ABSTRACT
Introduction: Heart failure (HF) remains a major public health problem worldwide, affecting approximately 23 million patients, and is predominantly a disease of the elderly population. Elderly patients mostly suffer from HF with preserved ejection fraction (HFpEF), which often presents with multiple co-morbidities and they require multiple medical treatments. This, together with the heterogeneous phenotype of HFpEF, makes it a difficult syndrome to diagnose and treat.
Areas covered: Although HF is most abundant in the elderly, this group is still underrepresented in clinical trials, which results in the lack of evidence-based medical regimens. The current review has focused on new potential therapies for this poorly studied population. The focus will be on several classes of drugs currently recommended or might be expected soon. These will include sacubitril/valsartan (former LCZ696), Omecamtiv mecarbil, Vericiguat, Ivabradine, mineralocorticoid receptor antagonists (MRAs) and potassium binders.
Expert opinion: We discuss promising new treatments and hypothesize that personalized approaches will be needed to treat elderly patients optimally. Medical doctors should not only focus on HF therapy, but comorbidities and polypharmacy should also influence therapeutic decision making. Furthermore, the importance of quality of life as a management endpoint should not be underestimated in the frail elderly.
1 Introduction
Heart failure (HF) is a detrimental disease, affecting approximately 23 million patients worldwide [Citation1]. It is a clinical syndrome, accompanied by typical symptoms like shortness of breath, orthopnea, ankle edema and fatigue, and signs like elevated jugular venous pressure, pulmonary crackles, and a third heart sound [Citation2]. These signs and symptoms are caused by a structural and/or functional cardiac abnormality. HF is associated with high morbidity and mortality rates as well with frequent hospitalization which have a huge impact on quality of life [Citation3]. Even though the cardiac treatment options have improved in the recent years, it remains to be linked to a poor prognosis of 50% mortality within 5 years [Citation2]. In this review, we will focus on new potential therapies that might improve outcome or reduce symptoms. We aim to discuss therapeutic options in the ageing population.
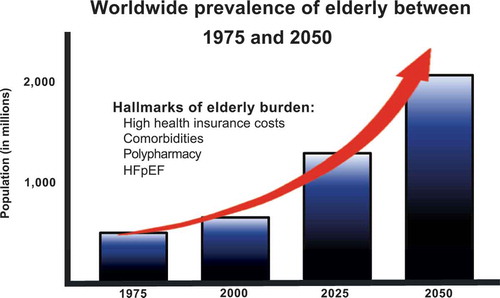
HF also drives the burden on health insurance in developed western countries. This is mostly due to a high 30-day rehospitalization rate [Citation4]. The US healthcare system current policy is to fine hospitals in which a HF patient is rehospitalized prior to 30 days after initial discharge [Citation5]. HF doctors are therefore focused on identifying patients at risk, to prevent this phenomenon. In all probability, the overall incidence and prevalence of HF will continue to rise, resulting in even higher healthcare expenditures [Citation6]. Beside pressure on healthcare, the World Health Organization (WHO) well defined another issue: ‘Population ageing is a triumph of humanity but also a challenge to society’ [Citation7]. Practically everywhere in the world, the number of people older than 80 years is growing faster than any other age group. As expected by the WHO, the elderly population will increase in threefold in the upcoming years [Citation8]. This implicates that we will be confronted with an elderly epidemic and their inherent problems ().
1.1. HF in the elderly population
HF occurs mainly in the elderly population. Old patients (≥65 years) account for 80% of HF hospitalizations and 90% of HF-related deaths [Citation9]. A lifetime risk of already 20% exists in healthy 40-year-old subjects to develop HF. At the age of 80, this risk remains unchanged, despite the fact elderly have a much shorter life expectancy [Citation10]. In addition, men have a higher probability to develop HF compared to women, though more women suffer from HF as a result of population demographics. The HF incidence rates of men nearly double every 10 years from the age of 65 and the incidence rates of women even triple [Citation10]. A higher incidence of HF in the elderly population can be explained to a large extent by the normal ageing process. Ageing results in cardiovascular changes, which may ultimately increase the risk of HF development [Citation11]. This underscores that the prevalence of HF will increase substantially due to ageing as well to developed drugs, which enable increased survival [Citation12,Citation13].
The prolonged life expectancy as well as the improved treatment options for sudden cardiac death and coronary artery disease (CAD) will result in a strong increase of newly diagnosed HF patients. In addition, the worldwide prevalence of diabetes and uncontrolled hypertension, which are major risk factors for HF development and progression, might lead to an even stronger increase in the number of HF patients [Citation14]. In conclusion, HF in the elderly differs clinically in many aspects from HF in the relatively young population. For example in the elderly (1) HF with preserved ejection fraction (HFpEF) is more common [Citation15] (2) often multiple comorbidities are present [Citation16] and (3) most have polypharmacy (≥5 medications) [Citation17]. However, it is important to note that a difference in terminology exists about the word ‘elderly’. Patients aged 65 probably have different characteristics than patients aged 75. The TRITON-TIMI study (prasugrel vs. clopidogrel in patients with acute coronary syndromes) [Citation18] showed less clinical efficacy and higher absolute levels of bleeding in patients aged 75 years and older compared to the overall cohort. The TRILOGY ACS investigators [Citation19] even pre-adjusted the prasugrel dose for patients aged 75 years and older, to reduce possible bleeding adverse events. The results from the TRITON-TIMI study are subgroup analyses. Multiple subgroup analyses according to age will be mentioned in this review. Of note, these data were obtained from post hoc analyses and should be considered exploratory.
1.2. Differentiating in HF phenotype
As briefly mentioned above, HFpEF is more common in elderly patients and the prevalence of HFpEF is increasing [Citation15]. HF characterization is mainly based on left ventricular ejection fraction (LVEF). Therefore, HF can be classified into two main subtypes. HF with reduced ejection fraction (HFrEF) and HFpEF. HFrEF, also known as systolic HF, is characterized by a lower-than-normal ejection fraction (LVEF <40%) due to inadequate muscle wall contraction. While HFpEF patients often have atrial fibrillation and hypertension in their history, HFrEF patients often endured a myocardial infarction [Citation2].
