ABSTRACT
Background: Data regarding the efficacy and safety of sodium–glucose cotransporter 2 inhibitors in the real-world setting in Japan are limited. The STELLA-LONG TERM study is an ongoing 3-year post-marketing surveillance study of ipragliflozin in type 2 diabetes (T2D) patients. Here, we report the interim results (including 3-, 12-, and 24-month data).
Research design and methods: All Japanese patients with T2D who were first prescribed ipragliflozin between 17 July 2014 and 16 October 2015 at participating centers in Japan were registered in STELLA-LONG TERM.
Results: At 3, 12, and 24 months, the safety analysis set comprised 11,053, 5475, and 138 patients, respectively; the efficacy analysis set comprised 8757 patients. Ipragliflozin treatment resulted in statistically significant improvements versus baseline in hemoglobin A1c, fasting plasma glucose concentration, body weight, blood pressure, heart rate, and serum concentrations of low-density lipoprotein cholesterol and triglycerides. The adverse drug reaction incidence rate was 10.71%, the most common reactions being renal and urinary disorders (5.06%), infections and infestations (1.24%), and skin and subcutaneous tissue disorders (1.14%).
Conclusions: Ipragliflozin was well tolerated and effective in Japanese patients with T2D; no new safety issues were identified.
1. Introduction
Sodium–glucose cotransporter 2 (SGLT2) inhibitors are a relatively new class of oral antihyperglycemic drugs that have been approved for the treatment of type 2 diabetes. These drugs inhibit the renal reabsorption of glucose and promote glycosuria, leading to a reduction in blood glucose independent from insulin activity [Citation1,Citation2]. Thus, SGLT2 inhibitors can improve glycemic control without risk of hypoglycemia and offer an added benefit of promoting weight loss via their glycosuric effect [Citation2].
In several randomized controlled trials [Citation3–Citation8], once-daily ipragliflozin was demonstrated to safely and effectively decrease hemoglobin A1c (HbA1c), fasting plasma glucose (FPG), and body weight in Japanese patients with type 2 diabetes. Such findings led to the approval of ipragliflozin for clinical use in Japan in January 2014 [Citation9].
Currently, there are limited data regarding the efficacy and safety of SGLT2 inhibitors in the real-world setting in Japan. To obtain additional safety and efficacy data of the long-term use of ipragliflozin in Japanese patients, Astellas Pharma Inc., under the guidance of the Japanese Pharmaceutical and Medical Device Agency, is conducting several post-marketing surveillance studies.
The Specified drug use resulTs survEy of IpragLifLozin treAtment in type 2 diabetic patients: LONG-TERM use (STELLA-LONG TERM) study is an ongoing 3-year prospective post-marketing surveillance study. This study aimed to register more than 10,000 patients first prescribed ipragliflozin under real-world settings at medical institutions in Japan.
A 3-month interim report of the STELLA-LONG TERM study [Citation10] was produced to assess the efficacy of ipragliflozin based on changes in glycemic control and other laboratory parameters. The safety of ipragliflozin was evaluated based on the incidence of adverse drug reactions (ADRs) within the first 3 months of treatment, as it is generally thought that most adverse events (AEs) occur during this period. Data from 3481 (30.5%) and 4360 (38.2%) patients were included in the efficacy and safety analyses, respectively. Significant improvements were observed in HbA1c and FPG with decreases of 0.67% and 28.8 mg/dL, respectively, at 3 months/final visit (both P < 0.001) from baseline (8.00% and 166.4 mg/dL, respectively). Overall, ADRs were observed in 194 patients (4.45%); the most common ADRs were renal and urinary disorders, which occurred in 110 patients (2.52%).
Safety data were available for a relatively small number of patients because a large number of case report forms had not been locked in the database prior to conducting the analysis of interim data. Thus, the present interim report of the ongoing 3-year post-marketing surveillance STELLA-LONG TERM study aims to present safety and efficacy data collected up to 24 months, including characteristics of AEs.
2. Patients and methods
The study design, patient selection criteria, and methods have been described in detail in the previous interim report [Citation10]. Briefly, the aim of STELLA-LONG TERM was to register all Japanese patients with type 2 diabetes first prescribed ipragliflozin between 17 July 2014 and 16 October 2015 at participating medical centers in Japan.
