ABSTRACT
Background: This post-marketing surveillance examined the safety and efficacy of long-term teneligliptin therapy in Japanese patients.
Research design and methods: We report interim results (cut-off date: 28 June 2017) of a 3-year PMS undertaken in subjects with type 2 diabetes mellitus (T2DM). Survey items included demographics, treatments, adverse drug reactions (ADRs), and laboratory variables. A subgroup analysis was also performed across three age groups (<65 years; 65 to <75 years; ≥75 years). Main outcome measures were incidence of ADRs, laboratory variables, and change in glycated hemoglobin (HbA1c) from baseline over time.
Results: Of 11,677 patients registered, data from 10,532 patients (6,338 males/4,194 females) were analyzed for the safety analysis set; the median administration period was 731 days. Overall, ADRs and serious ADRs were reported in 364 (3.46%) and 91 patients (0.86%), respectively. The most common ADRs were all hypoglycemia (0.32%), constipation (0.27%), and hepatic function abnormal (0.24%). No change in mean body weight occurred, and a reduction in mean HbA1c was observed until 2 years. The safety and efficacy profiles did not differ markedly among the three age groups.
Conclusions: These interim results show that teneligliptin was well tolerated and improved hyperglycemia in Japanese patients with T2DM in clinical practice.
1. Introduction
As in much of the world, the burden of type 2 diabetes mellitus (T2DM) is growing in Japan. In 2015, an estimated 8.37 million individuals in Japan had T2DM, and this is predicted to rise to 9.75 million in 2030, mainly due to population aging [Citation1].
Dipeptidyl peptidase-4 (DPP-4) inhibitors are a class of hypoglycemic drugs widely used in the management of T2DM. They stimulate insulin secretion and suppress glucagon secretion through inhibition of DPP-4, which catalyzes the degradation of incretins [Citation2]. DPP-4 inhibitors play a significant role in the management of T2DM in Japan. Over 70% of patients treated with antidiabetic drugs receive a DPP-4 inhibitor and, in 60% of cases, they receive a DPP-4 inhibitor as first-line therapy, according to the Japan Medical Data Center claims database [Citation2].
There are reports that DPP-4 inhibitors have greater glucose-lowering effects in Asian versus non-Asian individuals and in Japanese versus non-Japanese patients with T2DM [Citation3–Citation5], attributed to the different characteristics of T2DM in these populations. Such characteristics comprise pathophysiological differences (Asian patients, including Japanese patients, have reduced β-cell function and less insulin resistance); dietary factors, including a greater consumption of fish in the Asian diet; and possible genetic differences [Citation2,Citation5,Citation6]. Moreover, DPP-4 inhibitors have a low risk of hypoglycemia and are therefore a promising therapeutic option for glycemic control in elderly patients [Citation7,Citation8].
Given the widespread use of DPP-4 inhibitors in Japan, particularly in an increasingly elderly population, there is a real need for large-scale studies and real-world data (RWD) collection to examine the safety and efficacy of DPP-4 inhibitors in Japanese patients. To date, there have been large-scale studies of DPP-4 inhibitors undertaken [Citation9–Citation11] and RWD [Citation8,Citation12,Citation13] obtained in numerous countries. In Japan, studies that have reported RWD about the use of DPP-4 inhibitors have been relatively small in scale (500–1300 patients in each study) [Citation14–Citation16], compared with data from other countries (5000–17,000 patients in each clinical study [Citation9–Citation11] or 1700–120,000 patients in RWD studies [Citation8,Citation12,Citation13]).
Teneligliptin, an orally administered DPP-4 inhibitor, was approved for the management of T2DM in 2012 in Japan and in 2014 in Korea: the safety and efficacy of teneligliptin had been evaluated as monotherapy and as combination therapy with oral hypoglycemic agents and insulin [Citation17–Citation20]. The 3-year post-marketing surveillance (PMS) ‘RUBY’ (ExploRing the long-term efficacy and safety included cardiovascUlar events in patients with type 2 diaBetes treated bY teneligliptin in the real-world; JapicCTI-153047) is currently underway. The purpose of this PMS is to examine the safety and efficacy of long-term teneligliptin therapy administered in >10,000 Japanese patients in real-world clinical practice. To date, registration to the survey has been completed, almost all the baseline data have been obtained, and more than half of the survey period has elapsed. This is an interim report for more than 10,000 Japanese patients with T2DM, from whom case report forms had been received by the data cutoff date of 28 June 2017. In addition, a subgroup analysis was performed across three age groups (<65, 65–<75, and ≥75 years) because DPP-4 inhibitors are frequently used in elderly patients (≥65 years) in Japan [Citation15,Citation21].
