ABSTRACT
Introduction: Dry eye disease (DED) is a common ocular disorder that can have a substantial burden on quality of life and daily activities. Lifitegrast ophthalmic solution 5.0% is the first medication approved in the US for the treatment of the signs and symptoms of DED. The aim of this article is to summarize the preclinical and clinical data on lifitegrast and discuss how lifitegrast may fit into the current treatment landscape for DED.
Areas covered: A literature search of published preclinical and clinical data was conducted to review the chemistry, pharmacodynamics, pharmacokinetics, and clinical efficacy/safety of lifitegrast. The impact that lifitegrast may have on DED treatment practices is also discussed.
Expert opinion: The introduction of lifitegrast provides a potentially important additional option for eye care professionals treating DED. In clinical trials conducted in adults with DED, lifitegrast ophthalmic solution 5.0% improved both signs and symptoms of DED. Of note, in 2 phase 3 trials, symptom improvements were observed as early as 2 weeks, which may be explained by lifitegrast’s unique mechanism of action of blocking a specific signaling pathway in inflammation. Future research should include evaluation of whether lifitegrast can be used in combination with other DED treatments.
1. Introduction
Dry eye disease (DED) is a complex, multifactorial disease of the ocular surface that involves a loss of homeostasis of the tear film and can lead to ocular symptoms. Tear film instability and hyperosmolarity, ocular surface inflammation, and neurosensory abnormalities play roles in the etiology of the disease [Citation1]. Different types of DED exist, with the two major classes being aqueous tear-deficient DED and evaporative DED [Citation1]. As no single reliable test for DED is available, clinicians rely on a combination of DED symptoms and clinical signs to diagnose the condition. However, there is poor correlation between the signs and symptoms of DED [Citation2,Citation3], which, along with the multifactorial nature of the condition, complicates its diagnosis and management.
DED represents a public health concern due to its high prevalence and burden on the patient in terms of quality of vision, quality of life, physical/social functioning, and economic cost (due mainly to loss of work productivity) [Citation4,Citation5]. It is recognized that the prevalence of DED is higher in women and older individuals. In the Women’s Health Study and Physicians’ Health Study, the prevalence of DED (clinician diagnosed and/or severe symptoms of dryness and irritation constantly or often) was estimated at 7.8% in women and 4.3% in men aged 50 years or older [Citation6,Citation7]. Other studies have estimated the prevalence of DED (defined as subject-reported symptoms), in the range of 5–34% of the population aged over 50 years [Citation8].
Lifitegrast ophthalmic solution 5.0% received approval from the US Food and Drug Administration (FDA) in 2016 for treatment of the signs and symptoms of DED in adults. It was designated as the first in a new class of drugs called lymphocyte function-associated antigen-1 (LFA-1) antagonists. Lifitegrast blocks the interaction of cell surface proteins LFA-1 and intercellular adhesion molecule-1 (ICAM-1), and is believed to inhibit T-cell-mediated inflammation in DED.
In the interval between the approval of cyclosporine ophthalmic emulsion (CsA; Restasis®, Allergan, Dublin, Ireland) in 2003 and lifitegrast’s approval in 2016, numerous candidates were tested for the treatment of DED. Until the lifitegrast clinical trials program, none (including CsA) demonstrated statistical significance in primary end points for both signs and symptoms of DED. In a 2008 survey of ophthalmologists treating DED, almost all (94%) responded that more treatment options are needed for moderate or severe DED [Citation9]. Important attributes of a new therapeutic for DED, which lifitegrast may offer, are the treatment of both signs and symptoms of DED, protection of the ocular surface, relatively rapid onset of action, good tolerability, and long-term safety. In this review, we provide an overview of the pharmacological properties of lifitegrast and key data from clinical trials, and discuss how lifitegrast is likely to fit into the current treatment paradigm for DED.
2. Overview of the market
DED presents variable signs and/or symptoms, and available treatment options address different aspects of the disease. In the United States, CsA 0.05% (Restasis® and Restasis multidose™, Allergan, Dublin, Ireland) is approved to increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation. Other topical medications that are used to address DED in the United States [Citation10] include over-the-counter artificial tear substitutes that augment the tear film. Antibiotics are used off-label to treat meibomian gland dysfunction, a major cause of evaporative DED. Oral tetracyclines (e.g. doxycycline) inhibit the production of bacterial lipases [Citation11], thus improving the lipid profile of meibomian oils. Both tetracyclines and topical azithromycin are antibacterial and have anti-inflammatory properties [Citation12]. Short-term therapy with ophthalmic corticosteroids (e.g. loteprednol 0.5%; Lotemax®, Bausch & Lomb, Rochester, NY, USA) is also used off-label to reduce ocular surface inflammation.
