ABSTRACT
Objective: To assess the efficacy and safety of teneligliptin as add-on to insulin monotherapy in patients with type 2 diabetes mellitus (T2DM).
Research design and methods: In a 16-week, double-blind period, 148 Japanese T2DM patients with inadequate glycemic control with insulin and diet/exercise therapies were randomized to placebo or teneligliptin 20 mg. In a subsequent 36-week, open-label period, all patients received teneligliptin once daily. The primary outcome measure was change in HbA1c at the end of the double-blind period.
Results: The difference between placebo and teneligliptin in change in HbA1c in the double-blind period (least squares mean ± SE) was −0.80% ± 0.11%; teneligliptin was superior (ANCOVA, P < 0.001). The HbA1c-lowering effect of teneligliptin was maintained throughout the open-label period. The incidence of adverse events was 53.5% with placebo and 44.2% with teneligliptin in the double-blind period, 66.7% in the placebo/teneligliptin group in the open-label period, and 77.9% in the teneligliptin/teneligliptin group over both double-blind/open-label periods. The incidence of hypoglycemic symptoms was 11.1% in the placebo/teneligliptin group in the open-label period and 27.3% in the teneligliptin/teneligliptin group over both double-blind/open-label periods.
Conclusion: Teneligliptin was effective and well tolerated in Japanese T2DM patients with inadequate glycemic control.
Clinical trial registration: NCT02081599
1. Introduction
The incidence of type 2 diabetes mellitus (T2DM) is increasing rapidly worldwide, with the number of patients with diabetes expected to reach 642 million by 2040 [Citation1]. This is at least partly owing to the increasing number of T2DM patients in East Asian countries, which now comprise one-quarter of the global diabetes population [Citation2]. Insulin is widely used to treat T2DM, and in Japan and Korea, approximately 15% of patients with T2DM are prescribed insulin (either alone or in combination therapy) [Citation3,Citation4]. Insulin can prevent glucose toxicity through its strong blood glucose-lowering effect, and early introduction of insulin therapy has been reported to prevent the progression of diabetes mellitus [Citation5–Citation9]. Such effect has also been reported in Asian patients [Citation8,Citation9]. Insulin can be used in combination with other antidiabetic drugs and also for patients on dialysis. However, compared with other antidiabetic drugs, insulin requires careful attention with respect to administration timing and dose adjustment. Some patients do not adequately achieve their therapeutic goals despite insulin dose increases [Citation10]. In addition, insulin carries greater risk of hypoglycemia compared with other drugs, and is likely to cause weight gain [Citation5].
The use of dipeptidyl peptidase-4 (DPP-4) inhibitors, which act in a blood glucose-dependent manner, carries a low risk of hypoglycemia [Citation11]. In Japan, DPP-4 inhibitors, including teneligliptin (TNL), are the most commonly prescribed antidiabetic drugs [Citation12], and are suitable for both elderly and dialysis patients [Citation13]. In addition, DPP-4 inhibitors are weight neutral [Citation14–Citation16]. It has been reported that, for patients responding inadequately to insulin, addition of a DPP-4 inhibitor to insulin therapy is more effective than a dose increase of insulin [Citation10]. Taken together, a DPP-4 inhibitor may be the logical therapeutic option as an add-on to insulin therapy.
TNL has selective and long-lasting DPP-4 inhibitory activity, with an elimination half-life of 24.2 h, and ameliorates meal-related glycemic fluctuations throughout the day [Citation17]. In a prospective, unblinded, short-duration pilot study, addition of TNL to insulin therapy improved diurnal glucose and suppressed glycemic fluctuations assessed using continuous glucose monitoring, without increasing the frequency of hypoglycemia [Citation18]. However, no randomized double-blind, placebo-controlled studies have yet assessed the safety and efficacy of TNL add-on to insulin monotherapy in Japanese T2DM patients.
We examined the efficacy and safety of concomitant use of TNL in patients with T2DM who had inadequate glycemic control with insulin monotherapy. We also conducted a subgroup analysis according to the type of insulin regimen, dose of insulin, and age (over or under 65 years), as these factors may affect the safety or efficacy of TNL.
2. Patients & methods
2.1. Ethics
Prior to the conduct of this study, the Institutional Review Board reviewed and approved the contents of the study protocol and the informed consent document as well as the advisability of the conduct of this study, in terms of ethical, scientific, and medical/pharmaceutical viewpoints.
This study was conducted according to the principles of the Declaration of Helsinki, in accordance with the Pharmaceuticals and Medical Devices Act, the Ministerial Ordinance on Good Clinical Practice for Drugs, the Ministerial Ordinance on Good Post-marketing Study Practice for Drugs, the study protocol, and the relevant laws and regulations. Throughout the entire study, no serious protocol deviation was reported from study sites, and the safety of subjects and ethical conduct of the study were confirmed. All subjects provided written informed consent prior to participating in the study.