Ipragliflozin was prescribed and administered in accordance with the package insert. Accordingly, a once-daily dose of 50 mg was administered before or after breakfast. A lower dose was permitted at the attending physician’s discretion in patients with severe hepatic impairment but was to be used with caution. A dose increase to 100 mg was permitted if the treating physician judged the treatment efficacy as insufficient, but the patient’s clinical course was carefully monitored.
2.1. Study design
This post-marketing surveillance study was in compliance with Good Post-marketing Study Practice. The survey was performed as described in the 3-month interim report [Citation10]. Electronic case report forms were completed by physicians for each patient, separately from medical charts. Items in the survey included demographic and clinical characteristics of patients, medication data, laboratory variables, vital signs (blood pressure and heart rate), and safety data (AEs and ADRs). Patient-reported outcomes were not included in this surveillance study.
For this analysis of interim data, the cutoff date for the receipt of survey forms and data entry was 16 January 2017. Only patients whose data were locked in the study database at this time were included in the safety and efficacy analyses. Safety was evaluated based on AEs and ADRs that occurred during treatment with ipragliflozin. All ADRs were categorized according to system organ class and preferred term using MedDRA/J version 19.1. Efficacy outcome measures were the changes in glycemic control, body weight, and laboratory variables from baseline up to 24 months.
2.2. Statistical analysis
Details of sample size calculations and rationale for the length of the study are described in the previous interim report [Citation10].
Efficacy variables, vital signs, and laboratory variables are shown as means ± standard deviation, and changes from baseline were determined using paired t tests. Adjustments for type I error based on multiple hypothesis testing were not performed. Categorical variables, including baseline characteristics and ADRs, are shown as the number (n) and percentage (%) of patients. All statistical analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Patient disposition
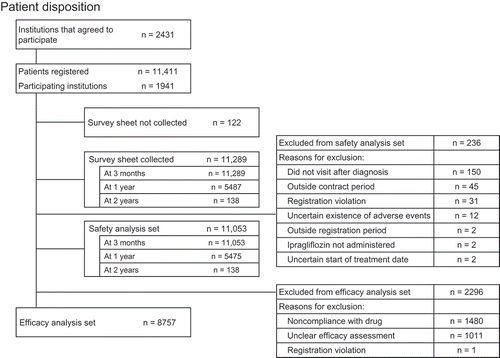
shows the disposition of patients registered in this surveillance study. Of 2431 institutions that agreed to participate in this study, 1941 participated and initially registered 11,411 patients. Survey forms were collected for 11,289 patients at 3 months, 5487 patients at 12 months, and 138 patients at 24 months. Out of 11,289 patients included in the locked database, the safety analysis set comprised 11,053 patients at 3 months, 5475 at 12 months, and 138 patients at 24 months. The efficacy analysis set comprised 8757 patients. The reasons for excluding patients from the safety and efficacy analysis sets are shown in .
3.2. Patient characteristics
shows the general characteristics of patients in the safety analysis set. Of 11,053 patients, 6714 (60.7%) were male. The mean (± standard deviation) age was 56.9 ± 12.2 years; body mass index (BMI), 29.14 ± 5.29 kg/m2; and estimated glomerular filtration rate (eGFR), 81.94 ± 21.75 mL/min/1.73 m2. The mean diabetes duration was 7.96 ± 6.45 years and was <5 years in 2593 patients (23.5%). Most of the patients (9246, 83.7%) had complications, most commonly arterial hypertension (6141, 55.6%) and dyslipidemia (6964, 63.0%). Diabetic nephropathy was reported in 1793 (16.2%) patients. Renal function was classified as normal in 9242 patients (83.6%), mildly impaired in 1372 patients (12.4%), moderately impaired in 123 patients (1.1%), and severely impaired in 10 patients (0.1%). Hepatic function was classified as normal in 8346 patients (75.5%), mildly impaired in 2087 patients (18.9%), moderately impaired in 297 patients (2.7%), and severely impaired in 11 patients (0.1%).
Table 1. Patient characteristics at baseline.
3.3. Treatment characteristics
summarizes the treatments received by patients at baseline and during the survey period. The majority (9614, 87.0%) of patients were initially prescribed 50 mg/day ipragliflozin. A lower dose (25 mg/day) was initially prescribed to 1408 patients (12.7%). Only 14 patients (0.1%) were initially prescribed 100 mg/day ipragliflozin. The majority (10,695, 96.8%) of patients remained on the initial dose of ipragliflozin.
Table 2. Treatments used at baseline and/or during the survey period.