A number of devices are available for the treatment of DED. These include the TrueTear™ Intranasal Tear Neurostimulator (Allergan, Dublin, Ireland) device, which temporarily increases tear production during neurostimulation in adult patients with aqueous tear deficiency, and the Lipiflow system (TearScience, Morrisville, NC, USA) which helps to clear blockages in the meibomian glands of patients with obstructive meibomian gland dysfunction. In Japan, the mucin secretagogues diquafosol ophthalmic solution (3% Diquas®; Santen Pharmaceutical, Osaka, Japan) and rebamipide (Mucosta® ophthalmic suspension unit dose 2%; Otsuka Pharmaceutical, Tokyo, Japan) are approved for the treatment of DED. These drugs promote the secretion of fluid and mucin from the ocular surface, thus improving tear film stability. Rebamipide also has anti-inflammatory properties [Citation13]. Drugs currently in development in the United States include preparations that use nanotechnology to improve the delivery of available drugs. These include KPI-121 (Kala Pharmaceuticals, Waltham, MA, USA), a nanoparticle formulation of the corticosteroid, loteprednol etabonate, and OTX-101 (Seciera™, Sun Pharma, Mumbai, India), a nanomicellar formulation of cyclosporine. In addition, RGN-259 (ReGenTree, Princeton, NJ, USA), a synthetic copy of the naturally occurring protein thymosin β4, which is predicted to promote corneal epithelial cell migration and decrease inflammation, is currently in phase 3 trials.
3. Introduction to the compound
3.1. Chemistry
Lifitegrast was designed to bind to LFA-1, a cell surface protein expressed on T lymphocytes (T cells), thus blocking the interaction of LFA-1 with its ligand, ICAM-1 [Citation14]. Lifitegrast was discovered through a rational design approach that focused on the binding epitope of ICAM-1, a region of six binding residues [Citation15,Citation16]. A tetrahydroisoquinoline analogue designated SAR 1118 (lifitegrast) was selected based on potency of binding in a HuT 78 T-cell adhesion assay, and the short half-life, fast clearance, and low exposure observed in rat pharmacokinetic studies [Citation17]. Lifitegrast is highly aqueous soluble (>100 mg/mL), which allows it to be formulated at 50 mg/mL (5.0%), thus maintaining physiological pH [Citation17]. Lifitegrast ophthalmic solution 5.0% is a preservative-free, sterile eye drop that has a target pH of 7.0–8.0, an osmolality range of 200–330 mOsmol/kg, and stability in aqueous solution at room temperature [Citation18].
3.2. Pharmacodynamics
Based on the available evidence, it is postulated that LFA-1/ICAM-1 interaction has roles in T-cell activation, through the formation of immunological synapses between T cells and antigen-presenting cells (e.g. dendritic cells), and in the migration of T cells to the ocular surface [Citation19]. In DED, research suggests that stress (environmental and/or microbial) to the ocular surface results in increased ICAM-1 expression in the ocular surface tissues [Citation20–Citation22]. Although the precise mechanism of action of lifitegrast in DED has not been established, it is thought that by blocking LFA-1/ICAM-1 interaction, lifitegrast inhibits T-cell migration, T-cell activation, and the release of pro-inflammatory cytokines (). This is supported by data from in vitro studies that demonstrated that lifitegrast inhibits Jurkat T-cell and HuT 78 T-cell adhesion to ICAM-1 (half-maximal inhibitory concentration [IC50] of 2.98 and 9 nmol/L, respectively) and inhibits the secretion of pro-inflammatory cytokines including interferon-γ, interleukin (IL)-1β, and tumor necrosis factor-α in a concentration-dependent manner in activated peripheral blood mononuclear cells (half maximal effective concentration [EC50] of 0.0016, 0.36, and 0.076 μM, respectively) [Citation17,Citation23]. Further, in a mouse model in which corneal inflammation was induced by epithelial abrasion and exposure to tobramycin-killed Pseudomonas aeruginosa or Staphylococcus aureus, lifitegrast ophthalmic solution reduced corneal inflammation [Citation24]. Lifitegrast also significantly increased tear production from baseline in dogs diagnosed with idiopathic keratoconjunctivitis sicca [Citation23].