The trial is registered with ClinicalTrials.gov (NCT02081599).
2.2. Study design
The study involved two treatment periods: a 16-week, randomized, double-blind, placebo-controlled period, and a 36-week, open-label period (Supplementary Figure 1).
After obtaining informed consent and confirming their eligibility, all patients were randomized with a block replacement method to placebo or TNL in a 1:1 ratio using a randomization key code. The randomization code was generated and kept until after all patients had completed the open-label period and the database was locked.
Prior to the double-blind period, patients entered a single-blind run-in period during which they received one placebo tablet once a day before breakfast for 4 weeks. During the double-blind period, patients received one TNL 20-mg (Tenelia®; Mitsubishi Tanabe Pharma Corporation; Osaka, Japan) or placebo tablet once a day before breakfast, and during the open-label period all patients received one TNL 20-mg tablet once a day before breakfast. Blinding was achieved by preventing all related personnel from knowing which patients were receiving which study drug, and by making the placebo and the investigational product visually indistinguishable, including the packaging. Patients with HbA1c ≥7.5% measured at the central laboratory from Week 28 onwards had the dose of TNL increased to 40 mg (the maximum dose; two tablets once daily before breakfast) starting at the next scheduled visit, provided there were no safety concerns. The dose increases were allowed only until Week 36. Mixed-meal tolerance tests (MMTTs) were conducted on the final day of the run-in period and at Weeks 16 and 52 of the treatment period. If the patient was to take the insulin dose just before or with a meal, the study drug was administered within 30 min before the meal together with the insulin dose. If the patient was not to take the insulin dose just before or with the meal, the study drug was administered 30 min before the meal loading.
2.3. Main inclusion criteria
Patients were included in the first day of the run-in period if they were outpatients aged ≥20 years at informed consent and had undergone diet and exercise therapies for diabetes for ≥8 weeks (≥56 days) before the first day of the run-in period. Patients also had to have maintained consistent insulin dosage and administration (daily dosage of ≥8 to ≤40 units) for treatment of diabetes mellitus for ≥8 weeks (≥56 days) prior to the first day of the run-in period, and to have not taken any prohibited antidiabetic agents for ≥8 weeks (≥56 days) before the first day of the run-in period. Patients who took any prohibited antidiabetic drug were allowed to enter the study when they discontinued the drug after providing consent and undergoing a washout period of ≥8 weeks (≥56 days).
The inclusion criteria for entry into the double-blind period were exercise and diet therapy regimens, dosage and administration of insulin products unchanged during the run-in period, no use of prohibited concomitant drugs during the run-in period, HbA1c on the first and final day of the run-in period of ≥7.5% and <10.5% (based on previous studies [Citation19,Citation20]), difference in HbA1c levels throughout the run-in period of <0.5%, fasting blood glucose of ≤270 mg/dL, and C-peptide of ≥0.6 ng/mL.
2.4. Insulin treatment
Temporary increases or decreases of insulin were permitted if the investigators decided it was necessary to avoid or treat hypoglycemia or to treat poor glycemic control (fasting blood glucose >240 mg/dL at two consecutive visits during the double-blind period or >200 mg/dL at two consecutive visits during the open-label and follow-up periods). The range of decrease or increase in the double-blind period was 1–4 units/day. During the open-label period, the range of dosage modification was determined by the investigators. Patients could continue in the trial even if the modifications to insulin dosage resulted in levels <8 units/day or >40 units/day. The types of insulin used were classified by their onset and duration of action. The classification of insulin was based on the definitions in the Treatment Guide for Diabetes by the Japan Diabetes Society [Citation21].
2.5. Mixed-meal tolerance test (MMTT)
At the end of the run-in period (baseline), the end of the double-blind period, and the end of the open-label period, patients underwent an MMTT after ≥10 h of fasting. A standard test meal contained carbohydrate 60%, lipid 25%, and protein 15% (total energy content 500 kcal). After blood sampling under fasting, blood samples were obtained 0.5 h, 1 h, and 2 h after starting the MMTT.