A total of 2036 patients (18.4%) who had not been treated with other antidiabetic drugs were administered ipragliflozin. The majority of patients (8943 patients, 80.9%) were prescribed ipragliflozin in combination with another antidiabetic drug, of which the most common were a dipeptidyl peptidase-4 inhibitor in 6131 patients (55.5%), metformin in 4580 patients (41.4%), a sulfonylurea in 3043 patients (27.5%), insulin in 1183 patients (10.7%), and an α-glucosidase inhibitor in 1120 patients (10.1%). At baseline, 3191 (28.9%) patients were receiving two concomitant antidiabetic drugs. Antihypertensive drugs (4875 patients, 44.1%) and statins (4032 patients, 36.5%) were the most commonly used concomitant drugs. Diuretics were prescribed to 818 patients (7.4%).
3.4. Efficacy
The efficacy of ipragliflozin was assessed at 3, 12, and 24 months. The baseline value of HbA1c was 8.11 ± 2.79% and that of FPG was 167.6 ± 59.8 mg/dL. As illustrated in , glycemic control improved significantly at 12 months, with mean reductions from baseline in HbA1c (−0.79%) and FPG (−31.0 mg/dL) (all P < 0.05). At 12 months, body weight decreased significantly with changes of −2.81 kg from a baseline value of 78.61 ± 17.21 kg (P < 0.05, ). As shown in , a significant decrease in fasting serum insulin concentration was observed: −5.82 µU/mL at 12 months from a baseline value of 16.46 ± 22.93 µU/mL (P < 0.05).
Figure 2. Changes in (a) hemoglobin A1c (HbA1c; National Glycohemoglobin Standardization Program [NGSP] units), (b) fasting plasma glucose (FPG), (c) body weight, and (d) fasting serum insulin from baseline. Results are presented as the mean and the error bars indicate standard deviation. *P < 0.05 vs. baseline (paired t test).
![Figure 2. Changes in (a) hemoglobin A1c (HbA1c; National Glycohemoglobin Standardization Program [NGSP] units), (b) fasting plasma glucose (FPG), (c) body weight, and (d) fasting serum insulin from baseline. Results are presented as the mean and the error bars indicate standard deviation. *P < 0.05 vs. baseline (paired t test).](/cms/asset/c72560ac-ddd6-448f-95b2-96a6e45eb96d/ieop_a_1408792_f0002_b.gif)
3.5. Vital signs
summarizes the changes in vital signs. Significant decreases were observed in systolic blood pressure, diastolic blood pressure, and heart rate: −4.2 mmHg (n = 2725), −2.6 mmHg (n = 2723), and −0.9 beats/min (n = 1832) at 12 months, respectively, from baseline values of 133.3 ± 15.2 mmHg, 78.4 ± 11.0 mmHg, and 78.0 ± 12.4 beats/min, respectively (all P < 0.05).
Table 3. Changes from baseline in vital signs and laboratory parameters.
3.6. Laboratory variables
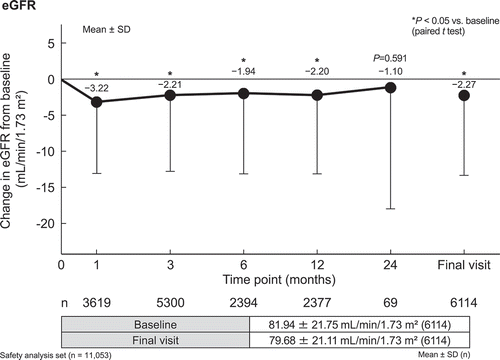
Laboratory variables are summarized in . Mean changes in serum concentrations of the liver enzymes aspartate aminotransferase (−4.6 ± 13.9 U/L) and alanine aminotransferase (−8.4 ± 18.9 U/L) showed a significant decrease from baseline to 12 months (all P < 0.05). Mean changes in serum concentrations of both low-density lipoprotein cholesterol (LDL-C; −3.8 ± 25.8 mg/dL) and triglycerides (−25.4 ± 119.5 mg/dL) showed a significant decrease from baseline to 12 months (all P < 0.05). Mean changes in high-density lipoprotein cholesterol (HDL-C; 2.9 ± 8.4 mg/dL) showed a significant increase from baseline to 12 months (P < 0.05). Mean changes in serum uric acid concentration (−0.26 ± 0.93 mg/dL) also showed a significant decrease from baseline to 12 months (P < 0.05). Mean changes in hematocrit (1.80 ± 2.62%) and blood urea nitrogen (1.07 ± 3.48 mg/dL) showed a significant increase from baseline to 12 months (P < 0.05). Changes in eGFR from baseline are shown in ; eGFR decreased significantly (−2.20 mL/min/1.73 m2) at 12 months from a baseline value of 81.94 ± 21.75 mL/min/1.73 m2 (P < 0.05).