2. Patients and methods
2.1. Patients and treatments
Adult subjects with T2DM, who were treatment-naïve for teneligliptin and who were subsequently prescribed teneligliptin by their physician for the treatment of T2DM, were eligible for registration to this PMS. Teneligliptin was prescribed in accordance with the approved label, which states that the usual adult dosage is 20 mg administered orally, once daily. If the efficacy of teneligliptin 20 mg/day was considered by the physician to be insufficient, the label permitted an increase in dosage up to 40 mg once daily, with close monitoring of the clinical course. All treatment decisions were made at the discretion of physicians. This survey involved the collection of anonymous data from clinical settings; thus, in compliance with Japanese regulations for PMS, it was not necessary to obtain informed consent from patients.
2.2. Survey design
This PMS conformed to Good Post-Marketing Study Practice guidelines. Patients were registered in a central system between May 2013 and February 2015; the survey is expected to run until August 2018, providing 3-year follow-up data for each patient. All data were recorded in electronic case report forms (eCRFs) for each patient. An electronic data-capture system was used for patient registration and data collection.
The survey collected baseline demographic information, followed by information about treatment status, clinical laboratory tests and adverse events at 6 months, 1 year, 2 years, and 3 years after registration. The survey included the following demographic characteristics: sex, age, body weight, body mass index (BMI), duration of diabetes, presence of diabetic complications (retinopathy, neuropathy, or nephropathy), presence of nondiabetic complications (renal disease, hepatic disease, hypertension, dyslipidemia, and dementia), and renal function (estimated glomerular filtration rate [eGFR] and presence/absence of dialysis) at the start of treatment. The survey also captured the initial daily dosage of teneligliptin, the average daily dosage of teneligliptin, the use of concomitant antidiabetic medication during treatment, the occurrence of adverse drug reactions (ADRs), body weight, and laboratory variables (glycated hemoglobin [HbA1c] and fasting plasma glucose [FPG]). ADRs were described using the Medical Dictionary for Regulatory Activities/Japanese, version 20.0. Hypoglycemia was categorized as follows: severe hypoglycemia was defined as a blood glucose level of ≤50 mg/dL, or diagnosed by the physician; and cases of mild or moderate hypoglycemia or ‘blood glucose decreased’ were determined according to physicians’ clinical judgment. Laboratory variables were assessed according to changes over time from baseline.
2.3. Statistical analysis
All statistical analyses were performed using SAS statistical software version 9.1.3 or later (BASE/SAS®, SAS/STAT®; SAS Institute Inc., Cary, NC, USA). A sample size of 3000 subjects was considered necessary to detect at least one case of an unknown ADR at an incidence of 0.1% to ensure a power of 95%. Treatment continuation rates of teneligliptin were assumed to be 70% at year 1, 50% at year 2, and 20–30% at year 3; thus, the sample size was set at 10,000, and 2000–3000 patients were expected to complete 3 years of treatment. Statistical analyses were performed on data pooled from all study centers. The report includes a subgroup analysis of patients divided into three age categories (<65; 65–<75; and ≥75 years). Categorical variables are presented as frequencies and percentages; continuous variables are presented using descriptive statistics. Changes in continuous variables from baseline were tested using paired t-tests, with a significance level of 5% (two-sided). Least squares mean (LS mean) and standard error (SE) for the change from baseline in HbA1c over time (months 3, 6, 12, 18, and 24) were calculated using analysis of covariance (the covariate variable was the value of HbA1c at baseline). Missing data for the variables under analysis were censored from the analysis.
3. Results
3.1. Demographic characteristics
By the data cutoff date of 28 June 2017, a total of 11,677 Japanese patients with T2DM were registered from 1755 participating institutions in Japan. Among these patients, eCRFs for 252 patients had not been retrieved at data cutoff; eCRF data were available for the remaining 11,425 patients. In total, 10,532 patients were included in the safety analysis set, while 893 patients were excluded. The most common reason for exclusion was lack of survey data after registration (n = 780). The efficacy analysis was undertaken in 10,062 patients (efficacy analysis set). In total, 470 patients were excluded because of either a lack of survey data about efficacy (n = 468) or off-label use (n = 2). The flow of patients through the survey is shown in .
