ABSTRACT
Introduction: Agents that target the vascular endothelial growth factor (VEGF) or mammalian target of rapamycin (mTOR) pathway as well as the PD-1 checkpoint inhibitor nivolumab are standard therapies for advanced renal cell carcinoma (RCC). Recently, cabozantinib, an inhibitor of MET, VEGF receptors, and AXL, was approved by the FDA and European Commission based on improved progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) compared to standard of care treatment with everolimus in a randomized phase 3 trial in advanced RCC after prior VEGFR-tyrosine kinase inhibitor (TKI) therapy.
Areas covered:The preclinical development and scientific rationale, pharmacokinetics, and clinical efficacy and safety of cabozantinib for the treatment of advanced RCC are reviewed. The use of cabozantinib in clinical practice with the growing number of available treatments for advanced RCC is discussed.
Expert opinion: Cabozantinib is the only therapy for advanced RCC that has improved PFS, ORR, and OS in a pivotal phase 3 trial after prior antiangiogenic therapy. While no clinical trials have been published comparing cabozantinib with another TKI, available clinical data suggest it could be the most efficacious TKI for second-line therapy. Preliminary encouraging results have also been reported in a phase 2 trial in untreated poor- and intermediate- risk patients with RCC, indicating that treatment with cabozantinib may also be beneficial in the first-line setting.
1. Introduction
Renal cell carcinoma (RCC) is diagnosed in 330,000 individuals each year worldwide and results in approximately 140,000 deaths annually [Citation1]. Agents that target the vascular endothelial growth factor (VEGF) or mammalian target of rapamycin (mTOR) pathway are standard therapies in both the first- and second-line setting in advanced RCC. First-line therapies include the antiangiogenic agents sunitinib, pazopanib, and bevacizumab plus interferon-alfa and the mTOR inhibitor temsirolimus. Second-line therapies include the antiangiogenic agents axitinib and sorafenib, the mTOR inhibitor everolimus, and more recently the PD-1 checkpoint inhibitor nivolumab and the combination therapy lenvatinib plus everolimus [Citation2,Citation3]. Most of these agents have been approved based on improved progression-free survival (PFS) when compared to placebo or an active comparator in randomized phase 3 trials. Nivolumab extended survival after prior antiangiogenic therapy but did not improve PFS compared with everolimus [Citation4]. Despite a number of approved therapies, there is no cure for the majority of patients with advanced RCC. The median PFS for RCC patients ranges from 8 to 11 months for first-line therapy with pazopanib or sunitinib [Citation5–Citation7] and from 3 to 5 months for second-line therapy with sorafanib or axitinib after progression with sunitinib [Citation8,Citation9]. In a large population-based study using the International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool, the estimated median overall survival (OS) from the start of targeted second-line therapy was 12.5 months [Citation10].
Cabozantinib, an oral tyrosine kinase inhibitor (TKI), was recently approved for the treatment of advanced RCC in patients after prior antiangiogenic therapy based on the randomized phase 3 METEOR trial which compared the efficacy and safety of cabozantinib with everolimus [Citation11,Citation12]. Cabozantinib is an inhibitor of MET and AXL in addition to all 3 VEGF receptor subtypes. In the METEOR trial, treatment with cabozantinib improved PFS and extended OS compared with everolimus in previously treated patients. With the growing number of agents available for the treatment of advanced RCC, selection of which patients might benefit the most from a particular agent and the optimal sequencing of agents are important considerations. This manuscript summarizes the currently available preclinical and clinical trial data on cabozantinib in advanced RCC and potential applications to clinical practice.
2. Chemistry
Cabozantinib is a small molecule inhibitor of tyrosine kinases () [Citation13]. Tablets (CABOMETYX™; Exelixis, Inc.) [Citation14] and capsules (COMETRIQ®; Exelixis, Inc.) [Citation15] contain the (S)-malate salt of cabozantinib; the chemical name is N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, (2S)-hydroxybutanedioate and the molecular formula is C28H24FN3O5·C4H6O5. The molecular weight of the cabozantinib (S)-malate salt is 635.6 g/mol.
Table 1. Drug summary.