3.7. Safety
In the present analysis of interim data, ADRs were assessed in 11,053 patients (safety analysis set). The ADRs and serious ADRs are listed in and by system organ class together with the rates of these ADRs in the clinical trials prior to approval [Citation3–Citation8]. Overall, 1612 ADRs were reported in 1184 patients with an incidence rate of 10.71% in this surveillance study. The most common class of ADRs was renal and urinary disorders, which occurred in 559 patients (5.06%) followed by infections and infestations, which occurred in 137 patients with an incidence of 1.24%, and skin and subcutaneous tissue disorders, which occurred in 126 patients with an incidence of 1.14%. All other classes of ADRs occurred in <1% of patients.
Table 4. Adverse drug reactions reported during the survey period.
Table 5. Serious adverse drug reactions reported during the survey period.
In the clinical trials prior to ipragliflozin approval, the incidence of ADRs was 32.89%. Additionally, prior to approval, more patients experienced ADRs, such as renal and urinary disorders (10.55%), investigations (7.97%), general disorders and administration site conditions (6.05%), gastrointestinal disorders (6.05%), infections and infestations (3.83%), skin and subcutaneous tissue disorders (2.88%), and nervous system disorders (2.52%). No new unreported safety concerns were observed in this survey.
The incidence of serious ADRs in the current survey was 0.56% (62 patients). These included nervous system disorders (13 cases total: cerebral infarction, 8; lacunar infarction, 2; and cerebral hemorrhage, depressed level of consciousness, and transient ischemic attack, 1 each); cardiac disorders (11 cases total: acute myocardial infarction, 3; unstable angina and myocardial infarction, 2 each; and angina pectoris, atrial fibrillation, congestive cardiac failure, and coronary artery disease, 1 each); benign, malignant, and unspecified neoplasms (including cysts and polyps; 8 cases total: pancreatic carcinoma, 3; colon cancer, 2; and lung neoplasm, malignant lung neoplasm, and intraductal proliferative breast lesion, 1 each); infections and infestations (6 cases total: urinary-tract infection, 2; and liver abscess, peritonsillar abscess, pneumonia, acute pyelonephritis, and genital herpes simplex, 1 each); metabolism and nutrition disorders (6 cases total: dehydration and hypoglycemia, 2 each; and hyperglycemia and hyperphagia, 1 each); renal and urinary disorders (5 cases total: hematuria, neurogenic bladder, renal disorder, renal vessel disorder, and ureterolithiasis, 1 case each); hepatobiliary disorders (4 cases total: hepatic cirrhosis, 2; and cholecystitis, cholestatic jaundice, and liver disorder, 1 each); skin and subcutaneous tissue disorders (3 cases total: drug eruption, eczema, and urticaria, 1 each); injury, poisoning, and procedural complications (3 cases total: fall, road traffic accident, subarachnoid hemorrhage, contusion, and brain contusion, 1 each); gastrointestinal disorders (2 cases total: duodenal ulcer, gastric ulcer hemorrhage, and gastroesophageal reflux disease, 1 each); endocrine disorders (1 case of hypothyroidism); psychiatric disorders (1 case of depression); eye disorders (1 case of retinal hemorrhage), and ear and labyrinth disorders (1 case of sudden hearing loss).