Figure 1. Patient disposition. *Number of patients with a finalized demographic characteristics form. Note that some excluded patients had more than one reason for exclusion. eCRF, electronic case report form; ADR, adverse drug reaction.
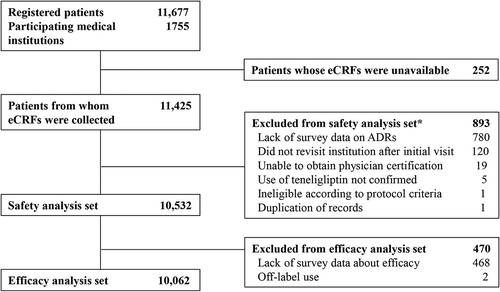
In the safety analysis set, the median administration period was 731 days. Baseline patient characteristics for the safety analysis set are shown in . More males (n = 6338; 60.2%) than females (n = 4194; 39.8%) were registered in the survey. The mean ± standard deviation (SD) age, BMI, and duration of diabetes were 65.4 ± 12.4 years, 25.26 ± 4.40 kg/m2, and 7.38 ± 7.86 years, respectively. Over half (57.0%) of the patients surveyed were ≥65 years of age, and about a quarter of patients (25.5%) were aged ≥75 years. The proportion of female patients in each group increased with age.
Table 1. Baseline characteristics (safety analysis set).
Patients in the <65-year age group had the highest mean BMI (26.47 ± 4.76 kg/m2) of all age groups and had the shortest mean duration of diabetes (5.50 ± 5.83 years). At the start of treatment, patients in the <65-year group had higher mean HbA1c levels and mean FPG levels compared with the 65–<75 and ≥75-year age groups: HbA1c (%), 8.15 ± 1.75, 7.57 ± 1.31, and 7.31 ± 1.14, respectively; FPG (mg/dL), 158.3 ± 57.7, 147.6 ± 46.9, and 145.8 ± 47.9, respectively. The frequency of diabetic complications, especially neuropathy and nephropathy, increased with age and was highest in the ≥75-year age group; conversely, eGFR at the start of treatment was lowest in the ≥75-year age group. More than 60% of patients had hypertension or dyslipidemia. Concomitant hypertension occurred with increasing frequency with age, while the frequency of dyslipidemia did not differ across the three age groups.
3.2. Prescribed treatments during the survey
Data about the concomitant use of antidiabetic drugs are shown in . At the start of the survey, approximately half of the patients received teneligliptin as monotherapy and almost all the patients were started at a dosage of 20 mg/day (n = 10,390; 98.7%).
Table 2. Concomitant use of antidiabetic drugs during the survey period (safety analysis set).
During the survey period, the mean daily dosage of teneligliptin was 20.38 ± 2.69 mg; this was similar across all three age groups. Overall, a total of 293 patients received a dosage of 40 mg/day teneligliptin at least once. Sulfonylureas were the most common concomitant antidiabetic drugs prescribed and were taken by 24.7% of all patients. The types and doses of sulfonylureas are shown in Table S1; the most commonly used sulfonylurea was glimepiride, at a daily dose of approximately 1.5 mg. Sulfonylureas were used similarly across all three age groups. In contrast, biguanides and sodium glucose co-transporter 2 inhibitors were more likely to be prescribed in patients aged <65 years compared with the other age groups.
