1. Introduction
In recent years, modern anticancer treatments have led to significant survival improvements; however, in premenopausal women, the administration of these therapies is also associated with potential unwanted side effects such as treatment-related premature ovarian failure (POF) and subsequent reduced fertility [Citation1]. At the time of cancer diagnosis, the maintenance of gonadal function and fertility has crucial importance for many patients. As recommended by international guidelines, all patients of reproductive age should be counseled about the potential treatment-related loss of ovarian function and fertility and helped with informed fertility preservation decisions [Citation2,Citation3].
For young female cancer patients, embryo/oocyte cryopreservation are standard strategies to preserve fertility [Citation2,Citation3]; however, they require a period of controlled ovarian stimulation with subsequent delay in the initiation of anticancer treatments and they cannot preserve gonadal function during cytotoxic therapy. Pharmacological protection of the ovaries with the use of temporary ovarian suppression with gonadotropin-releasing hormone analogs (GnRHa) during chemotherapy is a widely available option requiring no delay in the initiation of treatment. However, taking into account the controversial data available up to 2013, it was considered an experimental strategy in cancer patients [Citation2,Citation3].
Over the past few years, several important news have become available on the potential efficacy and safety of temporary ovarian suppression with GnRHa during chemotherapy as a strategy to preserve ovarian function and fertility in young patients with breast cancer and lymphoma.
2. Recent data in breast cancer patients
The two largest randomized trials investigating the role of temporary ovarian suppression with GnRHa during chemotherapy (the POEMS-SWOG S0230 [Citation4] and the PROMISE-GIM6 [Citation5] studies) have recently reported long-term results. In these studies, premenopausal women with early-stage breast cancer candidates to (neo)adjuvant chemotherapy were randomized to receive cytotoxic therapy alone or concurrently with GnRHa. While all the 218 evaluable patients randomized in the POEMS-SWOG S0230 trial were diagnosed with endocrine-insensitive breast cancer [Citation4], approximately 80% of the 281 included in the PROMISE-GIM6 study had hormone receptor-positive disease [Citation5,Citation6]. Both studies were designed to investigate the efficacy of this strategy in protecting gonadal function; hence, treatment-related POF was the primary end point and a composite end point including both menstrual function and hormonal values was used for its definition (). Major limitation of the POEMS-SWOG S0230 trial is represented by the lack of information on primary end point data for 83 patients (38% of the study population). Nevertheless, a similar significant absolute and relative magnitude of benefit was shown in the two studies. As compared to the use of cytotoxic therapy alone, the use of GnRHa during chemotherapy significantly reduced the risk of developing treatment-related POF (22% vs. 8%; odds ratio [OR], 0.30; 95% confidence intervals [CI], 0.09–0.97; p = 0.04 in the POEMS-SWOG S0230 study [Citation4]; 25.9% vs. 8.9%; OR, 0.28; 95% CI, 0.14–0.59; p < 0.001 in the PROMISE-GIM6 trial [Citation6]). A significant protective effect on gonadal function with the use of this strategy was also observed at long-term follow-up in the PROMISE-GIM6 trial (5-year cumulative incidence estimate of menstrual resumption: 72.6% vs. 64.0%; age-adjusted hazard ratio [HR], 1.48; 95% CI, 1.12–1.95) [Citation5]. Moreover, more patients treated with concurrent chemotherapy and GnRHa had a subsequent pregnancy (22 vs. 12 women; OR, 2.45; 95% CI, 1.09–5.51; p = 0.03 in the POEMS-SWOG S0230 study [Citation4]; 8 vs. 3 women; age-adjusted HR, 2.40; 95% CI, 0.62–9.22; p = 0.20 in the PROMISE-GIM6 trial [Citation5]).
Table 1. Main characteristics of the largest randomized studies investigating the role of temporary ovarian suppression with GnRHa during chemotherapy.