HFpEF, also known as diastolic HF, is a heterogeneous syndrome both in the field of etiology and pathophysiology and is characterized by a seemingly normal ejection fraction (LVEF ≥50%). However, wall thickening may cause the ventricle to hold an exceptionally small amount of blood. Due to contributing factors, nonspecific signs and symptoms and the absence of a dilated left ventricle, the HFpEF subtype is hard to dissect [Citation2]. A current review [Citation20] clearly described the clinical classification of HFpEF in which 7 subtypes could be identified. These subtypes were atrial fibrillation, right HF caused by pulmonary venous hypertension, CAD, hypertrophic cardiomyopathy, multivalvular lesions, restrictive cardiomyopathies, and finally the so-called ‘garden variety,’ which includes hypertension, diabetes/metabolic syndrome, obesity and/or chronic kidney disease. Although there is a lot of controversy surrounding HFpEF and its subtypes, abovementioned clinical classification clearly indicates differences in pathophysiology between HFpEF and HFrEF. HFpEF and HFrEF also seem to have different etiological profiles: HFpEF patients are generally older [Citation21,Citation22] and more often women [Citation22,Citation23] compared to HFrEF patients.
According to the most recent European Society of Cardiology (ESC) HF guidelines, the gray area between HFrEF and HFpEF is classified as a third HF phenotype as the group with a LVEF of 40–49%, also known as HF with mid-range ejection fraction (HFmrEF). HFmrEF patients are likely to suffer from mild systolic dysfunction together with characteristics of diastolic dysfunction [Citation2]. This also called middle child [Citation24] is currently under much investigation and might be depicted as a transient between HFpEF and HFrEF. ‘Identifying HFmrEF as a separate group will stimulate research into the underlying characteristics, pathophysiology, and treatment of this group of patients’, according to the most recent ESC HF guidelines [Citation2].
In addition to the physical examination and the medical history, biomarkers also showed to be indispensable tools in establishing HF diagnosis and provide an estimate of prognosis. Biomarkers are evaluated whether they could play a role in disease management strategies [Citation25]. The most studied and established HF biomarkers are high-sensitivity troponin T (hsTnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) [Citation26], which indicate myocyte injury and wall stress, respectively. Although both markers are elevated in HF, HFrEF patients tend to have higher levels compared to HFpEF. Nevertheless, both yield a comparable prognostic value regardless of LV ejection fraction [Citation27]. Beside the diagnostic and prognostic value of these biomarkers in HF management, it has been shown that biomarkers can be powerful in the identification of new onset HF [Citation28,Citation29] and can also identify HF patients with low risk of event [Citation30]. Additionally, early HF treatment based upon biomarker levels in patients with at least one comorbidity has proven to reduce the HF incidence [Citation30–Citation34]. Up until now, no concrete evidence to recommend them for routine clinical use is in place [Citation2]. Beside the established biomarkers, novel biomarkers might add some incremental value regarding differentiation between HFrEF and HFpEF [Citation35,Citation36]. It has been suggested that the fibrosis marker galectin-3 [Citation36,Citation37], suppression of tumorigenicity 2 (ST-2) [Citation38], and growth differentiation factor 15 (GDF-15) [Citation39] might play a prominent role in this phenotype distinction.
2 Therapeutic challenges in the elderly
Since elderly mainly suffer from the HFpEF phenotype, we attempted in this review to primarily focus on treatment options for HFpEF patients but also to provide an overview of important conducted studies and ongoing trials. Unfortunately, the established medical regimen in HFrEF patients has failed to improve outcome in HFpEF. This includes angiotensin converting enzyme (ACE) inhibitors [Citation40], angiotensin receptor blockers (ARB)s [Citation41], β-blockers [Citation42], and digoxin [Citation43]. This might be due to the nonspecific symptoms, the heterogeneous phenotype, and the lack of clarity regarding a well-defined definition of HFpEF. Although, in a specific subgroup of elderly with chronic HFpEF there is evidence that β-blockers might be of benefit [Citation44]. Beside medication, life style changes are also very important in HF treatment. More than 70% of HFpEF patients suffer from obesity [Citation45], an independent risk factor for HF development [Citation46,Citation47]. Despite the so-called ‘HF obesity paradox’ (obese HF patients show lower mortality rates) [Citation45], caloric restriction diet seems to have beneficial effects in HFpEF patients [Citation48]. The same applies to aerobic exercise training, since exercise intolerance is one of the primary symptoms of chronic HF [Citation2].
According to the ESC, HF diagnosis is based on clinical signs, chest X-rays and electrocardiograms (ECG) [Citation2]. A retrospective study [Citation49] with 116 old patients demonstrated that the specificity of abovementioned methods is rather worse, with only 50%, 20%, and 9%, respectively. Consequently, diagnosing HF in elderly is a difficult task and HF management regarding HFpEF patients is solely symptomatic [Citation50].
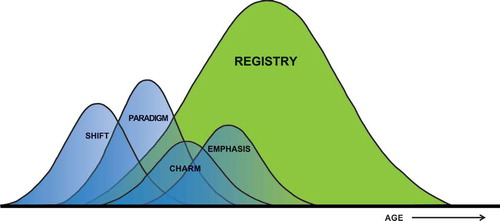
Despite the considerable prevalence of HF in the elderly, elderly are often underrepresented in clinical trials. As can be noted from , clinical trials only examine a part of the HF population compared to HF registries [Citation51]. Further, elderly have significantly worse outcomes compared to patients of the younger generation [Citation52]. Therefore, the importance of studies to the efficiency and safety of recommended treatments in elderly cannot be overemphasized.
Figure 2. The elderly population is often underrepresented in clinical trials, while the majority of HF patients consists of elderly, as can be noted from the HF registry.
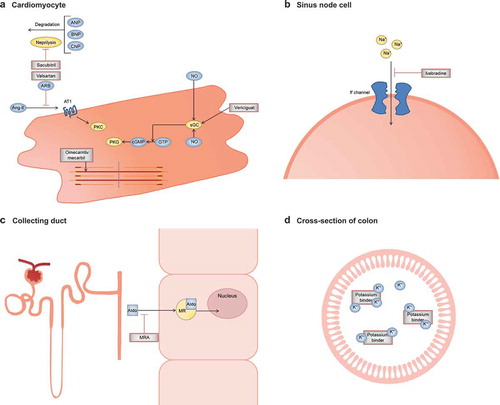
Although limitations exist to study this specific population, clinicians have to accept the fact that new therapeutic options are a necessity to treat and manage these patients in the future. The current review will focus on new medical treatment options that are recently recommended or might be expected soon. We will include sacubitril/valsartan (LCZ696), omecamtiv mecarbil, vericiguat, ivabradine, MRAs, and potassium binders (RLY5016) (for the mechanistic function see ).