2.6. Evaluation parameters
The primary endpoint was the difference between the TNL and the placebo group in change in HbA1c at the end of the double-blind period at Week 16. The secondary endpoints were difference in change in fasting plasma glucose (FPG) between the TNL and the placebo group at the end of the double-blind period, and the difference in the change in area under the glucose plasma concentration–time curve (AUC) 0–2-h and 2-h postprandial plasma glucose in the MMTT between the TNL and the placebo group at the end of double-blind period. Other endpoints were the change from baseline in HbA1c and FPG at the end of the open-label period; the change from baseline in postprandial plasma glucose at the end of the open-label period; the changes in fasting glucagon, C-peptide, glycoalbumin (GA), 1,5-anhydroglucitol (1,5-AG), and body weight at each evaluation point or at the end of the double-blind and open-label periods; the change in postprandial C-peptide at the end of the double-blind and open-label periods; and the proportion of patients who achieved HbA1c <7.0% and <8.0% at the end of the double-blind and open-label periods.
2.7. Safety
Safety was measured by recording adverse events, symptoms of hypoglycemia, vital signs, and electrocardiogram findings. If hypoglycemia symptoms occurred, the patients measured their blood glucose concentration by self-monitoring of blood glucose when possible, and recorded their symptoms and data of the blood glucose concentration in their diaries. Hypoglycemia was confirmed by investigators based on the diaries. The specific cutoff value of blood glucose concentration for hypoglycemia was not set in this study.
2.8. Statistical methods
2.8.1. Analysis sets
The analysis set for both efficacy (full analysis set; FAS) and safety (safety analysis set) consisted of all randomized patients who had taken at least one dose of the study drug and had any post-randomization efficacy or safety data, respectively. Patients who did not have T2DM were excluded from the FAS.
2.8.2. Efficacy during the double-blind period
The baseline was defined as the final day of the run-in period.
The primary endpoint of change from baseline in HbA1c between the placebo and TNL group at the end of the double-blind period was analyzed in the FAS. An analysis of covariance (ANCOVA) model was used with treatment group as a fixed effect and the baseline HbA1c as a covariate. The missing data were imputed using the last observation carried forward (LOCF) method.
The secondary endpoints and other endpoints (excepting the proportion of patients who achieved HbA1c <7.0% and <8.0%) were analyzed in the same manner as that for the primary analysis of the primary endpoint using the FAS. The missing data in the MMTT were not imputed.
The proportion of patients who achieved HbA1c <7.0% and HbA1c <8.0% in the placebo and TNL groups were compared using Fisher’s exact test.
2.8.3. Efficacy during the double-blind and open-label periods
The baseline was defined as the final day of the run-in period for the TNL/TNL group and the first day of Week 16 for the placebo/TNL (P/TNL) group (open-label period).
For the changes in parameters other than the proportion of patients who achieved HbA1c <7.0% and <8.0%, a paired t-test was used for a comparison from the baseline to the end of open-label period.
2.8.4. Safety
Safety was analyzed in the safety analysis set. Data were summarized in the double-blind and open-label periods. Adverse events in the TNL/TNL group that occurred in the double-blind, open-label, and follow-up periods are summarized. Adverse events that occurred in the open-label and follow-up periods are summarized for the P/TNL group. An adverse event that continued from the double-blind period was also included in the summary.
2.8.5. Sample size
The difference in mean HbA1c change between the 20 mg TNL group and the placebo group at Week 16 (double-blind period) was assumed to be −0.5% and the standard deviation for each treatment group, 0.8. Fifty-five patients in each group were required (t-test, two-sided significance level of 0.05%) to ensure 90% power. Allowing for 20% of patients to withdraw, the sample size was determined to be 140 patients (70 patients in each group).
2.8.6. Statistical software
Statistics were calculated using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
A flow diagram of patients is shown in Supplementary Figure 2 and the demographic and clinical characteristics of patients in the FAS at baseline are shown in . In the placebo and TNL groups, the mean age was 57.4 and 60.1 years, respectively. The respective mean HbA1c levels were 8.73% and 8.70% in the placebo and TNL groups. The mean daily dose of insulin was 21.4 units in the placebo group and 21.2 units in the TNL group. There were no clear differences in the mean insulin dose or number of patients for each regimen type in the placebo and TNL groups. In the double-blind period, 97.2% of patients in the placebo group and 100% of patients in the TNL group either did not change or reduced their insulin dose. Throughout the treatment period with TNL, 87.3% of patients in the P/TNL group and 83.1% of patients in the TNL/TNL group did not change or reduced their insulin dose.
Table 1. Patient characteristics in the double-blind period (FAS)
3.1. Efficacy
The difference between the TNL and placebo groups in the change in HbA1c levels from baseline to the end of the double-blind period (LS mean ± SE) was −0.80% ± 0.11%, and the TNL group was confirmed as being superior to the placebo group (ANCOVA, P < 0.001) (). The HbA1c-lowering effect was maintained throughout the open-label period or double-blind and open-label periods during TNL treatment ( and Supplementary Table 1).
