Abstract
Fertility restoration potential of immature testicular tissue (ITT) depends on the number of spermatogonial cells in the retrieved tissue prior to cryopreservation in oncofertility programme. There are limited data on the association between type of malignancy and testicular germ cell population. Hence, this study is aimed to investigate the spermatogonial and Sertoli cell population in ITT retrieved from 14 pre-pubertal boys who opted for fertility preservation. Histopathological and immunochemical analysis of seminiferous tubules from haematological (N = 7) and non-haematological (N = 7) malignant patients revealed 3.43 ± 2.92 and 1.71 ± 1.81 spermatogonia per tubular cross section (S/T), respectively. The Sertoli cell number was comparable between haematological and non-haematological group (18.42 ± 3.78 and 22.03 ± 10.43). Spermatogonial quantity in ITT did not vary significantly between haematological and non-haematological cancers. This observation, though preliminary, would contribute to the limited literature on paediatric male oncofertility.
Introduction
Cancer treatment–induced gonadotoxicity has been extensively reported in the literature; however, limited data are available to demonstrate the association between the cancer pathophysiology and testicular population in prepubertal boys. Cancer pathology can affect reproductive health through mechanisms such as hypothalamus–pituitary gonadal axis disruption or through direct impact on the testicular environment by cancer cell invasion or the pro-inflammatory responses associated with the underlying malignancy (Delgouffe et al., Citation2022). While this impairment of gonadal function can be reflected in the sperm quality in adolescents or young adult males (Poorvu et al., Citation2019), in prepubertal boys, quantification of germ cells or spermatogonia can be an important determinant in risk prediction of male infertility (Poganitsch-Korhonen et al., Citation2017). However, there is a dearth of literature on understanding the effect of cancer disease on the prepubertal germ cell pool in the testis. Though cancer or a haematologic disorder can negatively affect the testicular function and germ cell quantity in prepubertal boys (Gille et al., Citation2021; Stukenborg et al., Citation2018; Wigny et al., Citation2016), the results from these studies are inconsistent. However, a recent report has shown that spermatogonial quantity is reduced in the testes of prepubertal boys diagnosed with cancer or severe haematological disorder compared to healthy controls and the decline is disease and age dependent (Masliukaite et al., Citation2023).
From the oncofertility perspective, understanding the impact of age or cancer type on the testicular population in paediatric patients would allow for optimization of fertility preservation and restoration techniques as well as enable effective fertility preservation counselling. Hence, in this study, spermatogonial and Sertoli cell quantities and other testicular characteristics in ITTs from prepubertal boys undergoing fertility preservation were compared between haematological and non-haematological cancers.
Material and methods
Parents or legal guardians of prepubertal patients were counselled along with their wards for ITT cryopreservation as a part of the fertility preservation programme and informed consent was taken in accordance with the Kasturba Medical College and Kasturba Hospital Institutional Ethics Committee guidelines (IEC#32/2015). Immature testicular tissue was collected in Leibovitz medium (L-15 Medium; # 11415064, Invitrogen, UK), supplemented with 1% human serum albumin (# A9511, Sigma-Aldrich, USA) on ice. ITT cryopreservation was performed by the slow-freezing method at Centre for Fertility Preservation. Prior to cryopreservation, approximately 10% of tissue was fixed for histopathology, to rule out malignancy and to assess the pre-freeze spermatogonial, Sertoli cell quantities and other testicular characteristics. One part of the ITT fragments was used for immunostaining and other part subjected to haematoxylin–eosin (H&E) staining. H and E stained slides were observed under a light microscope and images were captured (DP20 and DP25; DP2-BSW version 1.2, Olympus corporation, Japan) for histological assessment of the seminiferous tubules. The spermatogonia and Sertoli cells within the histology cross-section of seminiferous tubules were identified based on cell shape, location, and appearance of nuclei (). Spermatogonia were identified as cells located in the basal region, round to oval nuclei with open chromatin (Type Apale) or dark condensed chromatin (Type Adark or Type B). In this study type A and B spermatogonia were not counted separately. Sertoli cells were identified as cells located directly on the tubular basement membrane, with dark round nuclei and were surrounded by germ cells in different stages of maturation (Fietz & Bergmann, Citation2017).