3.3. Safety
ADRs that occurred in at least five patients and serious ADRs that occurred in at least two patients are shown in . Of all the patients surveyed, 364 patients (3.46%) experienced ADRs, and 91 patients (0.86%) had serious ADRs. The incidence of ADRs was similar across all age groups: <65 years, 2.98%; 65–<75 years, 4.01%; and ≥75 years, 3.58%. The most frequently experienced ADRs were all hypoglycemia (hypoglycemia, blood glucose decreased, and hypoglycemic unconsciousness, n = 34; 0.32%), constipation (n = 28; 0.27%), hepatic function abnormal (n = 25; 0.24%), rash (n = 12; 0.11%), and dizziness (n = 11; 0.10%). Of note, the incidence of all hypoglycemia was higher in patients aged 65–<75 years (n = 18; 0.54%) compared with patients aged <65 years (n = 9; 0.20%) or ≥75 years (n = 7; 0.26%). The concomitant drug most associated with the incidence of hypoglycemia was insulin (incidence rate ratio 7.08, 95% confidence interval 3.41–14.69), with sulfonylurea not identified as a risk factor. The incidence of serious ADRs was higher in the 65–<75-year group (n = 34; 1.02%) and in the ≥75-year age group (n = 35; 1.30%) compared with the <65-year age group (n = 22; 0.49%). The most frequently experienced serious ADR was all hypoglycemia (n = 9; 0.09%). Among the affected patients, eight patients were receiving concomitant therapy with insulin or sulfonylureas: six patients were receiving insulin, three patients were receiving sulfonylureas, and one patient who experienced hypoglycemic unconsciousness was receiving both insulin and a sulfonylurea. Pancreatic carcinoma was reported in five patients (0.05%) and cerebral infarction, ileus, and death were reported in three patients (0.03%) each. Any relationship between death and teneligliptin was unknown. During treatment with teneligliptin 40 mg/day (293 patients), 10 patients (3.41%) and three patients (1.02%) experienced ADRs and serious ADRs, respectively (detailed data not shown). There was no significant change in body weight in any age group over 2 years (Figure S1).
Table 3. Incidence of ADRs and serious ADRs (safety analysis set).
3.4. Efficacy
The change in HbA1c from baseline is shown in ). A reduction in HbA1c was observed at 3 months of treatment and was maintained over 2 years; the change in HbA1c from baseline at 2 years (mean ± SD for all patients) was −0.75 ± 1.36%. The extent of the decrease in HbA1c was greater in subgroups with higher baseline HbA1c values ()). Among the patients who had 2 years of HbA1c data, approximately 70% of patients had HbA1c ≥7.0% at baseline, and the proportion of patients achieving HbA1c <7.0% at 2 years was 48.3%. The LS mean changes in HbA1c from baseline for each age group also indicated that a reduction in HbA1c over time was observed in all age groups (). No marked differences were observed among the age groups: the changes in HbA1c from baseline at 2 years (LS mean ± SE) were −0.72 ± 0.02%, −0.76 ± 0.02%, and −0.77 ± 0.03% in the <65, 65–<75, and ≥75 years age groups, respectively. The time courses of HbA1c and FPG in all patients and age groups are shown in Figures S2 and S3.
Figure 2. Change in HbA1c from baseline to 2 years: (a) all patients (b) according to baseline HbA1c, in the efficacy analysis set. Data are expressed as the mean ± SD. The number of patients [N] at each point (a) and the number of patients and actual value (at baseline and after 2 years of treatment) in each group (b) are shown in parentheses. *p < 0.001 vs baseline, by paired-t test.
![Figure 2. Change in HbA1c from baseline to 2 years: (a) all patients (b) according to baseline HbA1c, in the efficacy analysis set. Data are expressed as the mean ± SD. The number of patients [N] at each point (a) and the number of patients and actual value (at baseline and after 2 years of treatment) in each group (b) are shown in parentheses. *p < 0.001 vs baseline, by paired-t test.](/cms/asset/6f3321d7-e9b1-4bb5-b2e5-792a733796d8/ieop_a_1420165_f0002_oc.jpg)
4. Discussion
This report provides the first preliminary RWD from the largest-scale survey on the long-term use of teneligliptin in Japanese patients with T2DM. Overall, the results demonstrated that teneligliptin, administered as monotherapy or combination therapy for up to 2 years, was well tolerated and reduced hyperglycemia in Japanese patients with T2DM, with a low incidence of hypoglycemia and no change in body weight. There were no remarkable differences among the three age groups (<65; 65–<75; and ≥75 years).
Pooled data from clinical studies undertaken in 1645 Japanese patients with T2DM reported an ADR incidence for teneligliptin of 9.5% (n = 156) across all the reviewed studies [Citation22]. Although the overall incidences of ADRs (3.46%) and serious ADRs (0.86%) in this interim analysis were low compared with those in the earlier clinical studies, the most common ADRs, all hypoglycemia (0.32%) and constipation (0.27%), were also the most common ADRs reported in pooled data from clinical trials (2.6% and 0.9%, respectively) [Citation22]. Conversely, hepatic function abnormal was rarely reported in the pooled analysis (0.1%) but was a common ADR in this survey (0.24%), albeit mild in its severity. This is consistent with previous post-marketing reports of hepatic dysfunction associated with teneligliptin, which resulted in revision of the package insert in 2014 [Citation23]. The Pharmaceuticals and Medical Devices Agency of Japan has also requested the inclusion of ‘hepatic dysfunction’ in the clinically significant adverse reactions section of the package inserts of other DPP-4 inhibitors (sitagliptin [Citation24], vildagliptin [Citation25], alogliptin [Citation26], and linagliptin [Citation27]).