Table 2. Effects of teneligliptin added to insulin on primary endpoints, secondary endpoints, and other parameters at Week 16 (double-blind period; FAS)
Figure 1. Time-course of HbA1c. Data are presented as mean ± SD. The table describes the number of patients remaining in the study at each week
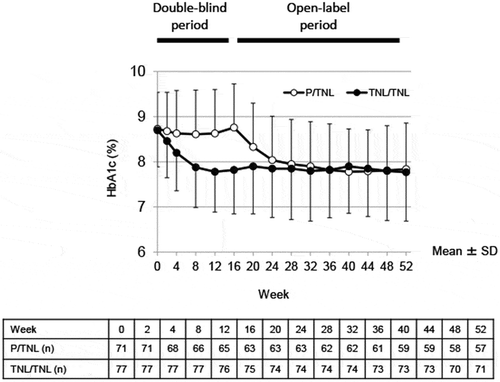
The difference between the TNL and placebo groups in the change in HbA1c did not differ according to the insulin regimen/dose or age in the double-blind period (Supplementary Table 2). The HbA1c-lowering effect with TNL in each subgroup was maintained at Week 52 (Supplementary Table 3).
The proportion of patients who achieved HbA1c <7.0% at the end of the double-blind period was 2.8% (2/71 patients) in the placebo group and 19.5% (15/77 patients) in the TNL group, and the proportion of patients who achieved HbA1c <8.0% was 14.0% (8/57 patients) in the placebo group and 50% (30/60 patients) in the TNL group. The proportions of patients achieving HbA1c <7.0% and <8.0% were both significantly higher in the TNL group than those in the placebo group (Fisher’s exact test, P = 0.001 and P < 0.001, respectively). These proportions were further increased at the end of the open-label period, with the percentage of patients achieving <7.0% in the P/TNL group and TNL/TNL group being 12.9% and 20.8%, respectively. The proportions of patients achieving <8.0% in the P/TNL group and TNL/TNL group were 52.1% and 51.7%, respectively.
Fasting blood glucose was significantly decreased in the TNL group compared with the placebo group (). The fasting blood glucose-lowering effect with TNL treatment was maintained to Week 52 (Supplementary Table 1).
The time-course of the plasma glucose concentrations in the MMTT is shown in . The difference in the changes of 2-h postprandial blood glucose and postprandial blood glucose AUC0–2h from baseline to Week 16 (double-blind period) between the TNL and placebo groups was significant ().
Figure 2. Time-course of plasma glucose concentration in the meal tolerance test. Data are presented as mean ± SD. The table describes the number of patients remaining in the study at each week
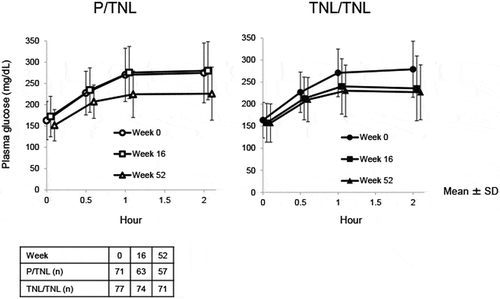
The effect of TNL on 2-h postprandial blood glucose and postprandial blood glucose AUC0–2h was maintained throughout the double-blind and open-label periods (Supplementary Table 4). Changes in postprandial C-peptide and postprandial C-peptide AUC0–2h from baseline to Week 16 of the double-blind period were not significantly different between the TNL and placebo groups (). A statistically significant increase in postprandial C-peptide and postprandial C-peptide AUC0–2h from baseline was observed in the TNL/TNL group but not the P/TNL group (Supplementary Table 4).
The change in body weight in the TNL group was significantly higher than that in the placebo group (). In the open-label period, increases in body weight at the end of the open-label period in the P/TNL and TNL/TNL groups were statistically significant compared with baseline (Supplementary Table 1).
Changes in fasting glucagon and C-peptide levels from baseline to the end of the double-blind period were not statistically different between the TNL and placebo groups. During the double-blind and open-label periods, no clear change in glucagon was observed through to Week 52 of TNL treatment (Supplementary Table 1).
3.2. Safety
3.2.1. Double-blind period
The incidence of adverse events was 53.5% (38/71 patients) in the placebo group and 44.2% (34/77 patients) in the TNL group (). TNL did not increase the incidence of adverse events in comparison with placebo during the double-blind period. Adverse events occurring at an incidence of ≥5% during the double-blind period in both the placebo and TNL groups were nasopharyngitis (placebo: 18.3%; TNL: 7.8%) and hypoglycemia (placebo: 7.0%; TNL: 11.7%). The incidence of serious adverse events was 2.8% (2/71 patients) in the placebo group and 1.3% (1/77 patients) in the TNL group. Adverse events resulting in treatment discontinuation occurred in 5.6% (4/71 patients) of patients in the placebo group and in no patients in the TNL group. The incidence of adverse drug reactions was 7.0% (5/71 patients) in the placebo group and 6.5% (5/77 patients) in the TNL group. No serious drug-related adverse events (e.g. hypoglycemic events) or deaths were observed.