The incidence rates of ADRs of special interest are shown in . These ranged from 0.01% (fracture) to 4.62% (polyuria/pollakiuria). Although 511 patients (4.62%) reported polyuria/pollakiuria, none of these events were considered serious. These included 400 pollakiuria ADRs (3.62%) and 272 polyuria ADRs (2.46%). Volume depletion was reported in 138 patients (1.25%), of which six cases were considered serious (0.05%). ADRs associated with volume depletion included pollakiuria (23 ADRs, 0.21%), dehydration (16 ADRs, 0.14%), polyuria (15 ADRs, 0.14%), thirst (15 ADRs, 0.14%), cerebral infarction (two ADRs, 0.02%), and lacunar infarction (one ADR, 0.01%). Skin complications were reported in 129 patients (1.17%), of which three cases (0.03%) were considered serious. Skin complication-related ADRs included drug eruption (41 ADRs, 0.37%), pruritus (27 ADRs, 0.24%), eczema (17 ADRs, 0.15%), and rash (15 ADRs, 0.14%). Of the 116 (1.05%) genital infections reported, one (0.01%) was considered serious. Genital infection-related ADRs included pruritus genital (47 ADRs, 0.43%), vulvovaginal candidiasis (21 ADRs, 0.19%), and female genital infection (15 ADRs, 0.14%). Urinary-tract infection was reported in 74 patients (0.67%), three of which were considered serious (0.03%). ADRs associated with urinary-tract infection included cystitis (37 ADRs, 0.33%) and urinary-tract infection (32 ADRs, 0.29%). Two of 67 cases of renal disorder (0.02%) were considered serious ADRs. Renal disorder-related ADRs included renal disorder (22 ADRs, 0.20%) and renal dysfunction (14 ADRs, 0.13%). Hepatic disorder was reported in 45 patients (0.41%), four cases of which were considered serious (0.04%). ADRs associated with hepatic disorder included hepatic disorder (18 ADRs, 0.16%) and hepatic dysfunction (14 ADRs, 0.13%). Twenty-four patients experienced hypoglycemia, of which three (0.03%) events were considered serious. ADRs associated with hypoglycemia included hypoglycemia (20 ADRs, 0.18%). Of the 14 patients reporting cerebrovascular disease (0.13%), 13 cases were considered serious (0.12%). The most common ADRs associated with cerebrovascular disease were cerebral infarction (eight ADRs, 0.07%), transient ischemic attack, and lacunar infarction (two ADRs each, 0.02%). Of the 13 patients reporting cardiovascular disease (0.12%), 11 cases were considered serious (0.10%). The most common ADRs associated with cardiovascular disease were acute myocardial infarction (three ADRs, 0.03%), angina pectoris, unstable angina pectoris, atrial fibrillation, congestive heart failure, and myocardial infarction (two ADRs each, 0.02%). Of the eight patients reporting malignant tumor (0.07%), seven cases were considered serious (0.06%). ADRs associated with malignant tumors included pancreatic carcinoma (three ADRs, 0.03%) and colon cancer (two ADRs, 0.02%). Three patients (0.03%) presented with ketone body-related events, none of which were considered serious. These included ketosis (two ADRs, 0.02%) and diabetic ketoacidosis (one ADR, 0.01%). The only ADR associated with fracture was ankle fracture (one case [0.01%], deemed as nonserious). Of 1612 ADRs, 1368 (84.9%) occurred within 90 days from the initial use of ipragliflozin (), and 1397 ADRs (86.7%) were classified either as resolved or in remission ().
Table 6. Adverse drug reactions of special interest.
Table 7. Time to onset of adverse drug reactions of special interest.
Table 8. Outcome of adverse drug reactions of special interest.
4. Discussion
Here, we report the interim results of the ongoing 3-year prospective post-marketing surveillance study, STELLA-LONG TERM, focusing on the safety of ipragliflozin in a large analysis set of 11,053 patients with data collected up to 24 months. To date, this is the largest post-marketing surveillance study of an SGLT2 inhibitor performed in Japanese patients with type 2 diabetes in real-world clinical practice. This post-marketing surveillance study is still in progress, and the final analysis will be reported at 3 years.
The data in the present analysis of interim data were similar to those reported previously at 3 months [Citation10] and to those reported in the clinical trials prior to approval [Citation3–Citation8]. There were differences in patient backgrounds between the present study and the clinical trials prior to approval, including the younger mean age (56.9 ± 12.2 vs. 59.0 ± 10.14 years prior to approval) and higher BMI (29.14 ± 5.29 vs. 25.98 ± 3.731 kg/m2 prior to approval). The incidence rate of skin complications with the short-term use of SGLT2 inhibitors was a safety concern raised by Yabe et al. [Citation12]. However, in the present study, the incidence was lower than that reported in the clinical trials prior to approval [Citation3–Citation8] and in the STELLA-ELDER surveillance study that assessed real-world clinical setting use of ipragliflozin in elderly patients (≥65 years old) with type 2 diabetes [Citation13].