In this interim analysis, combination therapy with insulin, but not sulfonylureas, was related to the incidence of hypoglycemia. The low rate of hypoglycemia associated with teneligliptin prescribed in combination with a sulfonylurea may be attributable to the adherence by physicians to recommendations published by the Committee for Appropriate Use of Incretin-Related Drugs (glucagon-like peptide-1 receptor agonists and DPP-4 inhibitors) in Japan, which recommended reducing the sulfonylurea dose when prescribed together with a DPP-4 inhibitor, especially in older patients or in patients with renal insufficiency [Citation28]. In the current survey, the most commonly used sulfonylureas, glimepiride and gliclazide, were prescribed at doses below the recommended dose.
Management of T2DM in elderly patients can be challenging because older patients are more likely to have underlying chronic disease or impaired cognitive or physical function. Moreover, they are at a higher risk, compared with younger patients, of developing severe hypoglycemia due to polypharmacy, decreased renal function, cognitive impairment or dementia, and hypoglycemia unawareness [Citation29–Citation31]. The Japan Diabetes Society and Japan Geriatrics Society Joint Committee recommends that glycemic targets for elderly patients should be defined on an individual basis and should take into consideration factors such as background characteristics and health status (e.g. age, cognitive function, physical function [basic and instrumental activities of daily living], comorbidities, risk for severe hypoglycemia, and life expectancy) [Citation32].
Results from the interim analysis of three subgroups of patients (<65, 65–<75, and ≥75 years) demonstrated no remarkable differences in the safety of teneligliptin between elderly and non-elderly patients. Although the incidence of all hypoglycemia was higher in patients aged 65–<75 years (n = 18; 0.54%) compared with that in patients aged <65 years (n = 9; 0.20%) and those aged ≥75 years (n = 7; 0.26%), the difference was small (0.28–0.34%). Therefore, the relationship between hypoglycemia and age remains to be addressed. No significant change in body weight for up to 2 years was observed across the three age groups. Likewise, no significant difference was seen in the efficacy of teneligliptin across the three age groups, with a sustained reduction in HbA1c (−0.72 to −0.77%) observed over 2 years. Similar results have been reported for other DPP-4 inhibitors in elderly patient populations. In a recent observational study (n = 831) [Citation15], a significant improvement in mean HbA1c was reported across all three age groups (<65, 65–<75, and ≥75 years) of Japanese patients with T2DM after 2 years of treatment with the DPP-4 inhibitor sitagliptin. In addition, consistent improvements in HbA1c in other populations of elderly patients with T2DM have been reported for sitagliptin [Citation33,Citation34], linagliptin [Citation35], saxagliptin [Citation36], alogliptin [Citation37], and vildagliptin [Citation38]. Interestingly, a meta-analysis of 63 randomized controlled trials revealed that DPP-4 inhibitors may be more effective in elderly than younger patients with mild/moderate fasting hyperglycemia [Citation39]. Nevertheless, to inform treatment decisions, additional research is required to fully determine the profiles of patients who are most likely to benefit from DPP-4 inhibitors.
Teneligliptin is eliminated by both hepatic metabolism, mediated by cytochrome P450 3A4 or flavin-containing monooxygenase 3, and renal excretion mechanisms; thus, no dosage reduction is required in patients with renal or hepatic impairment. Moreover, this dual mechanism of excretion may make teneligliptin less susceptible to drug–drug interactions [Citation40]. This may be beneficial for elderly patients in whom renal function is reduced and polypharmacy is common.
This survey has several limitations. First, the present report describes an interim analysis of survey results; thus, the results presented may not reflect final survey results. Second, this was a PMS with no control group, so it is not possible to determine the significance of the trends observed relative to no treatment. Finally, this survey included only Japanese patients, so our results cannot be generalized to other populations. However, our results remain clinically relevant, particularly in consideration of the differences in pathophysiology between Japanese and non-Japanese patients and of the wide use of DPP-4 inhibitors in Japan.