Table 3. Adverse events (safety analysis set)
The incidence of hypoglycemic symptoms was 7.0% (5/71 patients) in the placebo group and 11.7% (9/77 patients) in the TNL group, and there was no significant difference between the two groups (P = 0.406, Fisher’s exact test, ). The incidence rate of hypoglycemic symptoms in the TNL group was similar between the pre-mixed and long-acting insulin subgroups, and there was no imbalance between types of insulin regimen (). In the insulin dose subgroup analyses, the incidence rate of hypoglycemic symptoms in the TNL group was slightly higher than that in the <21.3 unit (mean daily dose of insulin) subgroup. In the subgroup analyses according to age, the incidence rate of hypoglycemic symptoms in the ≥65-year subgroup was lower than that in the <65-year subgroup.
Table 4. Hypoglycemic events (safety analysis set)
3.2.2. Double-blind and open-label period
The incidence of adverse events was 66.7% (42/63 patients) in the P/TNL group (open-label period) and 77.9% (60/77 patients) in the TNL/TNL group (double-blind and open-label periods) (). Adverse events occurring at an incidence of 5% or higher during the double-blind and open-label periods in all patients were nasopharyngitis (25.0%), hypoglycemia (20.0%), and constipation (7.9%). The incidence of serious adverse events was 7.9% (5/63 patients) in the P/TNL group (open-label period) and 6.5% (5/77 patients) in the TNL/TNL group. Adverse events resulting in treatment discontinuation occurred in 1.6% (1/63 patients) of patients in the P/TNL group (open-label period) and in no patients in the TNL/TNL group. The incidence of adverse drug reactions was 11.1% (7/63 patients) in the P/TNL group (open-label period) and 20.8% (16/77 patients) in the TNL/TNL group. No serious drug-related adverse events (e.g. hypoglycemic events) or deaths were observed.
The incidence of hypoglycemic symptoms was 11.1% (7/63 patients) in the P/TNL group (open-label period) and 27.3% (21/77 patients) in the TNL/TNL group (). There was no clear difference in the incidence of hypoglycemic symptoms after TNL treatment between types and doses of insulin in either the P/TNL (open-label period) and TNL/TNL groups (). There was no trend of higher incidence of hypoglycemia after a prolonged treatment with TNL in a particular time period, or for patients <65 years old or ≥65 years old (). There was also no effect of TNL dose increase from 20 to 40 mg/day on the incidence rate of hypoglycemia (data not shown).
There were no clinically significant changes in any laboratory parameters or clinically relevant findings/changes in resting standard 12-lead electrocardiogram throughout the double-blind and open-label periods.
4. Discussion
4.1. Blood glucose-lowering effects
TNL has been reported to lower HbA1c levels when used alone and in combination therapy [Citation19,Citation22,Citation23]. A similar glycemic-lowering effect was observed when TNL was used in combination with insulin in this study. TNL is expected to exert its blood glucose-lowering effect regardless of the type of combined antidiabetic drugs.
TNL 20 mg showed a significant blood glucose-lowering effect compared with placebo in patients with T2DM responding inadequately to insulin. In addition, a consistent HbA1c-lowering effect was observed up to Week 52 after TNL administration. The HbA1c-lowering effect was observed regardless of the type of insulin. This is further supported by improved 1,5-AG levels following TNL treatment, which reflects postprandial glucose levels more closely than HbA1c. TNL lowered not only HbA1c levels but also 2-h postprandial blood glucose and postprandial blood glucose AUC0-2h in the MMTT over 52 weeks. These findings are consistent with studies that assessed the effects of TNL as an add-on to pioglitazone [Citation19] or glimepiride [Citation20].
Because insulin was administered in this study, C-peptide was used as the index of endogenous insulin production. In the MMTT, changes in C-peptide over time in the TNL group were not different from those observed in the placebo group. In a previously reported study of TNL in which TNL was used either alone or in combination with pioglitazone, no obvious change in postprandial insulin levels was observed compared with placebo [Citation19]. The changes in C-peptide in this study were consistent with the above study results Furthermore, a study investigating sitagliptin as an add-on to insulin monotherapy demonstrated that postprandial blood glucose levels decreased without a change in the postprandial C-peptide levels. It was suggested that β-cell responsiveness was improved with sitagliptin treatment [Citation24]. Thus, the increase in C-peptide levels relative to postprandial glucose levels with TNL in this study may be a class effect, similar to that with sitagliptin.
