1. Historical notes on spironolactone (SP) as antiandrogen
Charles Masayuki Kagawa passed away on 9 May 2015 at the age of 92. He is considered the father of SP [Citation1]. From 1957, he studied a steroid compound (Aldactone-SC9420) which caused sodium depletion and moderate retention of potassium depending on the action of the mineralocorticoids. In 1963, Janigan showed cytoplasmatic inclusions, called ‘SP bodies’, in the glomerulosa of patients treated with SP, hypothesizing a direct effect on the adrenocortical hormone secretion. In 1959, Kagawa reported that SP could block the effect of testosterone propionate on the seminal vesicles and prostate of castrated rats.
In 1985, we evaluated the mechanism of antiandrogen activity of SP measuring the androgen receptor (AR) active metabolites in plasma of adrenalectomized and castrated mice [Citation2]. The study showed that plasma of mice chronically administered SP contained 10 times higher levels of AR active materials than from mice administered potassium canrenoate or canrenone. We concluded that some metabolites of SP have a powerful effect on AR. Moreover, in parallel bioassays (antagonism of the effect of testosterone on seminal vesicle weight), SP was four times more potent than potassium canrenoate and canrenone as antiandrogen.
Actually eplerenone, potassium canrenoate and canrenone are mainly used for treatment of hypermineralocorticism like Conn’s syndrome, secondary hyperaldosteronism [Citation3], and other diseases associated with pseudohyperaldosteronism [Citation4].
In the early 1980s, some studies reported the use of SP to treat hirsutism and subsequently a lot of other studies confirmed this new utilization of SP [Citation5,Citation6]. Recently, SP has raised importance for the treatment of women affected by polycystic ovary syndrome (PCOS) after the finding of a proinflammatory state and an increased cardiovascular risk in these patients ().
Figure 1. Extra-renal effects of spironolactone. The different actions of spironolactone are complex, being mediated not only by the direct antagonism of aldosterone and androgen receptors, but also indirectly through the reduction of hyperandrogenism and systemic inflammation. AR: androgen receptor; 17-OH-DH: 17-hydroxysteroid dehydrogenase; SHBG: sex hormone binding globulin; HbA1c: glycated haemoglobin.
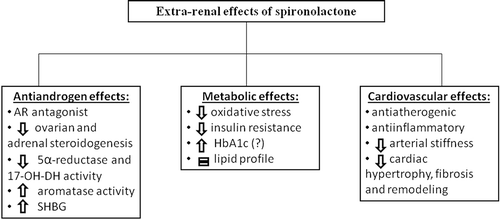
SP is the only antiandrogen approved for hirsutism in the United States and has been in use for this reason for more than three decades with close to 300 entries in Pub Med. The weakness of research in this field is the small sample of women enrolled in each study and a meta-analysis of all these studies should be performed. In this editorial, we aim to focus on the importance of the associated antiandrogen and antiinflammatory effects of SP in the treatment of PCOS.
2. PCOS: the role of aldosterone
PCOS is the most common endocrine disease of reproductive-aged women, with a prevalence of about 15%. The main characteristics of the syndrome are oligo-amenorrhea, clinical and/or biochemical symptoms of hyperandrogenism, and polycystic ovaries on the ultrasound examination. Following the criteria of Rotterdam consensus, the diagnosis is done upon two of these three characteristics, after having excluded other causes of hyperandrogenism [Citation7]. PCOS patients frequently have obesity, insulin resistance, and an increased risk of developing dyslipidemia, hypertension, type 2 diabetes, metabolic syndrome, and cardiovascular disease [Citation8]. Insulin resistance affects about 70% of patients independently of body mass index (BMI) and is considered the most important factor involved in the pathogenesis and progression of the disease [Citation9].
Women with PCOS have frequently a hyperaldosteronism (30% of the cases). In a previous study, we found that both the aldosterone to renin ratio and blood pressure values are normal in PCOS but significantly higher compared with healthy controls, comparable in age and BMI [Citation10]. Moreover, PCOS is characterized by an increased prevalence of different pathological conditions associated with hyperaldosteronism and/or increased aldosterone activity, like hypertension, Hashimoto’s thyroiditis, diabetes, obesity, and preeclampsia, particularly after menopause.