Figure 1. Representative images of histology (A) and immunofluorescence with DDX4/VASA (B) used for spermatogonial and Sertoli cell quantification. White stars and black arrows in figure A depict spermatogonia and Sertoli cells, respectively. Scale bar in A represents 100 µm; in B represents 50 µm.
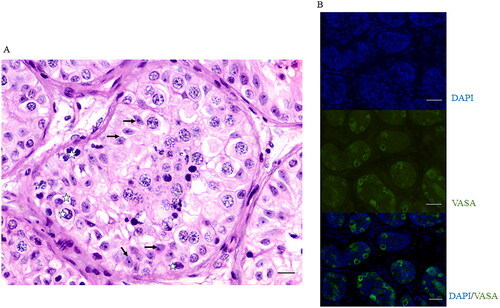
Immunostaining was done using germ cell marker Rabbit anti-DDX4/VASA, (#ab13840, Abcam, UK) and then stained with Alexa flour 488, Goat anti-Rabbit IgG (A11011, Invitrogen UK). ImageJ/Fiji software (ImageJ, USA) was used to manually count the germ cells that showed positive signals for DDX4/VASA, per seminiferous tubule in a cross section. Tubules that did not show any signal for germ cells were designated as germ cell negative tubules.
In each cross-section of the testis stained through H&E or immunofluorescence, the total number of seminiferous tubules, number of spermatogonia and number of Sertoli cells per tubular cross section, were scored (). Spermatogonia in a minimum of 25 seminiferous tubules per cross section were counted to determine the spermatogonia per tubular cross section (S/T). Descriptive statistics such as mean ± SD and further statistical analysis including Normality test or Shapiro Wilk test, Independent samples T-test were performed using Jamovi (Version 2.3, Open-source software; GNU Affero general public license) and graphs were prepared with Origin 6 (Microcal Software. Inc, USA) where the significance between the groups was assessed. p < 0.05 was set for statistical significance.
Results
A total of 14 patients were recruited for ITT banking between December 2015 to September 2020, fulfilling the Edinburgh selection criteria (Anderson et al., Citation2015). Patients with previous chemotherapy or radiotherapy exposure were excluded from the study. The mean age of the patients was 8 ± 3.5 years. Patients presented with different stages of cancer at the time of diagnosis, with 67% of the patients diagnosed at stage III of cancer, 22% at stage II and 11% at stage I of cancer (). The mean time interval from time of diagnosis to fertility preservation was 6.5 ± 8.3 days. None of the patients had a history of exposure to cancer therapy.
Table 1. Type of cancer and testicular cell quantities in study subjects.
Of the 14 patients included histopathology reports of two patients (11 and 12-month-old) showed dense, fibrous, and inadequate tissue to appreciate the seminiferous tubules or assess spermatogonia, hence were excluded from the analysis. Spermatogonial quantification in the remaining patients was done through scoring of S/T on H and E sections () and complemented with DDX4/VASA immunostaining (). To maintain uniformity, a minimum of 25 seminiferous tubules were counted per patient section, wherever possible (). The average number of S/T scored using H&E sections for all the patients was 2.72 ± 2.58 compared to manual counting of DDX4/VASA positive germ cells with the average S/T as 5.31 ± 5.5. Spermatogonial quantification between H and E and DDX4/VASA staining showed no statistically significant difference (). The number of S/T for the study subjects were compared with that of normative spermatogonial values for healthy prepubertal boys established agewise (Masliukaite et al., Citation2016) (). The S/T in 5–7 years age group was 0.65 ± 0.21 (range 0.48–0.96) which was 2- to 3-fold lower than the normative values reporting 2.5 ± 0.07 (range 2.1–3.1). However, statistical significance was not demonstrated due to small sample size.
Figure 2. Spermatogonial and Sertoli cells in immature testicular tissue of prepubertal boys according to age. (A) Comparison of Spermatogonia per tubular cross-section (S/T) between the two methods of scoring, according to age, with that of the established reference values. Cyan coloured squares represent number of S/T through H&E staining, pink circles represent number of S/T through DDX4/VASA immunostaining and the black triangles represent the normative values established in healthy prepubertal boys. No statistical significance was seen between the methods of scoring, according to age. (B) Sertoli cells per tubular cross section in the study subjects showed no statistical significance according to age.