4.2. Weight gain
Body weight increased from baseline with TNL treatment. Esposito et al. reported a tendency of a slight increase in body weight with the use of DPP-4 inhibitors [Citation25], and the extent of the weight gain was similar to that observed in this study. Meta-analyses have reported that DPP-4 inhibitors, even when used as an add-on to insulin, are almost weight neutral [Citation26–Citation28]. In patients receiving insulin, weight gain was likely to occur [Citation5]. This may be attributable to the facilitated insulin effect due to a reduction in glucose toxicity with the lowered blood glucose level following TNL administration. During the double-blind period in this study, significant weight gain was observed in patients receiving TNL compared with those receiving placebo, but no further weight gain was observed even after extending the treatment period. Therefore, the weight gain that occurred in this study is not considered to pose a clinical problem.
4.3. Adverse events and hypoglycemic symptoms
The main AEs in both groups were nasopharyngitis, hypoglycemia, and constipation for the entire study period. Serious adverse events were observed in three patients during the double-blind period and 10 patients during the double-blind and open-label periods; none of these events were attributed to the study drug. There was no difference in the incidence of hypoglycemia between the TNL and placebo groups in the double-blind period. The incidence of hypoglycemic symptoms was 11.1% in the P/TNL group (open-label period) and 27.3% in the TNL/TNL group. All of the hypoglycemia events were mild. The incidence of hypoglycemic symptoms in long-term combination use of TNL and oral antihyperglycemic agents was reported to be 10.1% in combination with sulfonylureas [Citation22], 1.9% with pioglitazone [Citation19], 3.8% with glimepiride [Citation20], 1.3% with α-glucosidase inhibitors [Citation22], and 1.1% with biguanides [Citation22]. The incidence of hypoglycemic symptoms with TNL in combination with insulin was higher than the incidences with these antihyperglycemic agents. Sulfonylureas must also be used with caution because of the risk of hypoglycemia, weight gain, and the lower durability of the glycemic response. It is stated in the package insert of Tenelia 20 mg tablets that combination use with sulfonylurea drugs or insulin may increase the risk of hypoglycemia [Citation29].
DPP-4 inhibitors are frequently used in elderly patients because they are associated with a low incidence of hypoglycemia. In general, the risk of hypoglycemia tends to increase in older patients, in those taking medications that can raise insulin levels, such as sulfonylureas or exogenous insulin, and for those who want strict glycemic control [Citation30]. This study also examined the efficacy and safety in patients aged ≥65 years, and revealed no elevation in the incidence of hypoglycemia in elderly patients treated with TNL compared with non-elderly patients. In addition, this study showed no clear relation between insulin type/daily dose and hypoglycemia.
The duration of illness was longer in patients receiving insulin and TNL in our study, compared with the reported studies on TNL used in combination with other antidiabetic drugs, such as pioglitazone and sulfonylureas [Citation19,Citation20,Citation22]. Long duration of diabetes is one of the risk factors for development of hypoglycemia [Citation31]; the difference in the duration of diabetes mellitus may also affect the incidence of hypoglycemia.
4.4. Limitations
In this study, only five patients were on intermediate-acting insulin, while most patients were on pre-mixed or soluble long-acting insulin. Therefore, the analysis included limited data on intermediate-acting insulin. Comparisons of the proportions of Asian patients classified by type of insulin have shown variations from country to country [Citation32]. In addition, a comparison with Western countries [Citation33] revealed obvious differences in demographic characteristics of patients receiving insulin therapy. Therefore, for extrapolation of the results of this study to non-Japanese patients with T2DM, it is necessary to take into account differences in the medical environment and clinical conditions of patients.
5. Conclusion
In summary, TNL demonstrated good tolerability and efficacy in T2DM patients with inadequate glycemic control with diet/exercise therapy plus insulin monotherapy. TNL is safe, effective, and tolerable for treatment of T2DM in combination with insulin.