Figure 1. Current understanding of the mechanism of action of lifitegrast in DED. (a) In DED, stress to the ocular surface can result in overexpression of ICAM-1. This leads to increased migration of T cells to the ocular surface, activation of T cells through the formation of immunological synapses, and the release of pro-inflammatory cytokines, which can cause damage to the ocular tissues. (b) Lifitegrast is thought to reduce T-cell migration, T-cell activation, and pro-inflammatory cytokine release by blocking LFA-1/ICAM-1 interaction. DED: dry eye disease; ICAM-1: intercellular adhesion molecule-1; LFA-1: lymphocyte function-associated antigen-1; mAPC: mature antigen-presenting cell. Adapted with permission from Perez et al., Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul Surf; 2016; 14; 207–215 [Citation14]. DOI: http://dx.doi.org/10.1016/j.jtos.2016.01.001.
![Figure 1. Current understanding of the mechanism of action of lifitegrast in DED. (a) In DED, stress to the ocular surface can result in overexpression of ICAM-1. This leads to increased migration of T cells to the ocular surface, activation of T cells through the formation of immunological synapses, and the release of pro-inflammatory cytokines, which can cause damage to the ocular tissues. (b) Lifitegrast is thought to reduce T-cell migration, T-cell activation, and pro-inflammatory cytokine release by blocking LFA-1/ICAM-1 interaction. DED: dry eye disease; ICAM-1: intercellular adhesion molecule-1; LFA-1: lymphocyte function-associated antigen-1; mAPC: mature antigen-presenting cell. Adapted with permission from Perez et al., Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul Surf; 2016; 14; 207–215 [Citation14]. DOI: http://dx.doi.org/10.1016/j.jtos.2016.01.001.](/cms/asset/39aaf994-a11a-401f-bd91-135ace80ae86/ieop_a_1372748_f0001_oc.jpg)
3.3. Pharmacokinetics and metabolism
Following ophthalmic administration of radiolabeled lifitegrast to rats [Citation25] and dogs [Citation23], the highest concentration of lifitegrast (ng equivalents [14C]-lifitegrast/g) at 0.5 h post dose was detected in the bulbar conjunctiva (31,500, 4510, rats [Citation25] and dogs [Citation23], respectively), palpebral conjunctiva (26,300, 3790), and cornea (17,150, 2130). In a study by Rao et al., the plasma concentration of lifitegrast post dose reached a maximum at 0.25 h (194 ng equivalents [14C]-lifitegrast/g) and decreased to below the level of quantification from 12 h [Citation25]. Similarly, in rabbits that received twice-daily ophthalmic lifitegrast for 5 days, the highest concentrations of lifitegrast (ng/g) were localized in the ocular anterior segment tissues (bulbar conjunctiva [9370–14,200], palpebral conjunctiva [9620–11,900], cornea [5190–5930], and anterior sclera [5870–11,200]), whereas there was low exposure in the plasma (maximum plasma concentration [Cmax]: 9.5–17.4 ng/mL) and posterior segment tissues (posterior sclera [369–826], optic nerve, retina, and vitreous humor [0–36]), suggesting a low potential for off-target systemic or ocular effects [Citation26].
In a phase 1 trial [Citation27], twice-daily administration of lifitegrast ophthalmic solution 5.0% to healthy volunteers resulted in a tear fluid lifitegrast concentration that exceeded the target therapeutic level in the eye of >1 μM (~600 ng/mL), i.e. substantially higher than the IC50 and EC50 values demonstrated in vitro for LFA-1/ICAM-1 binding and cytokine inhibition, respectively (Section 3.2). In participants administered lifitegrast twice daily for 10 days, a maximum tear fluid lifitegrast concentration (91,413 ng/mL) was achieved in a median time of 0.3 h, and there was no evidence of lifitegrast accumulation in tears during repeated ocular dosing. In the same study, low levels of lifitegrast in the plasma were detected within 5 min of instillation and were cleared from the plasma within 1–4 h. The Cmax of lifitegrast following ocular administration was <5 nM (<3 ng/mL) and there was no indication of systemic accumulation with repeated ocular dosing [Citation17,Citation27]. In the SONATA (Safety Of a 5.0% coNcentrATion of lifitegrAst ophthalmic solution) 1-year safety trial [Citation28], plasma concentrations of lifitegrast were low, and there was no accumulation in plasma over time (mean concentration of lifitegrast in plasma was below the lower limit of quantification [0.5 ng/mL] at days 0, 180, and 360).
In tests of in vitro toxicology, lifitegrast was negative in the Ames mutagenicity test (a bacterial assay for identifying potential carcinogens) and demonstrated low potency in human liver microsomal studies that assessed metabolism by cytochrome P450 enzymes, CYP3A4 (IC50 > 20 μM) and CYP2C9 (IC50 = 3.0 μM) [Citation17]. In the human ether-à-go-go-related gene (hERG) patch clamp assay, which tests for the potential of cardiac arrhythmia, lifitegrast had an IC50 of >20 μM [Citation17]. These IC50 values are several orders higher than those demonstrated in vitro for LFA-1/ICAM-1 binding and cytokine inhibition (Section 3.2). Thus, given the very low lifitegrast plasma levels in human trials following ocular administration (<5 nM), the potential for systemically absorbed lifitegrast to cause drug–drug interactions mediated by CYP450 or hERG ion channel inhibition is very low.