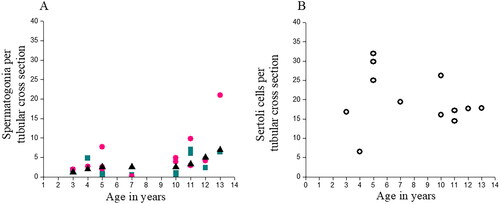
Sertoli cells were also quantified per tubular cross section through H and E images and the average number of Sertoli cells was 19.93 ± 7.13 (range 6.52–31.92) per tubular cross section. The number of cells increased between 5 and 10 years (range 19.44–31.92) with a reduction at 11–13 years (range 14.48–17.84). No statistical significance was observed ().
Spermatogonial quantity was assessed between haematological or non-haematological cancers to determine the impact of systemic effects of underlying malignancies on the testicular population. The average spermatogonial quantity (S/T) in the haematological and non-haematological groups was 3.43 ± 2.92 and 1.71 ± 1.81, respectively. Similarly, the Sertoli cell quantity was 18.42 ± 3.78 and 22.03 ± 10.43 per tubular cross section of haematological and non-haematological cancers respectively. The number of germ cell negative tubules was also comparable between the groups (5.1 in haematological vs 6.6 in non-haematological). There was no statistically significant difference in any of the above parameters between the two cancer groups.
Of the 14 patients, 10 of them are alive and in remission (71.4%), while two patients were lost to follow-up (14.2%) and two patients expired (14.2%). None of the patients have visited the centre for fertility restoration, at the time of the manuscript preparation.
Discussion
The findings of the present study showed that age-based S/T in the patients up to 7 years was approximately 1.5 which then increased to 3.9 at the age of 11–13 years. The increase in spermatogonial quantity with age was comparable to the normative values in healthy prepubertal males which reported 2.5–2.6 (0–7 years) and up to 7.0 (at puberty) (Masliukaite et al., Citation2016). Though, a reduction in the S/T was observed in 5- to 7-year age group compared to the normative values, the difference was non-significant which agrees with a recent study (Masliukaite et al., Citation2023). These observations suggest that spermatogonial quantity is not likely to be affected in the prepubertal boys diagnosed with cancer compared to healthy controls.
When the prepubertal patient data was compared between haematological or non-haematological cancer, it was revealed that the spermatogonial quantity was comparable between the two cancer types. This observation is in agreement with an earlier report where no significant variation in germ cell numbers was found between malignancies or non-malignant haematological disorders in prepubertal patients recruited for fertility preservation (Valli-Pulaski et al., Citation2019). On the contrary, an overall reduction in S/T was found in prepubertal patients diagnosed with cancer or severe haematological disorders when compared to controls, in another study (Masliukaite et al., Citation2023). Specifically, patients with CNS tumours and haematological disorders, such as sickle cell disease presented with the lowest spermatogonial quantity (Masliukaite et al., Citation2023).
About half of the patients included in the present study had malignant solid tumours such as Wilm’s tumour, Ewing’s sarcoma, Pheochromocytoma and cancer of the neck, none of which have shown to have a direct effect on the testis (Costabile, Citation1993). When testicular tissue in prepubertal patients diagnosed with lymphoma or sarcomas was compared with that of patients with Klinefelter’s syndrome, cryptorchidism or patients who have undergone chemotherapy, no significant changes in spermatogonial population was observed (Heckmann et al., Citation2018). This could be one of the reasons the spermatogonial quantity was comparable with that of the normative values in our patient cohort.
In the context of prepubertal oncofertility, it is important to determine the Sertoli cell number in cancer affected prepubertal boys according to age and malignancy. In this study, Sertoli cell quantity per tubular cross section according to age showed an increasing number of Sertoli cells between the age of 5–10 years which then declined at the age of 11–13 years. While this finding is in line with previous reports showing an inverse correlation between increasing age and Sertoli cell number (Petersen et al., Citation2015; Sharpe et al., Citation2003), it contrasts with earlier studies reporting a constant number of Sertoli cells between 0 and 3 months of age up to 10–11years with the total Sertoli cell number being 750 × 106 per testis (Cortes et al., Citation1987; Nistal et al., Citation1982).