Figure 3. Mechanism of function of new treatment options for HF. (a). The ARNI Entresto consists of the angiotensin II receptor antagonist valsartan and the neprilysin inhibitor sacubitril. Valsartan inhibits binding of angiotensin II and sacubitril prevents breakdown of endogenous natriuretic peptides. Omecamtiv mecarbil selectively activates cardiac myosin, resulting in increased myocardial contractility. Vericiguat stimulates sGC, resulting in production of cGMP and beneficial cardiovascular effects through the PKG pathway. (b). Ivabradine specifically inhibits the If channel of the sinus node cells, resulting in reduction of the slow depolarization action potential with decreased heart rate as a result. (c). MRAs function as antagonists of aldosterone in principal cells of the collecting duct, preventing the effects of sodium retention, cardiac hypertrophy and cardiac fibrosis. (d). Potassium binders prevent intestinal potassium uptake by binding potassium, thereby preventing a major problem in HF; hyperkalemia. Abbreviations: Aldo, aldosterone; Ang-II, angiotensin II; ANP, A-type natriuretic peptide; ARB, angiotensin-receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; AT1, type-1 angiotensin II receptor; BNP, B-type natriuretic peptide; cGMP, cyclic guanosine monophosphate; CNP, C-type natriuretic peptide; GTP, guanosine-5ʹ-triphosphate; If channel, funny channel; K+, potassium; MR, mineralocorticoid receptor; MRA, mineralocorticoid receptor antagonist; Na+, sodium; NO, nitric oxide; PKC, protein kinase C; PKG, protein kinase G, sGC, soluble guanylate cyclase.
2.1. Sacubitril/valsartan – LCZ696
A hallmark in HF management is the renin-angiotensin-aldosterone system (RAAS) inhibition. The CONSENSUS trial in 1987 [Citation53] already demonstrated the enormous benefit (40% mortality reduction) RAAS inhibition had on cardiovascular death. The importance of RAAS inhibition was also observed in the CHARM trial [Citation54], in which the ARB candesartan resulted in a survival benefit. The ELITE trial [Citation55] showed in the elderly HF patient that ARBs were better tolerated compared to ACE inhibitors. In the (small) ELITE I study, treatment with ARBs was associated with an unexpected reduction in mortality of 46% (losartan vs. captopril; 4.8% mortality vs. 8.7% mortality). Prospectively, however, the (well-powered) ELITE II trial [Citation56] did not validate the superiority of losartan over captopril. But, also in this study ARBs had a favorable safety profile compared to ACE inhibitors. Recently, a combination therapeutic of the angiotensin II receptor antagonist valsartan and the neprilysin inhibitor sacubitril (LCZ696) has been studied, also known as an angiotensin receptor-neprilysin inhibitor (ARNI) [Citation57].
The PARAMOUNT study [Citation57], a phase II randomized, double-blind multicenter trial, studied the efficacy of sacubitril/valsartan (n = 149) in HFpEF patients (LVEF >45%) compared to valsartan (n = 152). Sacubitril/valsartan resulted after 12 weeks of treatment in reduced NT-proBNP levels compared to valsartan and was also well tolerated in this study group. Regarding secondary end points, sacubitril/valsartan did correlate with left atrial reverse remodeling and showed a slightly significant improvement regarding New York Heart Association (NYHA) class after 36 weeks. Changes in NT-proBNP were not significantly different in prespecified subgroups according to age (years): <65 (n = 207) and ≥65 (n = 59). However, in the elderly patients sacubitril/valsartan may lead to lower NT-proBNP levels. Whether these effects might translate into improved outcomes needs to be tested prospectively. The PARAGON-HF study, recently announced, will investigate the latter.
Expectations are high, because the PARADIGM-HF study [Citation58], a randomized, double-blind trial, in HFrEF patients (LVEF ≤40%) was terminated early because an overwhelming benefit regarding sacubitril/valsartan was observed. On both end points, cardiovascular mortality and HF hospitalization, sacubitril/valsartan was proven to be superior to enalapril. The PARADIGM-HF trial included a larger number of patients with a broader range of ages compared to previous trials in HFrEF. A sub-analysis of the PARADIGM-HF trial examined the prespecified efficacy and safety outcomes according to age category (years): <55 (n = 1624), 55–64 (n = 2655), 65–74 (n = 2557), and ≥75 (n = 1563). Although the rate of primary end point increased across the age categories, LCZ696 remained to reduce the risk of an adverse event in all age groups with an overall risk reduction of 20% [Citation59]. The PARADIGM-HF trial was designed to obtain evidence to support the replacement of ACE inhibitors or ARBs in the management of chronic HF. Although this study implicates a greater effect of sacubitril/valsartan compared to RAAS inhibitors, one should keep in mind that sacubitril/valsartan treatment has not been studied in comparison with an ARB alone. Additionally, it has to be mentioned that the PARADIGM-HF trial consisted of a single-blind run-in period during which all patients received enalapril, followed by a single-blind run-in period during which all patients received LCZ696. This was done to ensure an acceptable side effect profile, but also constitutes a selection bias: Only eligible patients could continue the study, while elderly HF patients might be more susceptible to suffer from side effects and might be excluded based on these side effects.
Besides the PARAGON-HF, also two other trials are being conducted with sacubitril/valsartan namely (1) PARADISE-MI, a randomized, double-blind trial, which will investigate the efficacy and safety of sacubitril/valsartan in patients with acute myocardial infarction compared to ramipril and (2) TRANSITION, a randomized single-blind study, which will compare predischarge and post-discharge treatment with sacubitril/valsartan in HFrEF patients hospitalized for acute decompensation. In contrast to the above-described trials, the PARAMETER study (ARNI vs. ARB) [Citation60,Citation61] specifically focused on the elderly population (aged ≥60 years). The efficacy of sacubitril/valsartan in patients with essential hypertension (SBP ≥150 to <180) was studied. Primary and secondary end points included changes in central aortic systolic pressure (CASP) and central aortic pulse pressure (CAPP) after 12 weeks of treatment. Sacubitril/valsartan showed to be superior to olmesartan in reducing both CASP and CAPP in high-risk older patients.
2.2. Omecamtiv mecarbil
In HF patients, myocardial contractility is impaired. Several pharmaceuticals [Citation62–Citation66] are developed to improve this contractility, by increasing the intracellular calcium levels. Extra calcium results in an increased force and velocity of contraction, but increased myocardial oxygen consumption is needed to actively transport the calcium back into the sarcoplasmic reticulum. Furthermore, the elevations in intracellular calcium can increase the risk of arrhythmia [Citation63]. A possible therapeutic candidate is omecamtiv mecarbil (OM) because it is not focused on increasing the calcium levels in cardiomyocytes. OM is specifically designed to increase myocardial contractility, as a new selective cardiac myosin activator. OM accelerates the transition of cross-bridges between myosin and actin filaments and therefore mainly has an effect on the duration of systole instead of ventricular pressure [Citation67], which has been proven by Teerlink et al. in a study with 34 healthy men in 2011 [Citation68].