3. Preclinical development and rationale
Clear cell RCC is the most common histological subtype of RCC and is characterized by inactivation of the von Hippel–Lindau (VHL) tumor suppressor gene, resulting in upregulation of the VEGF signaling pathway [Citation16]. VHL loss also results in upregulation of MET and AXL which has been associated with tumor progression, development of resistance to VEGF pathway inhibition in preclinical models, and a poor prognosis in RCC patients [Citation17–Citation20]. Thus, agents that target alternative receptor tyrosine kinase signaling pathways in addition to the VEGF pathway may offer a therapeutic benefit in the treatment of advanced RCC.
Cabozantinib is a small molecule inhibitor of tyrosine kinases () including MET, VEGF receptors, and AXL [Citation13]. Cabozantinib also inhibits other tyrosine kinases involved in tumor pathology including RET, FLT3, KIT, and ROS1 [Citation13,Citation21]. Similar to other TKIs, cabozantinib is a reversible, ATP-competitive inhibitor.
Early reports on the activity of cabozantinib in preclinical studies demonstrated inhibition of MET and VEGFR2 activation. Oral administration of cabozantinib inhibited MET phosphorylation in H441 lung tumor xenografts which harbor constitutively phosphorylated MET [Citation13]. In separate experiments in naïve mice, cabozantinib treatment inhibited phosphorylation of MET by HGF in liver tissue and phosphorylation of VEGFR2 by VEGF in lung tissue. Consistent with this activity, significant tumor growth inhibition or tumor regression after cabozantinib treatment has been observed in multiple tumor xenograft models including medullary thyroid cancer (MTC), breast cancer, hepatocellular carcinoma, lung carcinoma, and glioblastoma [Citation13,Citation22,Citation23]. In a phase 3 trial, cabozantinib demonstrated efficacy compared with placebo in progressive, metastatic MTC, leading to approval in this indication [Citation24].
More recently, preclinical studies have been published showing that cabozantinib treatment can overcome sunitinib resistance and inhibit tumor growth in RCC models [Citation20]. Chronic sunitinib treatment was shown to increase MET and AXL signaling in addition to promoting prometastatic effects and angiogenesis in RCC cell models, supporting a role for these targets in the development of resistance to VEGF-targeted therapy. Treatment with cabozantinib resulted in reduced MET and AXL signaling in these sunitinib-treated cell lines and inhibition of tumor growth in a sunitinib-treated xenograft model that had acquired resistance. These results support a role for targeting MET and AXL in RCC.
4. Pharmacokinetics and metabolism
From a population pharmacokinetics (PK) analysis of cabozantinib supporting the evaluation of RCC, the predicted terminal half-life is approximately 99 h, the oral volume of distribution (VZ/F) is approximately 319 L, and the clearance (CL/F) at steady-state is estimated to be 2.2 L/h [Citation14]. Following oral administration of cabozantinib, median time to maximum plasma concentration (Tmax) for cabozantinib ranged from 2 to 3 h postdose. Repeat daily dosing of cabozantinib at 140 mg for 19 days resulted in four to fivefold higher mean cabozantinib accumulation (based on area under the plasma concentration–time curve [AUC]) compared with a single-dose administration; steady state was achieved by Day 15 [Citation25]. Cabozantinib is highly protein bound in human plasma (≥99.7%) [Citation14]. Within a 48-day collection period after a single oral dose of 14C-cabozantinib in healthy subjects, approximately 81% of the total administered radioactivity was recovered with 54% in feces and 27% in urine [Citation26]. Exposure (AUC0–inf) was approximately 30% and 6% higher in subjects with mild and moderate renal impairment, respectively, compared to subjects with normal renal function [Citation27]. Exposure (AUC0–inf) to cabozantinib was increased by about 81% and 63% in subjects with mild and moderate hepatic impairment, respectively, compared to subjects with normal hepatic function.