The efficacy of temporary ovarian suppression with GnRHa during chemotherapy has been recently confirmed also by a large meta-analysis including all the 12 available randomized studies that investigated this procedure in 1231 young women with breast cancer [Citation9]. In this meta-analysis, GnRHa administration showed to be associated with a significant reduced risk of developing treatment-related POF (OR, 0.36; 95% CI, 0.23–0.57). However, a significant heterogeneity (I2 = 47.1%; p = 0.026) was observed probably due to the different definition of POF and timepoint of its evaluation used in the included studies. Nevertheless, when the analysis was repeated to include only those studies with available information on the standard definition of amenorrhea 1 year after the end of chemotherapy, the use of GnRHa confirmed to be protective (OR, 0.55; 95% CI, 0.41–0.73), and no heterogeneity was observed (I2 = 0.0%; p = 0.936). Furthermore, although the absolute numbers remained low, a significantly higher chance of subsequent pregnancies for women treated with GnRHa was observed (OR, 1.83; 95% CI, 1.02–3.28; I2 = 0.0%; p = 0.629) [Citation9].
Following these results, the updated guidelines support temporary ovarian suppression with GnRHa during chemotherapy as an option to be discussed with all breast cancer patients interested in preserving ovarian function and/or fertility irrespective of the hormone receptor status of their disease [Citation10,Citation11]. In some countries like Italy, the coverage for the cost of GnRHa is granted to all young breast cancer patients who are candidates to (neo)adjuvant chemotherapy and are interested in ovarian function and/or fertility preservation [Citation11]. Final results from the MOMMY study, an ongoing individual patient data meta-analysis investigating the role of this strategy in breast cancer patients, are expected to provide further insights on this regard (PROSPERO registration number: CRD42014015638) [Citation1].
3. Recent data in lymphoma patients
Differently from the findings in young women with breast cancer, the recently updated results from the study by Demeestere and colleagues have shown no gonadal protection of GnRHa administration during chemotherapy in lymphoma patients [Citation7]. In this study, 129 premenopausal patients with Hodgkin or non-Hodgkin lymphoma were randomly assigned to receive alkylating agents-containing chemotherapy with either GnRHa plus norethisterone or norethisterone alone. At the primary analysis of the study, similar rates of treatment-related POF were observed in the two treatment arms (19% vs. 20%; p = 1.00); however, the anti-Mullerian hormone (AMH) values were higher in patients treated with GnRHa (1.4 ± 0.35 vs. 0.5 ± 0.15 ng/mL; p = 0.040) [Citation8]. At the updated analysis of the study after 5 years of follow-up, only 67 patients (52%) remained evaluable [Citation7]. A total of 19.4% of the patients treated with GnRHa during chemotherapy and 25% of those who underwent cytotoxic therapy alone experienced treatment-related POF (p = 0.763). At the multivariate logistic regression analysis, no significant protective effect with the use of GnRHa during chemotherapy was observed (OR, 0.70; 95% CI, 0.15–3.24; p = 0.651). As expected, the main factors significantly associated with an increased risk of treatment-related POF were age (p = 0.047), the conditioning regimen administered for hematopoietic stem cell transplantation (HSCT, p = 0.002), and the cumulative dose of cyclophosphamide >5 g/m2 (p = 0.019). No significant difference was observed in terms of ovarian reserve (assessed using AMH and follicle-stimulating hormone [FSH] levels) and pregnancy rates (53% vs. 43%; p = 0.467) between patients who underwent chemotherapy with or without GnRHa, respectively [Citation7].
Although providing important insights on long-term outcomes with the use of this strategy, several limitations should be considered in the interpretation of these updated results [Citation12]. This was an underpowered and exploratory analysis with approximately half of the study population lost to follow-up. To define treatment-related POF, the authors did not use a composite end point but relied only on FSH or AMH levels with a possible increased false positive rate. Moreover, in both treatment arms, patients could receive other forms of hormonal treatments (norethisterone acetate and hormonal contraceptives) with potential diminished observed protective effect of GnRHa. Nevertheless, although reported as a negative study, the results in terms of both OR for treatment-related POF and pregnancy rate observed in this study are consistent with the protective effect of GnRHa administration seen in the breast cancer trials (). Hence, the results may not have achieved statistical significance only due to lack of power [Citation12].
4. Expert opinion
Although the debate around this topic has continued [Citation12–Citation15], long-term follow-up data from well-designed large randomized studies support the efficacy and safety of temporary ovarian suppression with GnRHa during chemotherapy. Besides the limitations previously highlighted and the discrepancies among studies (), the most important difference between the study by Demeestere and colleagues and the breast cancer trials is represented by their study population. Lymphoma patients are characterized by a younger age at diagnosis and are treated with regimens having a high (e.g. HSCT conditioning regimens) or low (e.g. ABVD) gonadotoxic potential [Citation1]. On the contrary, breast cancer patients are characterized by an older age at diagnosis and are treated with regimens having an intermediate gonadotoxic effect [Citation1]. In this scenario, the modest but clear benefit with the use of temporary ovarian suppression with GnRHa during chemotherapy may not be seen in lymphoma patients while it becomes evident in women with breast cancer.