Cleland et al. [Citation69] studied the safety, tolerability, and efficacy of OM in patients with stable chronic systolic HF (LVEF ≤40%) in a double-blind phase II trial. Patients (n = 45) received infusions with different doses of OM or placebo. OM showed both a dose-dependent as well as a concentration-dependent increase in duration of systole and increased stroke volume. In addition, the ATOMIC-AHF investigators [Citation70] evaluated the efficacy and safety of intravenous OM treatment in patients with systolic dysfunction (LVEF ≤40%) who were admitted with acute symptoms of HF. The primary efficacy end point was the improvement of dyspnea. Secondary outcomes of interest evaluated changes in NT-proBNP, incidence of worsening HF, and short-term clinical outcomes. No improvement regarding both the primary and secondary end points was observed. Although OM showed a significant increase in LV systolic ejection time, an increase in stroke volume could not be observed. Nevertheless, OM was found to be well tolerated and showed a trend toward dyspnea improvement in the high-dose group.
In addition to intravenous OM treatment studies, the COSMIC-HF trial [Citation71] investigated oral OM treatment in a randomized, double blind phase II safety/efficacy study in patients with chronic HF and LV systolic dysfunction (LVEF ≤40%). Enrolled patients were randomized in either a fixed-dose regimen (n = 150) or dose based upon pharmacokinetics (n = 149). Efficacy endpoints included systolic ejection time, left ventricular end-systolic and end-diastolic diameters, heart rate, stroke volume and plasma concentration of NT-proBNP. Pharmacokinetic-titration based on plasma concentrations showed significant improvement regarding systolic ejection time, stroke volume and LV fractional shortening after 24 weeks of treatment compared to placebo.
Contractility issues might also be of importance in HFpEF, since Paulus et al. [Citation72] described a new paradigm in HFpEF development. High prevalence of comorbidities can induce a systemic proinflammatory state, resulting in structural and functional alterations of the myocardial tissue in which the protein titin plays a major role. Based on this hypothesis, an effect of OM may also be anticipated in HFpEF patients.
2.3. Vericiguat
HF is associated with increased inflammation and vascular dysfunction, accompanied by reduced nitric oxide (NO) levels, which ensures vasodilation in healthy individuals by binding its ‘receptor’ soluble guanylate cyclase (sGC) [Citation73]. Vericiguat is an oral sGC stimulator, which induces the production of the second messenger cyclic guanosine monophosphate (cGMP). HFpEF is associated with increased LV and peripheral vascular wall stiffness and right and left ventricular diastolic dysfunction, which results in altered LV end-diastolic pressures and ventricular relaxation. Since the cGMP pathway is an important regulator of endothelial function, cardiac performance and myocardial energetics, targeting the cGMP pathway provides compelling rationale as a therapeutic option in HFpEF patients [Citation74,Citation75]. Treatment with sCG stimulators might result in a maximal sGC activation, despite submaximally active NO levels in HF patients.
The SOCRATES study [Citation76] consists of two multicenter, randomized, double-blind, dose-finding phase II trials, namely SOCRATES-REDUCED and SOCRATES-PRESERVED. The recently published SOCRATES-REDUCED trial [Citation77] examined the efficacy and tolerability of vericiguat in patients with worsening chronic HF after clinical stabilization and LVEF <45%. The dose effect of vericiguat was examined based upon NT-proBNP levels and this was considered as the primary end point. 351 patients were randomized to different doses of vericiguat or placebo. Vericiguat treatment showed to be well tolerated, but failed to significantly reduce NT-proBNP levels compared to placebo. However, vericiguat might still be a promising agent. Prespecified secondary analyses demonstrated a significant increase in LVEF after 12 weeks of treatment with the 10-mg dose compared to placebo and the 10-mg dose was also associated with a trend toward reduction of clinical cardiovascular events. The SOCRATES-PRESERVED trial studied the same end points in HFpEF patients (LVEF ≥45%). This study is finished, but study results have not been published yet. The phase III VICTORIA trial (NCT02861534), currently enrolling HFrEF patients, aims to determine the efficacy of vericiguat compared to placebo on cardiovascular death or HF hospitalization.
2.4. Ivabradine
Ivabradine (corlanor or procoralan) specifically inhibits the funny (If) channel, which regulates pacemaker activity in the sinoatrial node. Inhibition leads to reduction of the slow depolarization action potential and thereby resulting in decreased heart rate. As far as known, ivabradine does not directly modify other cardiovascular parameters and, therefore, will not cause reduction of blood pressure, in contrast to β-blockers [Citation78,Citation79].
The SHIFT trial [Citation80], a randomized, double-blind, placebo-controlled trial studied the efficacy of ivabradine treatment in patients with HFrEF (LVEF ≤35%) compared to placebo. Reduction in heart rate (approximately 15 bpm from a baseline value of 80 bpm) by ivabradine resulted in an 18% relative risk reduction of the primary end point, a composite of cardio-vascular deaths and HF rehospitalization. Treatment with ivabradine was not significant different between elderly (≥65 years) and the younger population (<65 years). However, the younger patients seemed to have more benefit from ivabradine. The SHIFT trial investigated symptomatic HF patients, the BEAUTIFUL and SIGNIFY trial investigated subjects with CAD. The BEAUTIFUL investigators [Citation81] demonstrated that ivabradine treatment does not result in better outcome regarding the primary end point. This was a composite of (1) cardiovascular death, (2) admission for acute myocardial infarction, and (3) new onset or worsening HF in patients enrolled with CAD and LVEF <40%. Although ivabradine might be a therapeutic option, the randomized, double-blind, SIGNIFY trial [Citation82], which investigated the efficacy of ivabradine in patients with stable CAD without clinical HF (LVEF >40%) did not result in a reduced risk of cardiovascular death or death from nonfatal myocardial infarction compared to placebo regardless of age.
Kosmala et al. [Citation83] proved that short-term ivabradine treatment (n = 30) leads to improvement of exercise tolerance by improvement of LV filling pressure in symptomatic patients with HFpEF (LVEF ≥50%) compared to placebo (n = 31). Because many HFpEF patients only show HF symptoms during exercise, ivabradine treatment may be beneficial in HFpEF patients. However, a randomized crossover study [Citation84] examined the effect of ivabradine treatment on exercise capacity in 22 symptomatic HFpEF patients with objective evidence of exercise limitation compared to placebo. Study results were compared with 22 similarly treated matched asymptomatic hypertensive volunteers. Change in VO2 peak was set as primary end point. Ivabradine treatment significantly worsened the change in VO2 peak compared to placebo in HFpEF patients and significantly reduced submaximal exercise capacity, which was determined by the oxygen uptake efficiency slope. These results question the role of heart rate reduction in improving symptoms in HFpEF patients.