Cabozantinib most potently inhibits CYP isozyme CYP2C8 in vitro (Ki,app = 4.6 µM) but had no effect on the PK of rosiglitazone (a CYP2C8 substrate) when coadministered at clinically relevant plasma concentrations [Citation28]. Cabozantinib is primarily metabolized by CYP3A4, and coadministration of the strong CYP3A4 inhibitor ketoconazole or the strong CYP3A4 inducer rifampin resulted in increased and decreased cabozantinib plasma exposures, respectively. A high-fat meal increased the maximum plasma concentration (Cmax) and AUC values by 41% and 57%, respectively, relative to fasted conditions in healthy subjects administered a single 140-mg oral cabozantinib dose [Citation29]. Administration of the proton pump inhibitor esomeprazole resulted in no clinically relevant effect on cabozantinib plasma PK in healthy subjects. Cabozantinib is an inhibitor (IC50 = 7.0 μM), but not a substrate, of P-glycoprotein (P-gp) transport activities in a bidirectional assay system using MDCK-MDR1 cells [Citation26]. In addition, cabozantinib was shown to be a substrate of drug transporter multidrug-resistance-associated protein 2 in an in vitro assay. Cabozantinib is available in two formulations, tablets and capsules. Tablets (CABOMETYX; Exelixis, Inc.) and capsules (COMETRIQ; Exelixis, Inc.) are not bioequivalent, nor interchangeable. Following a single 140-mg dose, a 19% increase in the Cmax of the tablet formulation compared to the capsule formulation was observed; the difference in the AUC was less than 10% [Citation30].
5. Clinical efficacy and safety
Cabozantinib was first evaluated in RCC in a phase 1b drug–drug interaction study that included a cohort of 25 patients with advanced RCC who had failed standard therapy [Citation31]. The assigned dose was 140 mg cabozantinib orally once daily based on the maximum-tolerated dose determined in the first in-human study [Citation25]; patients also received a single dose of rosiglitazone, a CYP2C8 substrate, at day 1 and day 22. Eighty percent (20/25 patients) of the cohort reported at least one dose reduction from the starting dose of 140 mg/day, resulting in a median average daily dose of 75.5 mg/day. The most common final dose was 60 mg/day (11/25 patients) which was the assigned dose for the subsequent phase 3 trial in RCC. The median number of prior therapies in the RCC cohort was two with 88% having received at least one prior VEGF-targeting therapy and 60% having received prior mTOR inhibitor therapy. Treatment with cabozantinib in these patients resulted in an objective response rate (ORR) of 28%, and 52% had stable disease as their best response. The median PFS was 12.9 months and median OS was 15.0 months. Based on these preliminary clinical results and the scientific rationale for cabozantinib treatment in RCC, a larger phase 3 trial was commenced.
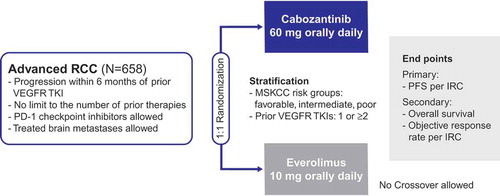
The international, randomized phase 3 METEOR trial compared the efficacy and safety of cabozantinib with everolimus in 658 patients with advanced RCC who experienced disease progression on or after prior VEGFR-TKI therapy [Citation11,Citation12] (). Eligible patients were 18 years of age or over and had measurable disease per RECIST version 1.1 criteria, clear cell histology, and a Karnofsky performance status score of at least 70%. Patients with treated brain metastases were allowed. Multiple types of prior therapies including cytokines, monoclonal antibodies (such as those targeting VEGF or PD-1), and chemotherapies were allowed with no limit on the number of prior therapies. The study excluded patients with prior mTOR inhibitor therapy or patients with uncontrolled hypertension or clinically significant cardiovascular, gastrointestinal, wound healing, or infectious comorbidities.
Patients were randomized 1:1 to receive either 60 mg cabozantinib or 10 mg everolimus once a day. Stratification factors were the number of prior VEGFR TKIs (1 or ≥2) and Memorial Sloan Kettering Cancer Center (MSKCC) risk group (favorable, intermediate, poor) [Citation32].
The primary end point was PFS as assessed by an independent radiology committee (IRC) in the first 375 randomized patients. Secondary end points included OS and ORR per IRC in all 658 randomized patients. The trial was designed to provide adequate power for assessment of both PFS and OS. To provide longer follow-up for the event-driven analysis of PFS, the primary end point analysis was prespecified to be conducted in the first 375 randomized patients.