4. Clinical efficacy and safety
4.1. Effect on signs and symptoms of DED
Four 12-week, multicenter, randomized-controlled trials conducted in the United States have evaluated the efficacy and safety of lifitegrast versus placebo in adults with DED. These were a phase 2 trial [Citation29] and three phase 3 trials: OPUS-1 [Citation30], OPUS-2 [Citation31], and OPUS-3 [Citation32]. Key inclusion criteria included subject-reported history of DED, Schirmer Tear Test without anesthesia of ≥1 and ≤10 mm in ≥1 eye, and corneal fluorescein staining score ≥2.0 in ≥1 eye (0–4 point scale). In addition, in OPUS-2 and OPUS-3, participants had more severe symptomatology (baseline eye dryness score [EDS, visual analogue scale (VAS), 0–100 point scale] ≥40 and artificial tear use within 30 days of study entry) than participants in the phase 2 and OPUS-1 trials. The use of ophthalmic medications, including corticosteroids and artificial tears, was not allowed during these studies. In all lifitegrast trials, the placebo comprised the same components of the investigational product solution minus lifitegrast.
In the phase 2 trial, participants were randomized 1:1:1:1 to lifitegrast ophthalmic solution 0.1%, 1.0%, or 5.0%, or placebo twice daily for 84 days. Based on the results of this trial, lifitegrast 5.0% was selected for investigation in the phase 3 trials. In OPUS-1, -2, and -3, following a 14-day open-label, placebo run-in period, participants were randomized 1:1 to twice-daily doses of lifitegrast ophthalmic solution 5.0% or placebo. The effect on DED signs and symptoms was assessed on days 14, 42, and 84. In the phase 2 trial, the primary outcome end point was inferior corneal staining score (ICSS; 0–4 point scale) at day 84. In the OPUS-1 trial the co-primary sign and symptom end points, respectively, were change from baseline to day 84 in ICSS and visual-related function subscale of a symptom scale (0–4 point scale). In OPUS-2 the co-primary sign end point was change from baseline to day 84 in ICSS; the co-primary symptom end point was change from baseline to day 84 in EDS (VAS), a patient-reported measure reported as a single score for both eyes. In OPUS-3, the primary efficacy end point was change from baseline to day 84 in EDS (VAS). In this trial, ICSS was analyzed ad hoc.
In relation to DED signs ()), lifitegrast significantly improved ICSS versus placebo in the phase 2 trial (secondary end point; treatment effect [TE] 0.35; 95% confidence interval [CI] 0.05–0.65; nominal p = 0.0209) and in OPUS-1 (co-primary end point; TE 0.24; 95% CI 0.10–0.38; p = 0.0007). In OPUS-3, ad hoc analysis of ICSS demonstrated a nominally significant mean reduction from baseline to day 84 that favored lifitegrast compared with placebo (study eye; TE 0.17; 95% CI 0.03–0.30; nominal p = 0.0144). In OPUS-2, the co-primary sign end point of ICSS did not achieve statistical significance.
Figure 2. Treatment effects in ICSS and EDS across the 12-week randomized controlled trials (ITT population with LOCF). Day 14 and day 42 TEs in ICSS are not available for the OPUS-2 and OPUS-3 trials. CI: confidence interval; EDS: eye dryness score; ICSS: inferior corneal staining score; ITT: intent-to-treat; LIF: lifitegrast; LOCF: last observation carried forward; NS: not significant; PBO: placebo; VAS: visual analogue scale.
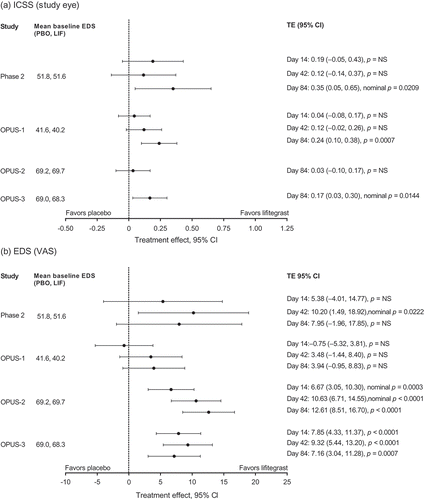
With respect to DED symptoms, lifitegrast significantly improved EDS (VAS) versus placebo in OPUS-2 (co-primary end point; TE 12.61; 95% CI 8.51–16.70; p < 0.0001) and OPUS-3 (primary end point; TE 7.16; 95% CI 3.04–11.28; p = 0.0007). In the OPUS-1 trial, the co-primary symptom end point of visual-related function subscale did not achieve statistical significance. It is noteworthy that in OPUS-2 and OPUS-3 lifitegrast significantly improved EDS (VAS) as early as 2 weeks after the start of treatment ()). A pooled analysis of data from these similarly designed trials demonstrated significant improvement in EDS (VAS) from baseline to day 84 (TE 9.92; 95% CI 7.01–12.83; p < 0.0001), day 42 (TE 9.75; 95% CI 6.99–12.50; p < 0.0001), and day 14 (TE 7.23; 95% CI 4.71–9.76; p < 0.0001) versus participants receiving placebo [Citation33].