2.5. Mineralocorticoid receptor antagonists
The physiological importance of aldosterone is indirect regulation of blood volume and blood pressure by sodium retention. However, aldosterone also plays an essential role in the pathogenesis of HF [Citation85]. By antagonizing aldosterone, mineralocorticoid receptor antagonists (MRAs) can prevent the pathophysiological effects of sodium retention, cardiac hypertrophy and cardiac fibrosis [Citation86]. At present, the success of several MRAs has already been established in HFrEF. First, the RALES trial [Citation87] determined the efficacy of co-therapy with spironolactone in patients with severe HF (LVEF ≤35%) compared to placebo. The primary efficacy end point evaluated all-cause mortality and secondary end points included cardiovascular death and hospitalization and change in NYHA class. Spironolactone treatment proved to be successful in reducing the risk of all-cause mortality (30% risk reduction) and prespecified secondary outcomes when compared to placebo regardless of age.
Second, the EPHESUS investigators [Citation88] studied the efficacy of eplerenone treatment in addition to optimal treatment in a multicenter, randomized, double-blind trial in patients with LV dysfunction (LVEF ≤40%) after acute myocardial infarction. Treatment with eplerenone led to reduction of overall mortality (15% risk reduction) and to reduction of cardiovascular death and hospitalization (13% risk reduction) in comparison with placebo. Although not significant, younger patients showed a tendency toward better outcome compared to elderly (p = 0.08).
Finally, the multicenter, randomized EMPHASIS-HF trial [Citation89,Citation90] also investigated the effectiveness of eplerenone in patients with systolic HF (LVEF ≤35%) and mild HF symptoms. The EMPHASIS-HF only studied patients with an age above 55 and revealed a risk reduction in primary end point (combination of cardiovascular death and HF hospitalization) by 37% and additionally a reduction in the rate of death from any cause and the rate of hospitalization for any reason compared to placebo.
The last few years, there is also an increasing number of studies suggesting a beneficial effect of MRAs in HFpEF patients. In the Aldo-DHF trial [Citation91], HFpEF patients (LVEF ≥50%) were randomized to spironolactone or placebo and followed up for 12 months. Spironolactone treatment was found to enhance diastolic function, but no significant differences were observed in maximal exercise capacity, HF symptoms or quality of life. However, in the Aldo-DHF trial, the clinical significance of the improved LV function was not examined. The TOPCAT study [Citation92], a randomized, double-blind, phase III trial studied the efficacy of spironolactone in patients with symptomatic HF and a LVEF of ≥45%. The primary end point was a composite of cardiovascular death, aborted cardiac arrest and HF hospitalization. Spironolactone treatment failed to show significant reduction in primary outcome compared to placebo. However, a post hoc analysis [Citation93] showed a markedly reduced rate of primary outcome, cardiovascular death and HF hospitalization in the Americas, suggesting a beneficial effect of spironolactone in elderly HFpEF patients.
2.6. Potassium binders
Hyperkalemia is a well-known problem in HF patients and is associated with both mortality and hospitalization [Citation94]. Especially elderly are vulnerable to hyperkalemia due to decreased aldosterone production and cardiac therapy [Citation95,Citation96], in which mainly RAAS inhibitors and MRAs play a role. The fear for occurrence of hyperkalemia might result in premature discontinuation of treatment or insufficient dosing [Citation97], resulting in increased cardiovascular risk. The current therapy options for reducing potassium levels carry quite some disadvantages like (1) patient adherence is low and (2) current pharmaceuticals might have low tolerability and entail unfavorable adverse effects [Citation98]. In the light of this knowledge, potassium binders, which can prevent intestinal potassium uptake, may offer a solution.
RLY5016, which is also known as patiromer sorbitex calcium, is the most studied potassium binder. In the past 50 years, RLY5016 is the first new drug approved for hyperkalemia treatment [Citation99]. The PEARL-HF investigators [Citation100] conducted a randomized, double-blind trial, in which the efficacy and safety of the potassium binder RLY5016 was investigated in patients with chronic HF (LVEF ~40%). Patients were treated with RLY5016 in addition to standard therapy, including an ACE inhibitor or ARB and a β-blocker, resulting in significant reduction of serum K+. As expected, RLY5016 has proven to reduce the incidence of hyperkalemia and to increase the number of patients which could be up-titrated with spironolactone compared to placebo. On the other hand, RLY5016 treatment resulted, as could be expected, in more cases in hypokalemia compared to placebo (6% vs. 0%).
3 Conclusion
A major concern for the next generation is the rise of elderly people, and specifically elderly patients with HF. As discussed in this review, most elderly HF patients can be characterized as HFpEF patients. Diagnosing, treating, and managing patients with HFpEF still remains a challenge. This is due to (1) the heterogeneous syndrome, (2) the associated lack of clarity of current diagnostic criteria, (3) the absence of established treatment, (4) the focus on HFrEF in most HF studies, and (5) the large discrepancy between enrolled patients in HF trials and registries, as depicted in . Intervention, to (1) prevent disease development (2) slowdown disease progression, (3) treat comorbid conditions, (4) improve quality of life, and (5) reduce HF rehospitalizations and cardiovascular death, should be a top priority for the upcoming years. In summary, these observations imply that studies relating novel therapeutic options for HFpEF are needed.
4 Expert opinion
In the last decades, the cardiology field has improved dramatically, especially with improved re-synchronization therapy [Citation101], ventricular assist devices [Citation102,Citation103], and heart transplantation [Citation104]. Scientists assemble to keep improving at the same pace. These new pharmaceuticals have been studied and seem promising, but concrete results for management of HFpEF are still lacking. Nevertheless, we might need to accept that new developed drugs will not be able to drastically reduce the primary end point of trials published in the 80s and 90s. Although we need to strive for smaller goals, for example vericiguat, which seemed a promising agent in HFrEF. Compared to placebo it resulted in an increase in LVEF and a trend toward reduction of clinical events. Although study results of vericiguat treatment in HFpEF patients are likely to be published on short notice, no current HFpEF trials are available at this moment. Also, not a single HFpEF study to the efficacy of OM and the potassium binder RLY5016 have been performed. Understanding the problems that we face, and the ‘lacks in our knowledge’ that currently exist, we are eager to hopefully see these kind of studies emerging on a short notice. Intravenous OM showed an increase in LV systolic ejection time and a trend toward dyspnea improvement and oral OM showed improvement in systolic ejection time, stroke volume, and LV fractional shortening, which all might benefit HFpEF patients. Tackling side effects that prohibit optimal uptitration of HF medication are also welcome. This could be a possible role for potassium binders. These drugs may be of great potential for elderly patients, as they are primarily vulnerable for hyperkalemia due to reduced aldosterone levels.