At the time of this review, the study was ongoing, and results for the primary end point of PFS and secondary end points of ORR and OS were available [Citation11,Citation12]. The study met its primary end point of prolonging the duration of PFS as assessed by an IRC with a median PFS of 7.4 months with cabozantinib and 3.8 months with everolimus (hazard ratio [HR] = 0.58, 95% confidence interval [CI] 0.45–0.74; p < 0.0001). The ORR in all randomized patients was improved with cabozantinib compared to everolimus (17% vs. 3%; p < 0.0001). A planned interim analysis of the secondary end point of OS in all 658 randomized patients was conducted at the time of the primary PFS analysis and demonstrated a trend for improved survival (49% information fraction: HR 0.67, 95% CI 0.51–0.89; p = 0.005) but did not reach the boundary for significance (p ≤ 0.0019) at this information fraction. A subsequent analysis of OS demonstrated a statistically significant improvement in survival with cabozantinib treatment: the median OS was 21.4 months with cabozantinib compared with 16.5 months with everolimus (HR = 0.66, 95% CI 0.53–0.83; p = 0.0003).
The baseline demographic and disease characteristics were similar between the cabozantinib and everolimus groups. The majority of patients were male (75%) with a median age of 63 years for the cabozantinib group and 62 years for the everolimus group. Seventy-one percent received only one prior VEGFR TKI, and the most common prior VEGFR TKIs were sunitinib (63%) and pazopanib (43%). Forty-six percent of patients were in the favorable - risk category, 42% in the intermediate - risk category, and 13% in the poor - risk category as defined by MSKCC criteria for RCC. OS and PFS were increased with cabozantinib compared to everolimus (HR < 1) for all subgroups analyzed including those defined by MSKCC risk groups, the number of prior VEGFR TKIs, duration of prior VEGFR-TKI treatment, prior sunitinib or pazopanib therapy, prior treatment with checkpoint inhibitors targeting PD-1, and MET expression level. Prolongation of OS and PFS with cabozantinib was also observed irrespective of tumor burden or metastatic site, including in patients with bone metastases, which are associated with a poor prognosis [Citation33].
As of the cutoff date of 31 December 2015, patients were on cabozantinib treatment for a median duration of 8.3 months compared with 4.4 months for everolimus. Adverse events were managed by dose reductions or interruptions. Allowed dose levels were 60, 40, and 20 mg for cabozantinib and 10, 5, and 2.5 mg for everolimus. Dose reductions due to adverse events were higher in the cabozantinib group (62% compared with 25%), but the rates of discontinuation due to adverse events not related to disease progression were similar (12% in the cabozantinib group and 11% in the everolimus group). The median daily dose was 43 mg for cabozantinib and 9 mg for everolimus. Adverse events of all causality (any grade) were observed in 100% of patients in the cabozantinib group and >99% of patients in the everolimus group. Common adverse events observed with cabozantinib were typical of those observed with other VEGFR TKIs in RCC patients [Citation34] and included diarrhea (75%), fatigue (59%), nausea (52%), decreased appetite (47%), palmar–plantar erythrodysesthesia syndrome (43%), and hypertension (37%). The incidence of grade 3 or 4 events was 71% with cabozantinib and 60% with everolimus. The most common grade 3 or 4 adverse events were hypertension (15%), diarrhea (13%), and fatigue (11%) with cabozantinib and anemia (17%), fatigue (7%), and hyperglycemia (5%) with everolimus.
6. Regulatory status
Cabozantinib (CABOMETYX; Exelixis, Inc.) tablets were approved by the FDA in April 2016 for the treatment of advanced RCC in patients who have received prior antiangiogenic therapy, and by the European Commission for the treatment of advanced RCC following VEGF-targeted therapy in September 2016. Cabozantinib (COMETRIQ; Exelixis, Inc.) capsules were previously approved by the FDA for the treatment of patients with progressive, metastatic MTC, and by the European Commission for the treatment of adult patients with progressive, unresectable locally advanced or metastatic MTC.