4.2. Safety and tolerability
Across the four 12-week trials, lifitegrast ophthalmic solution 5.0% was generally well-tolerated with no serious ocular treatment-emergent adverse events (TEAEs) reported. The TEAEs rarely led to discontinuation and most were mild to moderate in severity. Overall, the most common (>5% in either treatment group) ocular TEAEs reported in these trials were visual acuity reduced, instillation-site pain, instillation-site irritation, and instillation-site reaction. Each of these TEAEs occurred at a higher rate in the lifitegrast versus the placebo group, except for visual acuity reduced, which in three trials was slightly higher in the placebo group (placebo vs. lifitegrast: phase 2, 5.2% vs. 1.7%; OPUS-1, 5.1% vs. 4.8%; OPUS-2, 6.4% vs. 5.0%). The most common (>5% in either treatment group) non-ocular TEAE in all of the trials was dysgeusia, which also occurred at a higher rate in the lifitegrast group.
To assess the longer-term tolerability and safety of twice-daily lifitegrast, the SONATA 1-year safety study was conducted in DED patients (lifitegrast, n = 220; placebo, n = 111) [Citation28]. Key inclusion criteria were adults (aged ≥18 years) with self-reported DED, Schirmer Tear Test without anesthesia ≥1 and ≤10 mm in ≥1 eye, corneal fluorescein staining score ≥2.0 in ≥1 eye, and the use of and/or desire to use artificial tears in the last 6 months. Participants were randomized 2:1 to lifitegrast ophthalmic solution 5.0% or placebo. To reflect real-world use, after day 14, participants were allowed to use as required artificial tears (≤4 times daily, as needed), topical ophthalmic/nasal steroids (only loteprednol etabonate ≤4 weeks at a time), antihistamines, mast cell stabilizers, and contact lenses. The primary end point was the percentage and severity of ocular and non-ocular TEAEs. In SONATA, lifitegrast appeared safe and well-tolerated, with no serious ocular TEAEs, and a similar safety profile as the previous 12-week trials. The most frequent (>5% in either group) TEAEs were instillation-site irritation (lifitegrast, 15.0%; placebo, 4.5%), instillation-site reaction (lifitegrast, 13.2%; placebo, 1.8%), visual acuity reduced (lifitegrast, 11.4%; placebo, 6.3%), and dysgeusia (lifitegrast, 16.4%; placebo, 1.8%).
A pooled analysis of the safety data from all five clinical trials has also been completed, including 1287 participants who received at least one dose of lifitegrast (n = 1177, placebo), with total exposure to lifitegrast treatment of 415.65 person-years (332.15 person-years for placebo) [Citation34]. The results of the pooled analysis further support the tolerability of lifitegrast ophthalmic solution 5.0% across all trials. The most common adverse events in the pooled analysis were instillation-site irritation (lifitegrast, 15.2%; placebo, 2.8%), instillation-site reaction (lifitegrast, 12.3%; placebo, 2.3%), instillation-site pain (lifitegrast, 9.8%; placebo, 2.1%), and dysgeusia (lifitegrast, 14.5%; placebo, 0.3%) [Citation34]. To date, there have been no postmarketing adverse events reported.
Given the mechanism of action of lifitegrast on T-cell function, the effect of twice-daily lifitegrast ophthalmic solution 5.0% on whole blood CD3, CD4, and CD8 lymphocyte counts was assessed in SONATA in a subset of participants (n = 75) on days 0 (baseline), 180, and 360 [Citation28]. There were minimal and similar changes in lymphocyte counts in both treatment groups, suggesting that lifitegrast did not cause chronic suppression of lymphocytes. In addition, there was no pattern of adverse events to suggest systemic toxicities or localized infectious complications resulting from chronic T-cell suppression [Citation28] (similar to findings from trials of CsA 0.05% [Citation35]).