5. Conclusions
This interim report from a large-scale RWD PMS survey provides initial information about the safety and efficacy of long-term teneligliptin therapy for the treatment of T2DM in both elderly and non-elderly Japanese patients. The safety profile of teneligliptin in this interim analysis did not differ markedly from the established safety profiles of teneligliptin or other DPP-4 inhibitors.
Author contributions
T. Kadowaki, M. Haneda, and H. Ito supervised and contributed to the design and concept of this survey. M. Ueno contributed to the design and data collection. M. Matsukawa performed the statistical analysis. H. Iijima drafted the manuscript. All authors contributed to interpretation and discussion of the data, reviewing the manuscript and have approved the final draft.
Declaration of interest
T Kadowaki has received speaker honorarium/lecture fees from Astellas Pharma Inc., AstraZeneca K.K., Eli Lilly Japan K.K., Kowa Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd.; has received research grants from Daiichi Sankyo Co., Ltd., Novartis Pharma K.K., and Takeda Pharmaceutical Co., Ltd.; has received scholarship grants from Astellas Pharma Inc., Daiichi Sankyo Co., Ltd., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi K. K., Sumitomo Dainippon Pharma Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd.; and has belonged to courses endowed by Kowa Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd. M Haneda has received speaker honorarium/lecture fees from Astellas Pharma Inc., Kowa Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi K.K., Taisho Pharmaceutical Co., Ltd., and Taisho Toyama Pharmaceutical Co., Ltd.; and has received scholarship grants from Astellas Pharma Inc., Daiichi Sankyo Co., Ltd., Eli Lilly Japan K.K., Johnson & Johnson K.K., Kissei Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sanofi K.K., Shionogi & Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd. H Ito has received speaker honorarium/lecture fees from Daiichi Sankyo Co., Ltd. and Mitsubishi Tanabe Pharma Corporation, and has received scholarship grants from Daiichi Sankyo Co. Ltd. and Mitsubishi Tanabe Pharma Corporation. M Ueno, M Matsukawa, T Yamakura, K Sasaki, M Kimura, and H Iijima are employees of Mitsubishi Tanabe Pharma Corporation. The authors would like to acknowledge Emma Donadieu, MSc, and David Murdoch of Edanz Medical Writing, for providing medical writing support, which was funded by Mitsubishi Tanabe Pharma Corporation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have received an honorarium from Expert opinion on Pharmacotherapy for their review work, but have no other relevant financial relationships to disclose.
Supplementary_material.zip
Download Zip (948.3 KB)Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Charvat H, Goto A, Goto M, et al. Impact of population aging on trends in diabetes prevalence: a meta-regression analysis of 160,000 Japanese adults. J Diabetes Investig. 2015;6:533–542.
- Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7(Suppl 1):102–109.
- Kim YG, Hahn S, Oh TJ, et al. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708.
- Park H, Park C, Kim Y, et al. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: meta-analysis. Ann Pharmacother. 2012;46:1453–1469.
- Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Investig. 2015;6:495–507.
- Moller JB, Pedersen M, Tanaka H, et al. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care. 2014;37:796–804.
- Hinnen D. Dipeptidyl peptidase-4 inhibitors in diverse patient populations with type 2 diabetes. Diabetes Educ. 2015;41:19S–31S.
- Scheen AJ. Safety of dipeptidyl peptidase-4 inhibitors for treating type 2 diabetes. Expert Opin Drug Saf. 2015;14:505–524.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335.
- Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242.
- Montilla S, Marchesini G, Sammarco A, et al. Drug utilization, safety, and effectiveness of exenatide, sitagliptin, and vildagliptin for type 2 diabetes in the real world: data from the Italian AIFA Anti-diabetics Monitoring Registry. Nutr Metab Cardiovasc Dis. 2014;24:1346–1353.
- Sportiello L, Rafaniello C, Scavone C, et al. The importance of pharmacovigilance for the drug safety: focus on cardiovascular profile of incretin-based therapy. Int J Cardiol. 2016;202:731–735.
- Yuasa S, Sato K, Takai M, et al. Factor analysis of changes in hemoglobin A1c after 12 months of sitagliptin therapy in patients with type 2 diabetes. J Clin Med Res. 2016;8:461–471.
- Umezawa S, Kubota A, Maeda H, et al. Two-year assessment of the efficacy and safety of sitagliptin in elderly patients with type 2 diabetes: post hoc analysis of the ASSET-K study. BMC Endocr Disord. 2015;15:34.