In contrast to the above-mentioned drugs, data are available of sacubitril/valsartan, ivabradine and MRAs in HFpEF patients. Sacubitril/valsartan proved to be well tolerated and showed an overwhelming advantage in cardiovascular mortality and HF hospitalization in HFrEF patients. Also in HFpEF patients, sacubitril/valsartan appears promising. It was associated with left atrial reverse remodeling and an improvement in NYHA class. Additionally, the PARAMETER trial, which specifically focused on elderly, also showed positive results. We all await the results of the prospective PARAGON-HF study which will provide us with the evidence whether sacubitril/valsartan will lead to improved outcomes in HFpEF patients.
Ivabradine treatment showed to reduce cardiovascular deaths and HF rehospitalizations in HFrEF patients. Additionally, ivabradine may also prove useful in HFpEF patients, because of the proven increase in exercise tolerance. Finally, in recent years, an increasingly amount of clinical trials focused on the efficacy of MRAs in HFpEF patients and showed beneficial effects.
4.1. Challenges ahead
Ageing results in age-related physiological changes, not all cardiac related. Older HF patients often have to cope with multiple comorbidities. These diseases might involve each other, especially as hypothesized in HFpEF [Citation72]. Additionally, HF patients generally use multiple prescription drugs, up to 10 or 15 in one patient. Clearly the use of such combinations not only raises the likelihood of simple side effects, but drug–drug or drug–disease interactions are common and are often not considered. Specifically, the use of nonsteroidal anti-inflammatory drugs, antiarrhythmic drugs and statins is notorious when it comes to drug interactions [Citation105]. The heterogeneous phenotype of HFpEF is already a reason not to pursue the ‘one-size-fits-all’ approach in HFpEF patients. Comorbidities and polypharmacy make multidisciplinary approaches even more important to optimally treat elderly patients. For instance, age-related decline in kidney function has been recognized for decades, as reflected by estimated glomerular filtration rate (eGFR) [Citation106]. Coexistence of HF and poor renal function occurs regularly and is associated with an extremely bad prognosis [Citation107]. Therefore, it can be very important to adapt therapy dose to kidney function in treatment of elderly patients. We report important indices of pharmacokinetics and influences of kidney function in .
Table 1. The pharmacokinetic/dynamic profile of discussed novel medicaments.
The balance between the treatment of comorbidities, but simultaneously the relevance to be aware of polypharmacy is delicate and therefore the medical regimen for a HF patient has to be chosen wisely. Despite the complicated management of HF in elderly, clinical trials still underrepresent these typical HF patients. Furthermore, the exceedingly heterogeneous HFpEF phenotype might presumably contribute greatly to the failure of randomized clinical trials in HFpEF patients. Therefore, we would like to stimulate studies that shift the focus more on the elderly population and make a better differentiation between HFpEF phenotypes. Further, mortality and hospitalization are common primary and secondary study end points, while frail older patients are probably more interested in their functional capacity and quality of life. This emphasizes the importance of life style changes in addition to medical treatment. Taken together, personalized treatment mainly aimed at quality of life instead of quantity of life, may be a solution for the elderly population.
Article highlights
The current dilemma is that HF is primarily a disease of the elderly population. However, even though the major part of the HF cohort consists of elderly patients, elderly are often underrepresented in clinical trials.
Diagnosing and treating HFpEF patients continues to be a challenge, due to the heterogeneous phenotype, secondary comorbidities and multiple medication and the associated lack of clarity of current diagnostic criteria.
Several novel medicaments, as discussed in this article, have been studied in HFpEF patients and seem promising, although concrete results for management of HFpEF are still lacking.
Mortality and hospitalization are common study endpoints, while elderly patients are likely to have different goals of treatment in comparison with younger patients. Therefore, multidisciplinary approaches mainly focused on quality of life instead of quantity of life are a necessity to optimally treat elderly patients.
Declaration of interest
RA de Boer has received honoraria from Novartis. The University Medical Centre Groningen, which employs the authors, received research funding from AstraZeneca, Bristol-Myers Squibb, and Trevena for research projects, which have no relation to this work. DJ van Veldhuisen has received Board Memberships and/or travel expenses from Novartis and Corvia Medical for participation in studies in the field of heart failure with preserved ejection fraction. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002 Dec 10;106(24):3068–3072.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016 Aug;18(8):891–975.
- Kraai IH, Vermeulen KM, Hillege HL, et al. Perception of impairments by patients with heart failure. Eur J Cardiovasc Nurs. 2016 Apr;15(2):178–185.
- Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009 Sep;2(5):407–413.
- Zuckerman RB, Sheingold SH, Orav EJ, et al. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016 Apr 21;374(16):1543–1551.
- Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the united states: A policy statement from the american heart association. Circ Heart Fail. 2013 May;6(3):606–619.
- Active Ageing: A Policy Framework. World Health Organization, 2002. Available from: http://apps.who.int/iris/bitstream/10665/67215/1/WHO_NMH_NPH_02.8.pdf [Last accessed 15 December 2016].
- World Population Ageing. World Health Organization, 2015. Available from: http://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2015_Report.pdf [Last accessed 15 December 2016].
- Haldeman GA, Croft JB, Giles WH, et al. Hospitalization of patients with heart failure: national hospital discharge survey, 1985 to 1995. Am Heart J. 1999 Feb;137(2):352–360.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016 Jan 26;133(4):e38–360.
- Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. 2012 Jan;8(1):143–164.
- Khatibzadeh S, Farzadfar F, Oliver J, et al. Worldwide risk factors for heart failure: A systematic review and pooled analysis. Int J Cardiol. 2013 Sep 30;168(2):1186–1194.
- Jhund PS, Macintyre K, Simpson CR, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: A population study of 5.1 million people. Circulation. 2009 Feb 3;119(4):515–523.
- Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the united states: A policy statement from the American Heart Association. Circulation. 2011 Mar 1;123(8):933–944.
- Van Riet EE, Hoes AW, Wagenaar KP, et al. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time A systematic review. Eur J Heart Fail. 2016 Mar;18(3):242–252.
- Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003 Oct 1;42(7):1226–1233.
- Wong CY, Chaudhry SI, Desai MM, et al. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med. 2011 Feb;124(2):136–143.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007 Nov 15;357(20):2001–2015.