5. Regulatory affairs
Lifitegrast was discovered at Sunesis (South San Francisco, CA, USA) and developed clinically by Shire (Lexington, MA, USA). A New Drug Application was submitted to the FDA in March 2015 for the approval of lifitegrast ophthalmic solution 5.0% to treat DED in adults. Efficacy data from the phase 2, OPUS-1, and OPUS-2 trials were included in the NDA application. The application was granted priority review in April 2015. In October 2015, Shire received a complete response letter from the FDA for lifitegrast, indicating that the marketing application would not be approved without additional clinical data. Shire responded 3 months later with a resubmission that included data from the OPUS-3 trial, which had been initiated following pre-submission feedback from the agency; the aim of OPUS-3 was to demonstrate efficacy in a symptom end point, EDS. The FDA subsequently approved Xiidra® (lifitegrast ophthalmic solution 5.0%) for the treatment of signs and symptoms of DED in July 2016. The agency based its approval on the efficacy of lifitegrast for the treatment of DED as demonstrated by replication of the sign and symptom end point results in the four submitted efficacy/safety trials compared with vehicle. Lifitegrast was granted designation as the first in a new class of drugs, LFA-1 antagonists.
6. Conclusion
Lifitegrast is the first medication approved by the FDA for the treatment of both the signs and symptoms of DED. Key features of lifitegrast include high aqueous solubility, rapid absorption into the ocular tissues, rapid systemic elimination, and a unique mechanism of action. In clinical trials, lifitegrast ophthalmic solution 5.0% improved signs and symptoms of DED in adults with DED across four 12-week efficacy/safety trials. Most notably, symptom improvements were observed as early as 2 weeks. Lifitegrast was well tolerated in the 12-week trials and a 1-year safety study, with no serious ocular TEAEs reported.
7. Expert opinion
Lifitegrast ophthalmic solution 5.0% is the first pharmacological medication to be approved by the FDA for the treatment of DED since 2003, when CsA 0.05% was approved for the indication of increasing tear production in DED patients. The approval of CsA 0.05% was based on significant improvements in Schirmer wetting in clinical trials; CsA 0.05% is not indicated for the treatment of the symptoms of DED. Results from clinical trials [Citation35,Citation36], and clinical experience post approval, have shown that marked improvement with CsA 0.05% generally takes at least 3 months. In contrast, significant symptom improvements occurred with lifitegrast in three of four 12-week trials (phase 2, OPUS-2, and OPUS-3) by 6 weeks and as early as 2 weeks in two trials (OPUS-2 and OPUS-3). The faster time to efficacy for lifitegrast could be a key factor for eye care professionals and may potentially be explained by differences in the mechanism of action. CsA inhibits the protein phosphatase, calcineurin, which results in inhibition of IL-2 production and inhibition of T-cell activation [Citation37]. However, CsA does not inhibit activated T cells [Citation38,Citation39], so there is a delay for activated T cells to undergo apoptosis and for new unstimulated T cells to form. Estimates for the median life span of human T cells are reported as up to 164 days for CD4+ and 157 days for CD8+ memory T cells [Citation40]. In contrast to CsA, lifitegrast is expected to act on all circulating T cells because it blocks a specific pathway (LFA-1/ICAM-1) in the DED inflammatory process, which has a role in T-cell activation and the migration/recruitment of activated T cells to ocular surface tissues [Citation19].
The introduction of lifitegrast will provide an additional option for treating DED, potentially helping to fulfill an important unmet need for eye care professionals treating this condition [Citation9]. A summary of the status of lifitegrast is listed in Box 1. Current limitations of the clinical data are a lack of head-to-head comparisons between lifitegrast and CsA, limited published data on the real-world use of lifitegrast and on the predictors of the response to treatment with lifitegrast. In addition, given the heterogeneous nature of DED, it is not clear whether everyone with the symptoms of the condition would benefit from an agent that inhibits ocular surface inflammation. Further studies are also needed to investigate whether lifitegrast could be used in a combination pharmacotherapy approach utilizing medications with different mechanisms of action that reduce inflammation (e.g. lifitegrast, corticosteroids, CsA) and improve tear function/protection of the ocular surface (e.g. artificial tear substitutes, mucin and tear secretagogues). In particular, whether the combination of lifitegrast with topical CsA has synergistic/additive effects, as has been observed for CsA 0.05% plus loteprednol [Citation41], would be of interest. It would also be interesting to determine whether the incidence of dysgeusia (unpleasant taste/change in taste sensation), which has been observed in lifitegrast trials, could be reduced with punctal plugs. Other future research could also provide information on whether lifitegrast can penetrate other parts of the eye (e.g. the meibomian glands and lacrimal glands), which would be useful in understanding whether lifitegrast has a particular role in treating evaporative DED caused by meibomian gland dysfunction.