Actually aldosterone is considered the main inducer of inflammation, heart hypertrophy, and atherosclerosis. In previous studies, we have characterized mineralocorticoid receptors (MR) in mononuclear leukocytes (MNL) [Citation11], and later, we demonstrated that the incubation of MNL with aldosterone regulates the intracellular content of electrolytes and increases the expression of two oxidative stress-related proteins, PAI-1 and p22(phox) [Citation12]. All these effects were blocked by coincubation of MNL with canrenone, the main metabolite of SP. These results suggest that MNL would drive MR in the site of inflammation, allowing aldosterone to promote inflammation and atherosclerosis. The studies of Pitt et al. on the prevention of cardiovascular risk are important in this regard, SP being also able to decrease or block the relapse of new cerebral or cardiac events in patients with previous cerebral or cardiac ischemia [Citation13].
3. SP in the treatment of PCOS
Actually the treatment of PCOS can utilize various hormonal contraceptives (HCs) containing progestin with low or antiandrogen activity, able to inhibit ovarian steroid production. HCs are effective for menstrual abnormalities and the androgen-related symptoms in most of the cases; available data do not demonstrate a positive or negative influence of HCs on insulin resistance and glucose tolerance, mostly because of different criteria assessing insulin resistance and end points [Citation14]. These drugs should be prescribed with caution in case of familiarity for venous thromboembolism and in women with metabolic syndrome, hypertension, obesity, cerebro-cardiovascular disease, and breast or endometrial cancer. A very common side effect in PCOS is the persistent amenorrhea after withdrawal of HCs, due to the prolonged block of hypothalamus in patients with previous oligo-amenorrhea. The use of HCs is associated with an activation of the renin aldosterone system probably due to the stimulation of angiotensinogen synthesis by estrogens.
HCs are often prescribed in combination with an antiandrogen. Some authors have proposed HCs associated with SP, not only to avoid the risk of pregnancy (given potential fetal toxicity of antiandrogens), but also to block androgen action at the hair follicles and protect from some risks related to HCs, as, for example, the increase of aldosterone activity. Other antiandrogens like finasteride, cyproterone acetate, and flutamide have been used [Citation6] but none of these drugs possess an anti-inflammatory effect, thus reducing future metabolic and cardiovascular risk [Citation10,Citation15].
Treatment with 100 or rarely 200 mg daily of SP for 6–9 months is very effective in most of characteristics of PCOS, ameliorating hirsutism, acne, seborrhea, effluvium capillorum, and the effects persist after its withdrawal in most of the patients. The possible explanation of this finding could be related to the complex antiandrogen activity of SP. The drug binds to AR as an antagonist, partially inhibits ovarian and adrenal steroidogenesis, blocks 5α-reductase and 17-hydroxysteroid dehydrogenase at the ovarian and adrenal level, activates aromatase, and increases sex hormone binding globulin [Citation16]. SP could also present an intraovarian action similar to that reported in patients with idiopathic hyperaldosteronism that shows a restoration of normal reactivity of glomerulosa to aldosterone after withdrawal of prolonged treatment with potassium canrenoate or canrenone [Citation17]. These complex mechanisms of action can explain the finding of normal testosterone values during SP treatment in women: the block of AR could increase LH and androgens, while the direct effect at the ovarian level and the stimulation of aromatase could reduce the synthesis of testosterone.
When patients are not satisfied of the final results or an initial relapse of the esthetic problems is evident, SP treatment can be restarted only avoiding the risk of pregnancy. Many patients do not like to stop SP considering the important psychological impact of acne and hirsutism and the excellent results of the therapy.
4. Side effects of SP in PCOS
The most frequent side effects are reduced blood pressure, hyperkalemia, and polyuria, due to MR block in normokalemic and normotensive subjects. During the treatment it is worth measuring sodium, potassium, blood pressure and other necessary exams.
In women who complain of these side effects, we have established an innovative treatment of PCOS administering SP and pure licorice together [Citation18]. Licorice blocks 17-hydroxysteroid dehydrogenase and 17-20 lyase at the adrenal and/or ovarian level, and it could be considered an adjuvant therapy of hyperandrogenism in PCOS. Licorice derivatives have mineralocorticoid-like properties by binding to MR and blocking 11β-hydroxysteroid dehydrogenase type 2. For these reasons, licorice can reduce the side effects related to the diuretic activity of SP. It is also interesting to note that the extract of licorice root has both proinflammatory and anti-inflammatory properties due to the complex composition of the extract. The inflammatory effect is related to the mineralocorticoid effect of glycyrrhetinic acid, while the anti-inflammatory effect seems to be due to other metabolites of licorice root.