- Roe MT, Armstrong PW, Fox KA, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012 Oct 4;367(14):1297–1309.
- Shah SJ, Katz DH, Deo RC. Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Fail Clin. 2014 Jul;10(3):407–418.
- Fischer M, Baessler A, Hense HW, et al. Prevalence of left ventricular diastolic dysfunction in the community Results from a doppler echocardiographic-based survey of a population sample. Eur Heart J. 2003 Feb;24(4):320–328.
- Klapholz M, Maurer M, Lowe AM, et al. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol. 2004 Apr 21;43(8):1432–1438.
- Martinez-Brana L, Mateo-Mosquera L, Bermudez-Ramos M, et al. Clinical characteristics and prognosis of heart failure in elderly patients. Rev Port Cardiol. 2015 Jul-Aug;34(7–8):457–463.
- Lam CS, Solomon SD. The middle child in heart failure: heart failure with mid-range ejection fraction (40-50%). Eur J Heart Fail. 2014 Oct;16(10):1049–1055.
- Savarese G, Musella F, D’Amore C, et al. Changes of natriuretic peptides predict hospital admissions in patients with chronic heart failure: A meta-analysis. JACC Heart Fail. 2014 Apr;2(2):148–158.
- deFilippi CR, De Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010 Dec 8;304(22):2494–2502.
- Van Veldhuisen DJ, Linssen GC, Jaarsma T, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013 Apr 9;61(14):1498–1506.
- Brouwers FP, De Boer RA, Van Der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013 May;34(19):1424–1431.
- Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012 Sep 25;126(13):1596–1604.
- Meijers WC, De Boer RA, Van Veldhuisen DJ, et al. Biomarkers and low risk in heart failure. Data from COACH and TRIUMPH. Eur J Heart Fail. 2015 Dec;17(12):1271–1282.
- Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): A prospective randomized controlled trial. J Am Coll Cardiol. 2013 Oct 8;62(15):1365–1372.
- Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013 Jul 3;310(1):66–74.
- Meijers WC, De Boer RA, Ho JE. Biomarkers to identify and prevent new-onset heart failure in the community. Eur J Heart Fail. 2016 Nov;18(11):1351–1352.
- Brouwers FP, Van Gilst WH, Damman K, et al. Clinical risk stratification optimizes value of biomarkers to predict new-onset heart failure in a community-based cohort. Circ Heart Fail. 2014 Sep;7(5):723–731.
- De Boer RA, Daniels LB, Maisel AS, et al. State of the art: newer biomarkers in heart failure. Eur J Heart Fail. 2015 Jun;17(6):559–569.
- Meijers WC, Van Der Velde AR, De Boer RA. Biomarkers in heart failure with preserved ejection fraction. Neth Heart J. 2016 Apr;24(4):252–258.
- De Boer RA, Edelmann F, Cohen-Solal A, et al. Galectin-3 in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013 Oct;15(10):1095–1101.
- Meijers WC, Van Der Velde AR, De Boer RA. ST2 and galectin-3: ready for prime time? EJIFCC. 2016 Aug 1;27(3):238–252.
- Santhanakrishnan R, Chong JP, Ng TP, et al. Growth differentiation factor 15, ST2, high-sensitivity troponin T, and N-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. 2012 Dec;14(12):1338–1347.
- Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006 Oct;27(19):2338–2345.
- Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008 Dec 4;359(23):2456–2467.
- Hernandez AF, Hammill BG, O’Connor CM, et al. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (organized program to initiate lifesaving treatment in hospitalized patients with heart failure) registry. J Am Coll Cardiol. 2009 Jan 13;53(2):184–192.
- Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary Digitalis Investigation Group trial. Circulation. 2006 Aug 1;114(5):397–403.
- Van Veldhuisen DJ, Cohen-Solal A, Bohm M, et al. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: data from SENIORS (study of effects of nebivolol intervention on outcomes and rehospitalization in seniors with heart failure). J Am Coll Cardiol. 2009 Jun 9;53(23):2150–2158.
- Haass M, Kitzman DW, Anand IS, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the irbesartan in heart failure with preserved ejection fraction (I-PRESERVE) trial. Circ Heart Fail. 2011 May;4(3):324–331.
- Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002 Aug 1;347(5):305–313.
- Morkedal B, Vatten LJ, Romundstad PR, et al. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (nord-trondelag health study), Norway. J Am Coll Cardiol. 2014 Mar 25;63(11):1071–1078.
- Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2016 Jan 5;315(1):36–46.
- Lien CT, Gillespie ND, Struthers AD, et al. Heart failure in frail elderly patients: diagnostic difficulties, co-morbidities, polypharmacy and treatment dilemmas. Eur J Heart Fail. 2002 Jan;4(1):91–98.
- Vazir A, Solomon SD. Management strategies for heart failure with preserved ejection fraction. Heart Fail Clin. 2014 Oct;10(4):591–598.
- Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006 Nov 8;296(18):2217–2226.
- Herrera AP, Snipes SA, King DW, et al. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am J Public Health. 2010 Apr 1;100(Suppl 1):S105–12.
- The CONSENSUS trial study group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). the CONSENSUS trial study group. N Engl J Med. 1987 Jun 4;316:(23)1429–1435.
- Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-overall programme. Lancet. 2003 Sep 6;362(9386):759–766.
- Pitt B, Segal R, Martinez FA, et al. Randomised trial of losartan versus captopril in patients over 65 with heart failure (evaluation of losartan in the elderly study, ELITE). Lancet. 1997 Mar 15;349(9054):747–752.
- Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial–the losartan heart failure survival study ELITE II. Lancet. 2000 May 6;355(9215):1582–1587.
- Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: A phase 2 double-blind randomised controlled trial. Lancet. 2012 Oct 20;380(9851):1387–1395.
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014 Sep 11;371(11):993–1004.
- Jhund PS, Fu M, Bayram E, et al. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015 Oct 7;36(38):2576–2584.
- Williams B, Cockcroft JR, Kario K, et al. Rationale and study design of the prospective comparison of angiotensin receptor neprilysin inhibitor with angiotensin receptor blocker MEasuring arterial sTiffness in the eldERly (PARAMETER) study. BMJ Open. 2014 Feb 4;4(2):e004254,2013–004254.
- Williams B, Cockcroft JR, Kario K, et al. Effects of Sacubitril/Valsartan versus olmesartan on central hemodynamics in the elderly with systolic hypertension: the PARAMETER study. Hypertension. 2017 Mar;69(3):411–420.
- O’Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1999 Jul;138(1 Pt 1):78–86.
- Cohn JN, Goldstein SO, Greenberg BH, et al. A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure. Vesnarinone Trial Investigators. N Engl J Med. 1998 Dec 17;339(25):1810–1816.