Box 1. Drug summary
Table
Declaration of interest
E.D. Donnenfeld has been a consultant for AcuFocus, Alcon, Allergan, Abbott Medical Optics, Aquesys, Bausch & Lomb, CRST, Elenza, Glaukos, Icon Bioscience, Kala, Katena, LacriPen, Mati Therapeutics, Merck, Mimetogen, NovaBay, Novaliq, OcuHub, Odyssey, Omeros, Physician Recommended Nutriceuticals, RPS, Shire, Strathspey Crown, TearLab, TLC Laser Eye Centers, TrueVision, Versant Ventures, WaveTec, and Zeiss; and is an investor in AcuFocus, Aquesys, Elenza, Glaukos, LacriPen, Mati Therapeutics, Mimetogen, NovaBay, OcuHub, RPS, Shire/SARcode, Strathspey Crown, TearLab, TrueVision, Versant Ventures, and WaveTec. H.D. Perry has been a consultant for Alcon, Allergan, Blephex, Novabay, Omeros, and Physician Recommended Nutriceuticals. Medical writing assistance, provided by Nasser Malik, PhD, of Excel Scientific Solutions, was utilized in the production of this paper and funded by SARcode Bioscience, a fully owned company of Shire PLC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–283.
- Bartlett JD, Keith MS, Sudharshan L, et al. Associations between signs and symptoms of dry eye disease: a systematic review. Clin Ophthalmol. 2015;9:1719–1730.
- Baudouin C, Irkec M, Messmer EM, et al. Clinical impact of inflammation in dry eye disease: proceedings of the ODISSEY group meeting. Acta Ophthalmologica. 2017 Apr 8. doi:10.1111/aos. 13436. [Epub ahead of print].
- Li M, Gong L, Chapin WJ, et al. Assessment of vision-related quality of life in dry eye patients. Invest Ophthalmol Vis Sci. 2012;53:5722–5727.
- McDonald M, Patel DA, Keith MS, et al. Economic and humanistic burden of dry eye disease in Europe, North America, and Asia: a systematic literature review. Ocul Surf. 2016;14:144–167.
- Schaumberg DA, Dana R, Buring JE, et al. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. 2009;127:763–768.
- Schaumberg DA, Sullivan DA, Buring JE, et al. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326.
- DEWS. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:93–107.
- Asbell PA, Spiegel S. Ophthalmologist perceptions regarding treatment of moderate-to-severe dry eye: results of a physician survey. Eye Contact Lens. 2010;36:33–38.
- AAO. Cornea/External Disease PPP Panel, Hoskins Center for Quality Eye Care. Dry eye syndrome PPP – 2013. 2013 [cited 2017 May 12]. Available from: http://www.aao.org/preferred-practice-pattern/dry-eye-syndrome-ppp–2013#CAREPROCESS
- Dougherty JM, McCulley JP, Silvany RE, et al. The role of tetracycline in chronic blepharitis. Inhibition of lipase production in staphylococci. Invest Ophthalmol Vis Sci. 1991;32:2970–2975.
- Foulks GN, Borchman D, Yappert M, et al. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: a comparative clinical and spectroscopic pilot study. Cornea. 2013;32:44–53.
- Arakaki R, Eguchi H, Yamada A, et al. Anti-inflammatory effects of rebamipide eyedrop administration on ocular lesions in a murine model of primary Sjogren’s syndrome. PloS One. 2014;9:e98390.
- Perez VL, Pflugfelder SC, Zhang S, et al. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul Surf. 2016;14:207–215.
- Gadek TR, Burdick DJ, McDowell RS, et al. Generation of an LFA-1 antagonist by the transfer of the ICAM-1 immunoregulatory epitope to a small molecule. Science. 2002;295:1086–1089.
- Keating SM, Clark KR, Stefanich LD, et al. Competition between intercellular adhesion molecule-1 and a small-molecule antagonist for a common binding site on the alphal subunit of lymphocyte function-associated antigen-1. Protein Sci. 2006;15:290–303.
- Zhong M, Gadek TR, Bui M, et al. Discovery and development of potent LFA-1/ICAM-1 antagonist SAR 1118 as an ophthalmic solution for treating dry eye. ACS Med Chem Lett. 2012;3:203–206.
- Shire US Inc. Prescribing information (07/2016) Xiidra™ (lifitegrast ophthalmic solution) 5% [ cited 2017 Aug 30]. Available from: http://www.shirecontent.com/PI/PDFs/Xiidra_USA_ENG.pdf.