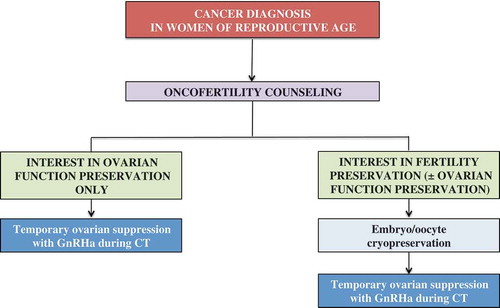
Embryo/oocyte cryopreservation remain the first options to be discussed with all young cancer patients interested in fertility preservation [Citation2,Citation3]. Temporary ovarian suppression with GnRHa during chemotherapy should not be seen as an alternative to embryo/oocyte cryopreservation (). On the contrary, this strategy should be considered as an option for women interested in preserving ovarian function only, and also for those interested in fertility preservation after cryopreservation procedures or when surgical strategies are contraindicated or not available.
Declaration of interest
M Lambertini acknowledges the support from the European Society for Medical Oncology (ESMO) for a Translational Research Fellowship at Institut Jules Bordet. L Del Mastro received honoraria from Takeda and personal fees from Ipsen and Takeda outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Lambertini M, Del ML, Pescio MC, et al. Cancer and fertility preservation: international recommendations from an expert meeting. BMC Med. 2016;14(1):1.
- Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–2510.
- Peccatori FA, Azim HA Jr, Orecchia R, et al. Cancer, pregnancy and fertility: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi160–170.
- Moore HCF, Unger JM, Phillips K-A, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015;372(10):923–932.
- Lambertini M, Boni L, Michelotti A, et al. Ovarian suppression with triptorelin during adjuvant breast cancer chemotherapy and long-term ovarian function, pregnancies, and disease-free survival: a randomized clinical trial. JAMA. 2015;314(24):2632–2640.
- Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011;306(3):269–276.
- Demeestere I, Brice P, Peccatori FA, et al. No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: final long-term report of a prospective randomized trial. J Clin Oncol. 2016;34(22):2568–2574.
- Demeestere I, Brice P, Peccatori FA, et al. Gonadotropin-releasing hormone agonist for the prevention of chemotherapy-induced ovarian failure in patients with lymphoma: 1-year follow-up of a prospective randomized trial. J Clin Oncol. 2013;31(7):903–909.
- Lambertini M, Ceppi M, Poggio F, et al. Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies. Ann Oncol. 2015;26(12):2408–2419.
- Paluch-Shimon S, Pagani O, Partridge AH, et al. Second international consensus guidelines for breast cancer in young women (BCY2). Breast. 2016;26:87–99.
- Lambertini M, Cinquini M, Moschetti I, et al. Temporary ovarian suppression during chemotherapy to preserve ovarian function and fertility in breast cancer patients: a GRADE approach for evidence evaluation and recommendations by the Italian Association of Medical Oncology. Eur J Cancer. 2017;71:25–33.
- Lambertini M, Falcone T, Unger JM, et al. Debated role of ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in women with cancer. J Clin Oncol. 2017;35(7):804–805.
- Del Mastro L, Lambertini M. Temporary ovarian suppression with gonadotropin-releasing hormone agonist during chemotherapy for fertility preservation: toward the end of the debate? Oncologist. 2015;20(11):1233–1235.
- Lambertini M, Peccatori FA, Moore HCF, et al. Reply to the letter to the editor “Can ovarian suppression with gonadotropin releasing hormone analogs (GnRHa) preserve fertility in cancer patients?” by Rodriguez-Wallberg et al. Ann Oncol. 2016;27(3):548–549.
- Rodriguez-Wallberg K, Turan V, Munster P, et al. Can ovarian suppression with gonadotropin-releasing hormone analogs (GnRHa) preserve fertility in cancer patients? Ann Oncol. 2016;27(2):257.