Although it has been reported that Japanese patients may be at increased risk of developing diabetic ketoacidosis in response to SGLT2 inhibitor therapy [Citation14], in the present study, we observed only one case of diabetic ketoacidosis (among the three ketone body-related ADRs). The patient was a male in his 40s with hypertension and mild hepatic impairment who had previously been treated with metformin. At the start of the present surveillance study, ipragliflozin and pioglitazone were added to his treatment. Twenty-nine days after the initial administration of ipragliflozin, he presented with diabetic ketoacidosis. According to the treating physician, the patient had been on a low-carbohydrate diet that may have been overly strict. The patient’s ketoacidosis later resolved. In this case, a causal relationship with ipragliflozin was not established; however, a similar case was reported previously in which a patient with type 2 diabetes and Prader–Willi syndrome on a low-carbohydrate diet developed ketoacidosis while being treated with ipragliflozin [Citation15]. The association of diabetic ketoacidosis with carbohydrate restriction has been previously reported [Citation16]; however, in our study, such an association could not be confirmed.
Peters et al. [Citation17] reported 13 cases of diabetic ketoacidosis associated with the use of the SGLT2 inhibitor canagliflozin from various practices across the United States. Given the association between SGLT2 inhibitors and diabetic ketoacidosis, the US Food and Drug Administration (FDA) issued a warning indicating that the SGLT2 inhibitors canagliflozin, dapagliflozin, and empagliflozin may lead to ketoacidosis [Citation18]. Recently, a retrospective study in the United States evaluated the risk of developing diabetic ketoacidosis among 50,220 patients newly prescribed an SGLT2 inhibitor and 90,132 patients newly prescribed a DPP-4 inhibitor [Citation19]. They concluded that shortly after initiating treatment with SGLT2 inhibitors, the risk of developing ketoacidosis was twice that with DPP-4 inhibitors [Citation19]. We are unable to provide clear reasons for the lower incidence of ketoacidosis with ipragliflozin observed in this post-marketing surveillance compared with the incidence reported for other SGLT2 inhibitors.
The CANVAS study recently reported an increased risk of lower limb amputations, mostly at the toe or metatarsal level, among patients treated with canagliflozin [Citation20]. Based on such findings, the European Medicines Agency issued a warning regarding the potential for increased risk of lower limb amputations among patients taking the SGLT2 inhibitors canagliflozin, dapagliflozin, and empagliflozin for type 2 diabetes [Citation21]. The FDA has requested the addition of this information to the drug label of canagliflozin [Citation22]. However, no such cases of amputations have been reported in this interim report or in the manufacturer’s pharmacovigilance database as of July 2017.
Although there was no significant difference between pre-approval clinical trials and the current surveillance in the overall incidence of hepatobiliary disorder ADRs, the incidence of serious hepatobiliary ADRs was higher in the current study. However, this difference may be explained by the fact that patients with hepatic dysfunction were excluded from the pre-approval clinical trials.
Based on the present observations, ipragliflozin treatment was associated with significant and clinically relevant improvements from baseline to 12 months in HbA1c (−0.79%), FPG (−31.0 mg/dL), and body weight (−2.81 kg) in real-world clinical practice. These results are consistent with the results of the 3-month interim report [Citation10], the clinical trials conducted for the new drug application in Japan [Citation3–Citation8], and a pooled analysis of these trials [Citation23]. In terms of other efficacy outcomes, we noted significant reductions from baseline to 12 months in blood pressure (systolic blood pressure: −4.2 mmHg; diastolic blood pressure: −2.6 mmHg), heart rate (−0.9 beats/min), LDL-C (−3.8 mg/dL), and triglycerides (−25.4 mg/dL), as well as a significant increase from baseline in HDL cholesterol (2.9 mg/dL). Although 1480 patients were excluded from the efficacy analysis in the current surveillance for ‘noncompliance with drug,’ it should be noted that this was unrelated to ADRs or insufficient efficacy. In Japan, off-label use of ipragliflozin is permitted at the discretion of the physician. In light of the safety issues that emerged following the launch of SGLT2 inhibitors in Japan, in the case of ipragliflozin, many physicians chose to initiate treatment at a lower dose than that specified in the package insert.
