimed.2021.114317
- Perrotta, M., & Hamper, J. (2023). Patient informed choice in the age of evidence-based medicine: IVF patients’ approaches to biomedical evidence and fertility treatment add-ons. Sociology of Health and Illness, 45(2), 225–241. https://doi.org/10.1111/1467-9566.13581
- Sauerbrun-Cutler, M. T., Brown, E. C., Huber, W. J., Has, P., & Frishman, G. N. (2021). Society for assisted reproductive technology advertising guidelines: How are member clinics doing? Fertility and Sterility, 115(1), 104–109. https://doi.org/10.1016/j.fertnstert.2020.07.001
- Spencer, E. A., Mahtani, K. R., Goldacre, B., & Heneghan, C. (2016). Claims for fertility interventions: A systematic assessment of statements on UK fertility centre websites. BMJ Open, 6(11), e013940. https://doi.org/10.1136/bmjopen-2016-013940
- The Competition and Markets Authority (CMA). (2020). Self-funded IVF Research: Qualitative Research Report. https://assets.publishing.service.gov.uk/media/5fa01b30e90e070420702a1b/IVF_Research_Final_Report.pdf
- The Competition and Markets Authority (CMA). (2021a). Fertility treatment: A guide for Clinics. https://www.gov.uk/government/publications/fertility-treatment-a-guide-for-clinics
- The Competition and Markets Authority (CMA). (2021b). Fertility treatment: A guide to your consumer rights. https://www.gov.uk/government/publications/fertility-treatment-a-guide-to-your-consumer-rights
- The Competition and Markets Authority (CMA). (2022a). Patients’ experiences of buying fertility treatment: Qualitative Research Report. https://assets.publishing.service.gov.uk/media/632c2fefe90e073721b08402/Consumer_research_report_160922.pdf
- The Competition and Markets Authority (CMA). (2022b). Consumer law compliance review of fertility clinics: Findings report. https://assets.publishing.service.gov.uk/media/632d65af8fa8f51d1f83391a/A._Final_findings_report.pdf
- van de Wiel, L., Wilkinson, J., Athanasiou, P., & Harper, J. (2020). The prevalence, promotion and pricing of three IVF add-ons on fertility clinic websites. Reproductive Biomedicine Online, 41(5), 801–806. https://doi.org/10.1016/j.rbmo.2020.07.021
- Wilkinson, J., & Stocking, K. (2021). Study design flaws and statistical challenges in evaluating fertility treatments. Reproduction and Fertility, 2(2), C9–C21. https://doi.org/10.1530/RAF-21-0015