Declaration of interest
T Kadowaki has received consulting fees and/or speakers’ bureau fees from Astellas Pharma Inc., AstraZeneca K.K., Merck Sharp & Dohme K.K., Mitsubishi Tanabe Pharma Corporation, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi K.K., Takeda Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., and Nippon Boehringer Ingelheim Co., Ltd.; research support from Daiichi Sankyo Co., Ltd. and Takeda Pharmaceutical Co., Ltd.; scholarship grants from Astellas Pharma Inc., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Sumitomo Dainippon Pharma Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Novo Nordisk Pharma Ltd., Sanofi K.K., and Ono Pharmaceutical Co., Ltd.; and belongs to endowed departments by Merck Sharp & Dohme K.K., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Kowa Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd. K Kondo, N Sasaki, K Miyayama, S Yokota, R Terata and M Gouda are employees of Mitsubishi Tanabe Pharma Corporation. Medical writing assistance, provided by Marion Barnett, Edanz Medical Writing, was utilized in the production of this manuscript and funded by Mitsubishi Tanabe Pharma Corporation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Participating institutions
Dr Kazuko Saito (Seiryo Naika Clinic); Dr Yoshiharu Kitakaze (Takamori Clinic); Dr Hiroshi Ohnuma (Sagae City Hospital); Dr Masakazu Mizutani (KOZAWA EYE HOSPITAL AND DIABETES CENTER); Dr Masayuki Noritake (Noritake Clinic); Dr Takeshi Inazawa (Kashiwa City Hospital); Dr Takahiko Tokuyama (Tokuyama Clinic); Dr Tatsushi Sugiura (Seiwa Clinic); Dr Kotaro Shimokawa (Medical Corporation Toyukai Yutenji Medicine); Dr Arihiro Kiyosue (Tokyo-Eki Center-building Clinic); Dr Yoshihiko Suzuki (HDC ATLAS CLINIC); Dr Katsunori Suzuki (SAISEIKAI NIIGATA DAINI HOSPITAL); Dr Michio Nakagawa (Matsumoto Nakagawa Hospital); Dr Nobuo Takahashi (Takahashi Family Clinic); Dr Takahiro Tosaki (TOSAKI Clinic for Diabetes and Endocrinology); Dr Kei Kotani (Kotani Diabetes Clinic); Dr Tomomi Hakoda (Nippon Kokan Fukuyama Hospital); Dr Kaoru Noda (Japan Community Healthcare Organization Shimonoseki Medical Center); Dr Yasuhiro Ono (Takagi Hospital); Dr Seiichi Tanaka (Japan Labour Health Welfare Organization Kyushu Rosai Hospital); Dr Masao Ohashi (Iryouhoujinshadan Houseikai Takayama Hospital); Dr Makoto Kunisaki (KUNISAKI MAKOTO CLINIC); Dr Yasuharu Ota (Medical Corporation Koseikai Ota Diabetes Internal Medicine Clinic); Dr Madoka Taguchi (Toshiba Rinkan Hospital); Dr Yuzuru Zaitsu (Zaitsu Internal Medicine Clinic); Dr Kotaro Iemitsu (Kounandaiiemitsu Clinic); Dr Ichiro Koizumi (Kaga Medical Center); Dr Hiroyuki Konya (Ashiya Municipal Hospital); Dr Shinichiro Ueda (Kawanishi City Hospital); Dr Satoru Naito (GENERAL SAGAMI KOSEI HOSPITAL); Dr Masaaki Miyauchi and Dr Takuji Yamada (Tomei-Atsugi Clinic).
100267_Supplementary_Information-_supp_tables_RE.DOCX
Download MS Word (34.5 KB)Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- IDF Diabetes Atlas 2015-7th Edition. International Diabetes Federation; 2015. Available at: http://www.diabetesatlas.org/.
- Yabe D , Seino Y , Fukushima M , et al. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015;15(6):602.
- Ko SH , Kim DJ , Park JH , et al. Task force team for diabetes fact sheet of the Korean diabetes association. Trends of antidiabetic drug use in adult type 2 diabetes in Korea in 2002-2013: nationwide population-based cohort study. Medicine (Baltimore). 2016;95(27):e4018.
- Kohro T , Yamazaki T , Sato H , et al. Trends in antidiabetic prescription patterns in Japan from 2005 to 2011. Int Heart J. 2013;54(2):93–97.
- ORIGIN Trial Investigators, Gerstein HC , Bosch J , Dagenais GR , et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012; 367(4):319–328.
- Ryan EA , Imes S , Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27(5):1028–1032.
- Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28(2):187–218.
- Li Y , Xu W , Liao Z , et al. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function. Diabetes Care. 2004;27(11):2597–2602.
- Weng J , Li Y , Xu W , et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicenter randomised parallel-group trial. Lancet. 2008;371(9626):1753–1760.
- Hong ES , Khang AR , Yoon JW , et al. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14(9):795–802.
- Rehman MB , Tudrej BV , Soustre J , et al. Efficacy and safety of DPP-4 inhibitors in patients with type 2 diabetes: meta-analysis of placebo-controlled randomized clinical trials. Diabetes Metab. 2017;43(1):48–58.