- Pflugfelder SC, Stern M, Zhang S, et al. LFA-1/ICAM-1 interaction as a therapeutic target in dry eye disease. J Ocul Pharmacol Ther. 2017;33:5–12.
- Gao J, Morgan G, Tieu D, et al. ICAM-1 expression predisposes ocular tissues to immune-based inflammation in dry eye patients and Sjögrens syndrome-like MRL/lpr mice. Exp Eye Res. 2004;78:823–835.
- Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjögren’s and non-Sjögren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–2614.
- Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32:19–41.
- Murphy CJ, Bentley E, Miller PE, et al. The pharmacologic assessment of a novel lymphocyte function-associated antigen-1 antagonist (SAR 1118) for the treatment of keratoconjunctivitis sicca in dogs. Invest Ophthalmol Vis Sci. 2011;52:3174–3180.
- Sun Y, Zhang R, Gadek TR, et al. Corneal inflammation is inhibited by the LFA-1 antagonist, lifitegrast (SAR 1118). J Ocul Pharmacol Ther. 2013;29:395–402.
- Rao VR, Prescott E, Shelke NB, et al. Delivery of SAR 1118 to the retina via ophthalmic drops and its effectiveness in a rat streptozotocin (STZ) model of diabetic retinopathy (DR). Invest Ophthalmol Vis Sci. 2010;51:5198–5204.
- Chung J-K, Spencer E, Hunt M, et al. Ocular distribution and pharmacokinetics of lifitegrast following repeat topical ocular dose administration to pigmented rabbits. Presented at the 2017 Annual Meeting of the Association for Research in Vision and Ophthalmology; May 7–11, 2017; Baltimore, MD.
- Semba CP, Swearingen D, Smith VL, et al. Safety and pharmacokinetics of a novel lymphocyte function-associated antigen-1 antagonist ophthalmic solution (SAR 1118) in healthy adults. J Ocul Pharmacol Ther. 2011;27:99–104.
- Donnenfeld ED, Karpecki PM, Majmudar PA, et al. Safety of lifitegrast ophthalmic solution 5.0% in patients with dry eye disease: a 1-year, multicenter, randomized, placebo-controlled study. Cornea. 2016;35:741–748.
- Semba CP, Torkildsen GL, Lonsdale JD, et al. A phase 2 randomized, double-masked, placebo-controlled study of a novel integrin antagonist (SAR 1118) for the treatment of dry eye. Am J Ophthalmol. 2012;153:1050.e1051–1060.e1051.
- Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014;121:475–483.
- Tauber J, Karpecki P, Latkany R, et al. Lifitegrast ophthalmic solution 5.0% versus placebo for treatment of dry eye disease: results of the randomized phase III OPUS-2 study. Ophthalmology. 2015;122:2423–2431.
- Holland EJ, Luchs J, Karpecki PM, et al. Lifitegrast for the treatment of dry eye disease: results of a phase iii, randomized, double-masked, placebo-controlled trial (OPUS-3). Ophthalmology. 2017;124:53–60.
- Matossian C, Karpecki P, Sall K, et al. Lifitegrast 5.0% versus placebo for dry eye disease: pooled symptom outcomes from the OPUS-2 and OPUS-3 phase 3 studies. Presented at the 2016 Annual Meeting of the American Academy of Ophthalmology; October 15–18, 2016; Chicago, IL.
- Shojaei A, Tauber J, Nichols KK, et al. Overview of clinical efficacy and safety of lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease. Presented at the 8th International Conference on the Tear Film & Ocular Surface; September 7–10, 2016; Montpellier, France.
- Sall K, Stevenson OD, Mundorf TK, et al. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–639.
- Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group. Ophthalmology. 2000;107:967–974.
- Yavuz B, Bozdağ Pehlivan S, Ünlü N. An overview on dry eye treatment: approaches for cyclosporin A delivery. Scientific World J. 2012;2012:194848.
- Kay JE, Benzie CR. Effects of cyclosporin A on the metabolism of unstimulated and mitogen-activated lymphocytes. Immunology. 1983;49:153–160.
- Strauss G, Osen W, Debatin KM. Induction of apoptosis and modulation of activation and effector function in T cells by immunosuppressive drugs. Clin Exp Immunol. 2002;128:255–266.
- Westera L, Drylewicz J, den Braber I, et al. Closing the gap between T-cell life span estimates from stable isotope-labeling studies in mice and humans. Blood. 2013;122:2205–2212.
- Sheppard JD, Donnenfeld ED, Holland EJ, et al. Effect of loteprednol etabonate 0.5% on initiation of dry eye treatment with topical cyclosporine 0.05%. Eye Contact Lens. 2014;40:289–296.