While the present study showed that the Sertoli cell number was comparable between haematological and non-haematological cancers, due to the paucity in literature on the Sertoli cell quantity in human immature testicular tissue of healthy controls or of other patients recruited for fertility preservation in other studies, it was difficult to compare our results. The present study is limited in that the nuclear volume and change in size of the Sertoli cells could not be determined to understand functionality of the Sertoli cells in cancer affected prepubertal testis.
Due to ethical restrictions, it was not possible to include healthy control group in this study, hence normative values from literature were used for reference (Masliukaite et al., Citation2016). The low sample size of the study could be attributed to a short duration between diagnosis and treatment, due to presentation of children at the advanced stage of cancer to the healthcare facility (Ganguly et al., Citation2021). The recruitment of patients for fertility preservation was further affected by the pandemic. Considering the low sample size, the data may have a component of bias. Post-thaw analysis and fertility restoration of ITT were not attempted hence it may be premature to state that the fertility outcomes between haematological and non-haematological cancers can be similar.
In conclusion, the study shows that spermatogonial and Sertoli cell quantities were comparable between haematological and non-haematological cancers. As the majority of the patients were in the stage II or III of cancer at the time of ITT retrieval, the severity of the malignancy is not likely to have an impact on the germ cell population which is reassuring in the scope of fertility preservation and restoration. Our findings, though small, could contribute to the emerging field of paediatric male oncofertility and would enable a positive approach for health professionals involved in fertility preservation counselling.
Author contribution
Conceived and designed the experiments: SKA. Performed the experiments and acquisition of data: PT, SLL. Analyzed and interpreted the data: PT, VLR. Wrote the manuscript: SKA, PT. Revised the manuscript critically for important intellectual content: VKB, VKP, SU, GK, NS. PT is the guarantor of this work and, as such, has full access to all the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have given final approval for publication.
Ethical approval
The study was approved by Kasturba Medical College and Kasturba Hospital Institutional Ethics Committee guidelines (IEC#32/2015).
Acknowledgements
The authors thank all the prepubertal cancer patients who gave consent to participate in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data can be made available on request.
Additional information
Funding
References
- Anderson, R. A., Mitchell, R. T., Kelsey, T. W., Spears, N., Telfer, E. E., & Wallace, W. H. (2015). Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. The Lancet. Diabetes & Endocrinology, 3(7), 556–567. https://doi.org/10.1016/S2213-8587(15)00039-X
- Cortes, D., Müller, J., & Skakkebaek, N. E. (1987). Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. International Journal of Andrology, 10(4), 589–596. https://doi.org/10.1111/j.1365-2605.1987.tb00358.x
- Costabile, R. A. (1993). The effects of cancer and cancer therapy on male reproductive function. The Journal of Urology, 149(5 Pt 2), 1327–1330. https://doi.org/10.1016/s0022-5347(17)36384-x
- Delgouffe, E., Braye, A., & Goossens, E. (2022). Testicular tissue banking for fertility preservation in young boys: Which patients should be included? Frontiers in Endocrinology, 13, 854186. https://doi.org/10.3389/fendo.2022.854186
- Fietz, D., & Bergmann, M. (2017). Functional anatomy and histology of the testis. In: M. Simoni, & I. Huhtaniemi (Eds.), Endocrinology of the testis and male reproduction. Endocrinology. (pp. 1–29). Springer. https://doi.org/10.1007/978-3-319-29456-8_9-1