The reduction in LDL-C was consistent with the results reported in the 3-month baseline paper [Citation10]. Further, these results support the findings of the study by Bando et al., in which treatment with ipragliflozin 50-mg once daily resulted in a significant reduction in the proportional LDL-C concentration, small dense LDL-C/large buoyant LDL-C ratio, and small dense LDL-C concentration, which are associated with increased LDL particle size [Citation24]. The changes in laboratory variables related to liver function, hematocrit, and kidney function in the current post-marketing surveillance showed similar trends to those observed in the 3-month interim report [Citation10], as well as the clinical trials prior to approval [Citation3–Citation8]. The increase in hematocrit level observed in the present study may not be a negative effect of ipragliflozin. It has previously been reported that increased hematocrit may translate into improvement in cardiac efficiency by releasing more oxygen to the muscle [Citation25]. Further, an improvement in cardiovascular event risk factors (plasma glucose, uric acid and lipid concentrations, blood pressure, and body weight) may be a class effect of SGLT2 inhibitors; thus, a reduction in cardiovascular events, such as that previously reported in studies on canagliflozin [Citation20] and empagliflozin [Citation26], may also be expected with ipragliflozin.
The strength of this study was that a safety analysis on ipragliflozin treatment beyond 12 months could be performed on a large sample of patients. The limitations of the study included potential bias from incorrect completion of the survey report forms and the lack of a control group for comparisons. Additionally, there are still large amounts of unlocked data for the 12-month period that were not included in the current analysis; although the data at 3, 12, and 24 months have been combined and analyzed together, data for a number of patients at the 12-month period (and beyond) are still pending analysis. Further, the number of patients analyzed at 24 months of treatment was small because few patients had received 24 months of treatment by the cutoff date for this study. Nevertheless, we believe it is important to present the currently available data in a timely manner so that clinicians are fully informed of all potential safety issues.
5. Conclusion
The results of this analysis of interim data for the STELLA-LONG TERM surveillance study indicate that ipragliflozin was well tolerated and effective in Japanese patients with type 2 diabetes in a real-world clinical setting. No new previously unreported safety concerns were observed, and the incidence of ADRs did not exceed those reported in the clinical trials prior to approval. The clinically relevant improvements in glycemic control, lipids, blood pressure, and body weight are consistent with the efficacy results of the clinical trials prior to approval.
Declaration of interest
Ichiro Nakamura, Hiromi Tabuchi, and Satoshi Uno are employees of Astellas Pharma Inc. Hiroshi Maegawa has received lecture fees from Astellas Pharma Inc., AstraZeneca K.K., Taisho Toyama Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., MSD K.K., Daiichi Sankyo Company Limited, Nippon Boehringer Ingelheim Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Sanofi K.K., Kowa Pharmaceutical Company Ltd., and Takeda Pharmaceutical Company Limited; research support from Astellas Pharma Inc., AstraZeneca K.K., and Nippon Boehringer Ingelheim Co., Ltd.; and grants from Takeda Pharmaceutical Company Limited, Astellas Pharma Inc., MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Kowa Pharmaceutical Company Ltd., Ono Pharmaceutical Co., Ltd., Daiichi Sankyo Company Limited, Sanofi K.K., Mitsubishi Tanabe Pharma Corporation, Sanwa Kagaku Kenkyusho Co., Ltd., Sunstar Inc., Sumitomo Dainippon Pharma Co., Ltd., Eli Lilly Japan K.K., AstraZeneca K.K., and Novo Nordisk Pharma Ltd. Kazuyuki Tobe has received lecture fees from Astellas Pharma Inc., AstraZeneca K.K., Kowa Pharmaceutical Company Ltd., Takeda Pharmaceutical Company Limited, and Novo Nordisk Pharma Ltd.; and grants from Kowa Pharmaceutical Company Ltd., Astellas Pharma Inc., Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Company Limited, Nippon Boehringer Ingelheim Co., Ltd., Takeda Pharmaceutical Company Limited, Sanofi K.K., and Fuji Chemical Industries Co., Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
We would like to thank the study investigators and participating patients at the 1941 contracted institutions.
Additional information
Funding
References
- Isaji M. SGLT2 inhibitors: molecular design and potential differences in effect. Kidney Int Suppl. 2011;120:S14–19.
- Abdul-Ghani MA, Norton L, DeFronzo RA. Efficacy and safety of SGLT2 inhibitors in the treatment of type 2 diabetes mellitus. Curr Diab Rep. 2012;12:230–238.
- Kadokura T, Akiyama N, Kashiwagi A, et al. Pharmacokinetic and pharmacodynamic study of ipragliflozin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Diabetes Res Clin Pract. 2014;106:50–56.