- Packer M, Carver JR, Rodeheffer RJ, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE study research group. N Engl J Med. 1991 Nov 21;325(21):1468–1475.
- Packer M, Colucci W, Fisher L, et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013 Apr;1(2):103–111.
- Krell MJ, Kline EM, Bates ER, et al. Intermittent, ambulatory dobutamine infusions in patients with severe congestive heart failure. Am Heart J. 1986 Oct;112(4):787–791.
- Malik FI, Hartman JJ, Elias KA, et al. Cardiac myosin activation: A potential therapeutic approach for systolic heart failure. Science. 2011 Mar 18;331(6023):1439–1443.
- Teerlink JR, Clarke CP, Saikali KG, et al. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: A first-in-man study. Lancet. 2011 Aug 20;378(9792):667–675.
- Cleland JG, Teerlink JR, Senior R, et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: A double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011 Aug 20;378(9792):676–683.
- Teerlink JR, Felker GM, McMurray JJ, et al. Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure: the ATOMIC-AHF study. J Am Coll Cardiol. 2016 Mar 29;67(12):1444–1455.
- Teerlink JR, Felker GM, McMurray JJ, et al. Chronic oral study of myosin activation to increase contractility in heart failure (COSMIC-HF): A phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet. 2016 Dec 10;388(10062):2895–2903.
- Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013 Jul 23;62(4):263–271.
- Stasch JP, Schmidt PM, Nedvetsky PI, et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest. 2006 Sep;116(9):2552–2561.
- Greene SJ, Gheorghiade M, Borlaug BA, et al. The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. J Am Heart Assoc. 2013 Dec 11;2(6):e000536.
- Dubin RF, Shah SJ. Soluble guanylate cyclase stimulators: A novel treatment option for heart failure associated with cardiorenal syndromes? Curr Heart Fail Rep. 2016 Jun;13(3):132–139.
- Pieske B, Butler J, Filippatos G, et al. Rationale and design of the SOluble guanylate cyclase stimulatoR in heArT failurE studies (SOCRATES). Eur J Heart Fail. 2014 Sep;16(9):1026–1038.
- Gheorghiade M, Greene SJ, Butler J, et al. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES-REDUCED randomized trial. JAMA. 2015 Dec 1;314(21):2251–2262.
- Bohm M, Swedberg K, Komajda M, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010 Sep 11;376(9744):886–894.
- Oliphant CS, Owens RE, Bolorunduro OB, et al. Ivabradine: A review of labeled and off-label uses. Am J Cardiovasc Drugs. 2016 Oct;16(5):337–347.
- Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet. 2010 Sep 11;376(9744):875–885.
- Fox K, Ford I, Steg PG, et al. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): A randomised, double-blind, placebo-controlled trial. Lancet. 2008 Sep 6;372(9641):807–816.
- Fox K, Ford I, Steg PG, et al. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014 Sep 18;371(12):1091–1099.
- Kosmala W, Holland DJ, Rojek A, et al. Effect of if-channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: A randomized trial. J Am Coll Cardiol. 2013 Oct 8;62(15):1330–1338.
- Pal N, Sivaswamy N, Mahmod M, et al. Effect of selective heart rate slowing in heart failure with preserved ejection fraction. Circulation. 2015 Nov 3;132(18):1719–1725.
- Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001 Dec 6;345(23):1689–1697.
- Pitt B, Pedro Ferreira J, Zannad F. Mineralocorticoid receptor antagonists in patients with heart failure: current experience and future perspectives. Eur Heart J Cardiovasc Pharmacother. 2017;3(1):48–57.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999 Sep 2;341(10):709–717.
- Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003 Apr 3;348(14):1309–1321.
- Zannad F, McMurray JJ, Drexler H, et al. Rationale and design of the Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure (EMPHASIS-HF). Eur J Heart Fail. 2010 Jun;12(6):617–622.
- Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011 Jan 6;364(1):11–21.
- Edelmann F, Wachter R, Schmidt AG, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013 Feb 27;309(8):781–791.
- Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014 Apr 10;370(15):1383–1392.
- Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation. 2015 Jan 6;131(1):34–42.
- Jain N, Kotla S, Little BB, et al. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012 May 15;109(10):1510–1513.
- Rosa RM, Silva P, Young JB, et al. Adrenergic modulation of extrarenal potassium disposal. N Engl J Med. 1980 Feb 21;302(8):431–434.
- Acker CG, Johnson JP, Palevsky PM, et al. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med. 1998 Apr 27;158(8):917–924.
- Dinsdale C, Wani M, Steward J, et al. Tolerability of spironolactone as adjunctive treatment for heart failure in patients over 75 years of age. Age Ageing. 2005 Jul;34(4):395–398.
- Chaaban A, Abouchacra S, Gebran N, et al. Potassium binders in hemodialysis patients: A friend or foe? Ren Fail. 2013;35(2):185–188.
- Vu BN, De Castro AM, Shottland D, et al. Patiromer: the first potassium binder approved in over 50 years. Cardiol Rev. 2016 Nov/Dec;24(6):316–323.
- Pitt B, Anker SD, Bushinsky DA, et al. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011 Apr;32(7):820–828.
- Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009 Oct 1;361(14):1329–1338.
- Schmitto JD, Hanke JS, Rojas SV, et al. First implantation in man of a new magnetically levitated left ventricular assist device (HeartMate III). J Heart Lung Transplant. 2015 Jun;34(6):858–860.
- Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012 Jun 26;125(25):3191–3200.
- Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: thirty-second official adult heart transplantation report–2015; focus theme: early graft failure. J Heart Lung Transplant. 2015 Oct;34(10):1244–1254.
- Mukete BN, Ferdinand KC. Polypharmacy in older adults with hypertension: A comprehensive review. J Clin Hypertens (Greenwich). 2016 Jan;18(1):10–18.
- Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest. 1950 May;29(5):496–507.
- Longhini C, Molino C, Fabbian F. Cardiorenal syndrome: still not a defined entity. Clin Exp Nephrol. 2010 Feb;14(1):12–21.
- Vu T, Ma P, Xiao JJ, et al. Population pharmacokinetic-pharmacodynamic modeling of omecamtiv mecarbil, a cardiac myosin activator, in healthy volunteers and patients with stable heart failure. J Clin Pharmacol. 2015 Nov;55(11):1236–1247.
- Greenberg BH, Chou W, Saikali KG, et al. Safety and tolerability of omecamtiv mecarbil during exercise in patients with ischemic cardiomyopathy and angina. JACC Heart Fail. 2015 Jan;3(1):22–29.