- Ganguly, S., Kinsey, S., & Bakhshi, S. (2021). Childhood cancer in India. Cancer Epidemiology, 71(Pt B), 101679. https://doi.org/10.1016/j.canep.2020.101679
- Gille, A.S., Pondarré, C., Dalle, J.H., Bernaudin, F., Chalas, C., Fahd, M., Jean, C., Lezeau, H., Riou, L., Drouineaud, V., Paye-Jaouen, A., Kamdem, A., Neven, B., Arnaud, C., Azarnoush, S., Yakouben, K., Sarnacki, S., de Montalembert, M., Comperat, E. M., … Barraud-Lange, V. (2021). Hydroxyurea does not affect the spermatogonial pool in prepubertal patients with sickle cell disease. Blood, 137(6), 856–859. https://doi.org/10.1182/blood.2020008146
- Heckmann, L., Langenstroth-Röwer, D., Pock, T., Wistuba, J., Stukenborg, J. B., Zitzmann, M., Kliesch, S., Schlatt, S., & Neuhaus, N. (2018). A diagnostic germ cell score for immature testicular tissue at risk of germ cell loss. Human Reproduction (Oxford, England), 33(4), 636–645. https://doi.org/10.1093/humrep/dey025
- Masliukaite, I., Hagen, J. M., Jahnukainen, K., Stukenborg, J. B., Repping, S., van der Veen, F., van Wely, M., & van Pelt, A. M. (2016). Establishing reference values for age-related spermatogonial quantity in prepubertal human testes: a systematic review and meta-analysis. Fertility and Sterility, 106(7), 1652–1657.e2. https://doi.org/10.1016/j.fertnstert.2016.09.002
- Masliukaite, I., Ntemou, E., Feijen, E. A. M., van de Wetering, M., Meissner, A., Soufan, A. T., Repping, S., Kremer, L. M. C., Jahnukainen, K., Goossens, E., & van Pelt, A. M. M. (2023). Childhood cancer and hematological disorders negatively affect spermatogonial quantity at diagnosis: a retrospective study of a male fertility preservation cohort. Human Reproduction (Oxford, England), 38(3), 359–370. https://doi.org/10.1093/humrep/dead004
- Nistal, M., Abaurrea, M. A., & Paniagua, R. (1982). Morphological and histometric study on the human Sertoli cell from birth to the onset of puberty. Journal of Anatomy, 134(Pt 2), 351–363.
- Petersen, P. M., Seierøe, K., & Pakkenberg, B. (2015). The total number of Leydig and Sertoli cells in the testes of men across various age groups - a stereological study. Journal of Anatomy, 226(2), 175–179. https://doi.org/10.1111/joa.12261
- Poganitsch-Korhonen, M., Masliukaite, I., Nurmio, M., Lähteenmäki, P., van Wely, M., van Pelt, A. M. M., Jahnukainen, K., & Stukenborg, J. B. (2017). Decreased spermatogonial quantity in prepubertal boys with leukaemia treated with alkylating agents. Leukemia, 31(6), 1460–1463. https://doi.org/10.1038/leu.2017.76
- Poorvu, P. D., Frazier, A. L., Feraco, A. M., Manley, P. E., Ginsburg, E. S., Laufer, M. R., LaCasce, A. S., Diller, L. R., & Partridge, A. H. (2019). Cancer treatment-related infertility: A critical review of the evidence. JNCI Cancer Spectrum, 3(1), pkz008. https://doi.org/10.1093/jncics/pkz008
- Sharpe, R. M., McKinnell, C., Kivlin, C., & Fisher, J. S. (2003). Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction (Cambridge, England), 125(6), 769–784. https://doi.org/10.1530/rep.0.1250769
- Stukenborg, J. B., Jahnukainen, K., Hutka, M., & Mitchell, R. T. (2018). Cancer treatment in childhood and testicular function: the importance of the somatic environment. Endocrine Connections, 7(2), R69–R87. https://doi.org/10.1530/EC-17-0382
- Valli-Pulaski, H., Peters, K. A., Gassei, K., Steimer, S. R., Sukhwani, M., Hermann, B. P., Dwomor, L., David, S., Fayomi, A. P., Munyoki, S. K., Chu, T., Chaudhry, R., Cannon, G. M., Fox, P. J., Jaffe, T. M., Sanfilippo, J. S., Menke, M. N., Lunenfeld, E., Abofoul-Azab, M., … Orwig, K. E. (2019). Testicular tissue cryopreservation: 8 years of experience from a coordinated network of academic centers. Human Reproduction (Oxford, England), 34(6), 966–977. https://doi.org/10.1093/humrep/dez043
- Wigny, K. M., van Dorp, W., van der Kooi, A. L., de Rijke, Y. B., de Vries, A. C., Smit, M., Pluijm, S. M., van den Akker, E. L., Pieters, R., Laven, J. S., & van den Heuvel-Eibrink, M. M. (2016). Gonadal function in boys with newly diagnosed cancer before the start of treatment. Human Reproduction (Oxford, England), 31(11), 2613–2618. https://doi.org/10.1093/humrep/dew234