Another frequent side effect of the treatment with SP is the intermenstrual bleeding which accounts for 30% of the cases. Recently, we studied the possible mechanisms of this side effect: SP can decrease the values of serum estradiol and the endometrial thickness in patients with PCOS compared with pretreatment, but PCOS patients with bleeding had pretreatment estradiol values lesser than the patients without this side effect [Citation16]. The mechanism underlying intermenstrual abnormalities is complex, involving several actions of SP, such as the effects on estradiol and progesterone production, on their receptors and the individual metabolism of SP in vivo. In our patients with intermenstrual abnormalities, the addition of 2.5 mg nomegestrol for 4–5 days from the 14th day of the cycle can block the bleeding and after some cycles of treatment the bleeding usually disappears (personal observation).
5. Conclusions/expert opinion
The actual renaissance of aldosterone receptor blockers follows the finding of cardiovascular protection of these drugs even in patients with normal aldosterone values. PCOS is an inflammatory disease and frequently presents a hyperaldosteronism and an increased cardiovascular risk.
A very old drug like SP is still very useful in the treatment of PCOS, utilizing a previously reported side effect reported during treatment of hyperaldosteronism. In our experience, long-term therapy with SP is the most effective treatment for androgen-related hirsutism, acne, and effluvium capillorum, but more importantly it can prevent the future complications due to the inflammatory status of PCOS, that is probably related to an increased effector mechanism of aldosterone.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Sabbadin C, Calò LA, Armanini D. The story of spironolactones from1957 to now: from sodium balance to inflammation. G Ital Nefrol. 2016;33(S66):33.S66.12.
- Armanini D, Karbowiak I, Goi A, et al. In-vivo metabolites of spironolactone and potassium canrenoate: determination of potential anti-androgenic activity by a mouse kidney cytosol receptor assay. Clin Endocrinol (Oxf). 1985;23(4):341–347.
- Armanini D, Sabbadin C, Donà G, et al. Aldosterone receptor blockers spironolactone and canrenone: two multivalent drugs. Expert Opin Pharmacother. 2014;15(7):909–912.
- Sabbadin C, Armanini D. Syndromes that mimic an excess of mineralocorticoids. High Blood Press Cardiovasc Prev. 2016. [Epub ahead of print]. doi:10.1007/s40292-016-0160-5.
- Shapiro G, Evron S. A novel use of spironolactone: treatment of hirsutism. J Clin Endocrinol Metab. 1980;51(3):429–432.
- Moghetti P, Tosi F, Tosti A, et al. Comparison of spironolactone, flutamide and finasteride efficacy in the treatment of hirsutism: a randomized, double blind, placebo-controlled trial. J Clin Endocrinol Metab. 2000;85(1):89–94.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25.
- Karaer A, Cavkaytar S, Mert I, et al. Cardiovascular risk factors in polycystic ovary syndrome. J Obstet Gynaecol. 2010;30(4):387–392.
- Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030.
- Armanini D, Bordin L, Donà G, et al. Polycystic ovary syndrome: implications of measurement of plasma aldosterone, renin activity and progesterone. Steroids. 2012;77:655–658.
- Armanini D, Strasser T, Weber PC. Characterization of aldosterone binding sites in circulating human mononuclear leukocytes. Am J Physiol. 1985;248:E388–E390.
- Calò LA, Zaghetto F, Pagnin E, et al. Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22(phox) in human mononuclear leukocytes. J Clin Endocrinol Metab. 2004;89:1973–1976.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592.
- Zulian E, Sartorato P, Benedini S, et al. Spironolactone in the treatment of polycystic ovary syndrome: effects on clinical features, insulin sensitivity and lipid profile. J Endocrinol Invest. 2005;28(1):49–53.
- Sabbadin C, Andrisani A, Zermiani M, et al. Spironolactone and intermenstrual bleeding in polycystic ovary syndrome with normal BMI. J Endocrinol Invest. 2016. Epub 2016 Apr 12. [Epub ahead of print]. doi:10.1007/s40618-016-0466-0.
- Armanini D, Fiore C, Pellati D. Spontaneous resolution of idiopathic aldosteronism after long-term treatment with potassium canrenoate. Hypertension. 2007;50(4):e69–e70.
- Armanini D, Castello R, Scaroni C, et al. Treatment of polycystic ovary syndrome with spironolactone plus licorice. Eur J Obstet Gynecol Reprod Biol. 2007;131(1):61–67.