- Kashiwagi A, Akiyama N, Shiga T, et al. Efficacy and safety of ipragliflozin as an add-on to a sulfonylurea in Japanese patients with inadequately controlled type 2 diabetes: results of the randomized, placebo-controlled, double-blind, phase III EMIT study. Diabetol Int. 2015;6:125–138.
- Kashiwagi A, Kazuta K, Goto K, et al. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2015;17:304–308.
- Kashiwagi A, Kazuta K, Takinami Y, et al. Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. Diabetol Int. 2015;6:8–18.
- Kashiwagi A, Shiga T, Akiyama N, et al. Efficacy and safety of ipragliflozin as an add-on to pioglitazone in Japanese patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study (the SPOTLIGHT study). Diabetol Int. 2015;6:104–116.
- Kashiwagi A, Takahashi H, Ishikawa H, et al. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17:152–160.
- Poole RM, Dungo RT. Ipragliflozin: first global approval. Drugs. 2014;74:611–617.
- Maegawa H, Tobe K, Tabuchi H, et al. Baseline characteristics and interim (3-month) efficacy and safety data from STELLA-LONG TERM, a long-term post-marketing surveillance study of ipragliflozin in Japanese patients with type 2 diabetes in real-world clinical practice. Expert Opin Pharmacother. 2016;17:1985–1994.
- Ministry of Health, Labour and Welfare. Classification criteria for seriousness of adverse drug reactions of pharmaceuticals. PAB/SD notification no.80. Tokyo: Ministry of Health, Labour and Welfare: 1992 Jun 29.
- Yabe D, Nishikino R, Kaneko M, et al. Short-term impacts of sodium/glucose co-transporter 2 inhibitors in Japanese clinical practice: considerations for their appropriate use to avoid serious adverse events. Expert Opin Drug Saf. 2015;14:795–800.
- Yokote K, Terauchi Y, Nakamura I, et al. Real-world evidence for the safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA-ELDER): final results of a post-marketing surveillance study. Expert Opin Pharmacother. 2016;17:1995–2003.
- Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7:135–138.
- Hayami T, Kato Y, Kamiya H, et al. Case of ketoacidosis by a sodium-glucose cotransporter 2 inhibitor in a diabetic patient with a low-carbohydrate diet. J Diabetes Investig. 2015;6:587–590.
- Yabe D, Iwasaki M, Kuwata H, et al. Sodium-glucose co-transporter-2 inhibitor use and dietary carbohydrate intake in Japanese individuals with type 2 diabetes: a randomized, open-label, 3-arm parallel comparative, exploratory study. Diabetes Obes Metab. 2017;19:739–743.
- Peters AL, Buschur EO, Buse JB, et al. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687–1693.
- FDA Drug Safety Communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. Silver Spring (MD): U.S. Food & Drug Administration. 2015 May 15 [cited 2017 Dec 5]; [five screens]. Available from: https://www.fda.gov/downloads/Drugs/DrugSafety/UCM446954.pdf
- Fralick M, Schneeweiss S, Patorno E. Risk of diabetic ketoacidosis after initiation of an SGLT2 inhibitor. N Engl J Med. 2017;376:2300–2302.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657.
- SGLT2 inhibitors: information on potential risk of toe amputation to be included in prescribing information. London (UK): European Medicines Agency. 2017 May 8 [cited 2017 Dec 5]; [one screen]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/SGLT2_inhibitors_(previously_Canagliflozin)/human_referral_prac_000059.jsp&mid=WC0b01ac05805c516f
- FDA Drug Safety Communication: FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR). Silver Spring (MD): U.S. Food & Drug Administration. 2017 May 16 [cited 2017 Jul 25]; [two screens]. Available from: https://www.fda.gov/Drugs/DrugSafety/ucm557507.htm
- Kashiwagi A, Yoshida S, Nakamura I, et al. Efficacy and safety of ipragliflozin in Japanese patients with type 2 diabetes stratified by body mass index: a subgroup analysis of five randomized clinical trials. J Diabetes Investig. 2015;7:544–554.
- Bando Y, Hitomi T, Keiko A, et al. Ipragliflozin lowers small, dense low-density lipoprotein cholesterol levels in Japanese patients with type 2 diabetes mellitus. J Clin Transl Endocrinol. 2016;6:1–7.
- Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39:1108−1114.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117−2128.