- Seino Y , Kuwata H , Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7(Suppl 1):102–109.
- Hinnen D . Dipeptidyl peptidase-4 inhibitors in diverse patient populations with type 2 Diabetes. Diabetes Educ. 2015;41(1 Suppl):19S–31S.
- de Wit HM , Te Groen M , Rovers MM , et al. The placebo response of injectable GLP-1 receptor agonists vs. oral DPP-4 inhibitors and SGLT-2 inhibitors: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(1):301–314.
- Karagiannis T , Paschos P , Paletas K , et al. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012;344:e1369.
- Aroda VR , Henry RR , Han J , et al. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther. 2012;34(6):1247–1258.e22.
- Eto T , Inoue S , Kadowaki T . Effects of once-daily teneligliptin on 24-h blood glucose control and safety in Japanese patients with type 2 diabetes mellitus: a 4-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2012;14:1040–1046.
- Tanaka S , Suzuki K , Aoki C , et al. Add-on treatment with teneligliptin ameliorates glucose fluctuations and improves glycemic control index in Japanese patients with type 2 diabetes on insulin therapy. Diabetes Technol Ther. 2014;16(12):840–845.
- Kadowaki T , Kondo K . Efficacy and safety of teneligliptin in combination with pioglitazone in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2013;4(6):576–584.
- Kadowaki T , Kondo K . Efficacy and safety of teneligliptin added to glimepiride in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study with an open-label, long-term extension. Diabetes Obes Metab. 2014;16(5):418–425.
- Fa.Kyorin.co.jp . The treatment guide for diabetes 2014-2015. Chapter 6. Pharmacotherapy, B Types of Insulin Preparations; p 33–34. Japan Diabetes Society. Japan; [cited 2017 Jul 10 ]. Available from http://www.fa.kyorin.co.jp/jds/uploads/Treatment_Guide_for_Diabetes_2014-2015.pdf
- Kadowaki T , Marubayashi F , Yokota S , et al. Safety and efficacy of teneligliptin in Japanese patients with type 2 diabetes mellitus: a pooled analysis of two Phase III clinical studies. Expert Opin Pharmacother. 2015;16(7):971–981.
- Kim MK , Rhee EJ , Han KA , et al. Efficacy and safety of teneligliptin, a dipeptidyl peptidase-4 inhibitor, combined with metformin in Korean patients with type 2 diabetes mellitus: a 16-week, randomized, double-blind, placebo-controlled phase III trial. Diabetes Obes Metab. 2015;17(3):309–312.
- Otsuka Y , Yamaguchi S , Furukawa A , et al. Addition of sitagliptin or metformin to insulin monotherapy improves blood glucose control via different effects on insulin and glucagon secretion in hyperglycemic Japanese patients with type 2 diabetes. Endocr J. 2015;62(2):133–143.
- Esposito K , Cozzolino D , Bellastella G , et al. Dipeptidyl peptidase-4 inhibitors and HbA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2011;13(7):594–603.
- Kim YG , Min SH , Hahn S , et al. Efficacy and safety of the addition of a dipeptidyl peptidase-4 inhibitor to insulin therapy in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2016;116:86–95.
- Chen C , Yu Q , Zhang S , et al. Assessing the efficacy and safety of combined DPP-4 inhibitor and insulin treatment in patients with type 2 diabetes: a meta-analysis. Int J Clin Exp Pathol. 2015;8(11):14141–14150.
- Min SH , Yoon JH , Hahn S , et al. Comparison between SGLT2 inhibitors and DPP4 inhibitors added to insulin therapy in type 2 diabetes: a systematic review with indirect comparison meta-analysis. Diabetes Metab Res Rev. 2016 May 7 Epub ahead of print. DOI:10.1002/dmrr.2818.
- Mitsubishi Tanabe Pharma Corp . Tenelia® tablets 20 mg Package Insert 2012.
- Amiel SA , Dixon T , Mann R , et al. Hypoglycaemia in type 2 diabetes. Diabet Med. 2008;25(3):245–254.
- Ahrén B . Avoiding hypoglycemia: a key to success for glucose-lowering therapy in type 2 diabetes. Vasc Health Risk Manag. 2013;9:155–163.
- Matsuba I , Sawa T , Kawata T , et al. Cross-national variation in glycemic control and diabetes-related distress among east asian patients using insulin: results from the MOSAIc study. Diabetes Ther. 2016;7(2):349–360.
- Polinski JM , Kim SC , Jiang D , et al. Geographic patterns in patient demographics and insulin use in 18 countries, a global perspective from the multinational observational study assessing insulin use: understanding the challenges associated with progression of therapy (MOSAIc). BMC Endocr Disord. 2015;15:46.