7. Conclusion
Treatment with cabozantinib, an inhibitor of MET, VEGFR receptors, and AXL, improved PFS, OS, and the ORR in previously treated patients with advanced RCC compared with everolimus in a randomized phase 3 trial. The safety profile of cabozantinib was acceptable and similar to other VEGFR TKIs in this patient population. Cabozantinib is an important new therapy for the treatment of advanced RCC in patients after prior antiangiogenic therapy.
8. Expert opinion
Cabozantinib is a new option for patients with advanced RCC who have received prior antiangiogenic therapy. Cabozantinib has a unique target profile and inhibits important targets involved in resistance mechanisms to VEGF-targeted agents, such as MET and AXL, in addition to inhibiting VEGFR. Cabozantinib is the only approved drug that has improved PFS, ORR, and OS in a pivotal phase 3 trial after one or more VEGFR TKIs.
The recommended dose of cabozantinib is 60 mg once daily with dose reductions to 40 or 20 mg to manage adverse events. In the METEOR trial, dose reductions occurred in more than half of the patients to improve individual tolerability. With application of these dose reductions, cabozantinib can be continued until patients experience radiographic progression or loss of clinical benefit.
Nivolumab has also been recently evaluated in a similar patient population against the comparator everolimus and has been approved based on improved OS and ORR [Citation4]. In addition, the combination of lenvatinib and everolimus was also recently approved in previously treated patients based on a randomized phase 2 trial [Citation35,Citation36]. However, the role of this combination remains controversial based on the size of the study and the tolerability of this regimen as an increased incidence of high-grade adverse events was observed relative to either agent alone. The respective role of nivolumab and of cabozantinib will be one of the major considerations in the near future.
Compared to axitinib and sorafenib, which are two TKIs considered after failure of a previous TKI therapy, cabozantinib efficacy appears to be more robust with longer median PFS, particularly when comparing subgroups of patients receiving only prior sunitinib TKI therapy [Citation9,Citation11]. Cabozantinib also demonstrated improved OS in the pivotal phase 3 trial [Citation12], while axitinib improved PFS compared with sorafenib, but without OS improvement [Citation9,Citation37]. Improvement in PFS and OS with cabozantinib was maintained in all reported subgroups, including in patients with bone metastases, suggesting that cabozantinib is a broadly applicable treatment option. Bone metastases are prognostic for poor outcomes in patients with advanced RCC, and treatment for these patients is an unmet medical need [Citation33].
Cabozantinib is also being evaluated as a first-line therapy in patients with advanced intermediate- or poor-risk RCC in the phase 2 CABOSUN trial (NCT01835158) [Citation38], a 150 patient study comparing cabozantinib with sunitinib. A recent press release reported that the primary end point of improved PFS with cabozantinib compared to sunitinib was met, and that safety with cabozantinib was consistent with that previously reported in advanced RCC. Further details and results of this trial are awaited. This recent announcement raises the possibility that cabozantinib could move to first-line therapy in the future.
Combination of cabozantinib with checkpoint inhibitors is also under investigation (NCT02496208) [Citation39] and represents an attractive treatment approach if the combination improves clinical benefit in patients with advanced RCC with an acceptable safety profile.
Overall, based on available clinical data, we believe that cabozantinib could be the most efficacious TKI for advanced RCC, not only after TKI therapy but perhaps also in the first-line setting, for patients who will receive VEGF-targeted agents as first-line treatment.
Declaration of interest
B Escudier has received honoraria from Novartis, Pfizer, Bristol-Myers Squibb, Exelixis and Roche. J Lougheed is an employee and stockholder of Exelixis. L Albiges has received honoraria from Novartis, Pfizer, Bristol-Myers Squibb, Sanofi, Roche and Astellas. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
We thank Steven Lacy, PhD and Christian Scheffold, MD, PhD (both from Exelixis, Inc.) for reviewing the manuscript and providing helpful suggestions.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
- Powles T, Staehler M, Ljungberg B, et al. Updated EAU guidelines for clear cell renal cancer patients who fail VEGF targeted therapy. Eur Urol. 2016;69:4–6.
- Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 3.2016. Available from: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. 2016
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813.
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731.
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068.
- Hutson TE, Escudier B, Esteban E, et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014;32:760–767.
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939.
- Ko JJ, Xie W, Kroeger N, et al. The international metastatic renal cell carcinoma database consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol. 2015;16:293–300.
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1814–1823.
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:917–927.
- Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298–2308.
- CABOMETYX United States Prescribing Information. Apr,2016.
- COMETRIQ United States Prescribing Information. May,2016.
- Shen C, Kaelin WG Jr. The VHL/HIF axis in clear cell renal carcinoma. Semin Cancer Biol. 2013;23:18–25.
- Gibney GT, Aziz SA, Camp RL, et al. c-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol. 2013;24:343–349.
- Gustafsson A, Martuszewska D, Johansson M, et al. Differential expression of Axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin Cancer Res. 2009;15:4742–4749.
- Rankin EB, Fuh KC, Castellini L, et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci U S A. 2014;111:13373–13378.
- Zhou L, Liu XD, Sun M, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35:2687–2697.
- Katayama R, Kobayashi Y, Friboulet L, et al. Cabozantinib overcomes crizotinib resistance in ROS1 fusion-positive cancer. Clin Cancer Res. 2015;21:166–174.
- Bentzien F, Zuzow M, Heald N, et al. In vitro and in vivo activity of cabozantinib (XL184), an inhibitor of RET, MET, and VEGFR2, in a model of medullary thyroid cancer. Thyroid. 2013;23:1569–1577.
- Xiang Q, Chen W, Ren M, et al. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin Cancer Res. 2014;20:2959–2970.
- Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639–3646.
- Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29:2660–2666.
- Lacy S, Hsu B, Miles D, et al. Metabolism and disposition of cabozantinib in healthy male volunteers and pharmacologic characterization of its major metabolites. Drug Metab Dispos. 2015;43:1190–1207.
- Nguyen L, Holland J, Ramies D, et al. Effect of Renal and Hepatic Impairment on the Pharmacokinetics of Cabozantinib. J Clin Pharmacol. 2016;56:1130–1140.
- Nguyen L, Holland J, Miles D, et al. Pharmacokinetic (PK) drug interaction studies of cabozantinib: effect of CYP3A inducer rifampin and inhibitor ketoconazole on cabozantinib plasma PK and effect of cabozantinib on CYP2C8 probe substrate rosiglitazone plasma PK. J Clin Pharmacol. 2015;55:1012–1023.
- Nguyen L, Holland J, Mamelok R, et al. Evaluation of the effect of food and gastric pH on the single-dose pharmacokinetics of cabozantinib in healthy adult subjects. J Clin Pharmacol. 2015;55:1293–1302.
- Nguyen L, Benrimoh N, Xie Y, et al. Pharmacokinetics of cabozantinib tablet and capsule formulations in healthy adults. Anticancer Drugs. 2016;27:669–678.
- Choueiri TK, Pal SK, McDermott DF, et al. A phase I study of cabozantinib (XL184) in patients with renal cell cancer. Ann Oncol. 2014;25:1603–1608.
- Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–463.
- McKay RR, Kroeger N, Xie W, et al. Impact of bone and liver metastases on patients with renal cell carcinoma treated with targeted therapy. Eur Urol. 2014;65:577–584.
- Eisen T, Sternberg CN, Robert C, et al. Targeted therapies for renal cell carcinoma: review of adverse event management strategies. J Natl Cancer Inst. 2012;104:93–113.
- Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473–1482.
- Motzer RJ, Hutson TE, Ren M, et al. Independent assessment of lenvatinib plus everolimus in patients with metastatic renal cell carcinoma. Lancet Oncol. 2016;17:e4–e5.
- Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–562.
- ClinicalTrials.gov. Cabozantinib-s-malate or Sunitinib Malate in Treating Patients With Previously Untreated Locally Advanced or Metastatic Kidney Cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT01835158. cited Oct 06, 2016
- ClinicalTrials.gov. Cabozantinib-s-malate and Nivolumab With or Without Ipilimumab in Treating Patients With Metastatic Genitourinary Tumors. Available from: https://clinicaltrials.gov/ct2/show/NCT02496208. cited Oct 06, 2016