- Kanatsuka A, Sato Y, Kawai K, et al. Relationship between the efficacy of oral antidiabetic drugs and clinical features in type 2 diabetic patients (JDDM38). J Diabetes Investig. 2016;7:386–395.
- Kadowaki T, Marubayashi F, Yokota S, et al. Safety and efficacy of teneligliptin in Japanese patients with type 2 diabetes mellitus: a pooled analysis of two phase III clinical studies. Expert Opin Pharmacother. 2015;16:971–981.
- Kadowaki T, Kondo K. Efficacy and safety of teneligliptin in combination with pioglitazone in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2013;4:576–584.
- Kadowaki T, Kondo K, Sasaki N, et al. Efficacy and safety of teneligliptin add-on to insulin monotherapy in Japanese patients with type 2 diabetes mellitus: a 16-week, randomized, double-blind, placebo-controlled trial with an open-label period. Expert Opin Pharmacother. 2017;18:1291–1300.
- Kadowaki T, Inagaki N, Kondo K, et al. Efficacy and safety of teneligliptin added onto canagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a multicentre, randomised, double-blind, placebo-controlled, parallel-group comparative study. Diabetes Obes Metab. 2017 Aug 8. DOI:10.1111/dom.13079
- Fujihara K, Igarashi R, Matsunaga S, et al. Comparison of baseline characteristics and clinical course in Japanese patients with type 2 diabetes among whom different types of oral hypoglycemic agents were chosen by diabetes specialists as initial monotherapy (JDDM 42). Medicine (Baltimore). 2017;96:e6122.
- Tenelia® 20 mg Tablets 2016 interview form. cited 2017 Oct 10. Available from: https://medical.mt-pharma.co.jp/di/file/if/tnl.pdf
- TENELIA® 20 mg tablets package insert. cited 2017 Oct 10. Available from: https://medical.mt-pharma.co.jp/di/file/dc/tnl.pdf
- JANUVIA® 12.5mg, 25mg, 50mg, 100mg tablets package insert. cited 2017 Oct 10. Available from: http://www.info.pmda.go.jp/downfiles/ph/PDF/170050_3969010F1034_2_27.pdf
- Equa® 50mg tablets package insert. cited 2017 Oct 10. Available from: www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/300242_3969011F1020_1_21#page=1
- Pharmaceuticals and Medical Devices Agency. Pharmaceuticals and medical devices safety information: alogliptin benzoate 2012. cited 2017 Oct 10. Available from: https://www.pmda.go.jp/files/000153459.pdf
- Pharmaceuticals and Medical Devices Agency. Summary of investigation results: linagliptin 2015. cited 2017 Oct 10. Available from: https://www.pmda.go.jp/files/000197853.pdf
- Yabe D, Seino Y. Dipeptidyl peptidase-4 inhibitors and sulfonylureas for type 2 diabetes: friend or foe? J Diabetes Investig. 2014;5:475–477.
- International Diabetes Federation. Managing older people with type 2 diabetes. cited 2017 Oct 10. Available from: https://www.ifa-fiv.org/wp-content/uploads/2014/02/IDF-Guideline-for-Older-People.pdf
- Bremer JP, Jauch-Chara K, Hallschmid M, et al. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care. 2009;32:1513–1517.
- Bruderer SG, Bodmer M, Jick SS, et al. Incidence of and risk factors for severe hypoglycaemia in treated type 2 diabetes mellitus patients in the UK-a nested case-control analysis. Diabetes Obes Metab. 2014;16:801–811.
- Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes, Haneda M, Ito H. Glycemic targets for elderly patients with diabetes. Diabetol Int. 2016;7:331–333. doi:10.1007/s13340-016-0293-8
- Barzilai N, Guo H, Mahoney EM, et al. Efficacy and tolerability of sitagliptin monotherapy in elderly patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Curr Med Res Opin. 2011;27:1049–1058.
- Bethel MA, Engel SS, Green JB, et al. Assessing the safety of sitagliptin in older participants in the trial evaluating cardiovascular outcomes with sitagliptin (TECOS). Diabetes Care. 2017;40:494–501.
- Barnett AH, Huisman H, Jones R, et al. Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382:1413–1423.
- Karyekar CS, Ravichandran S, Allen E, et al. Tolerability and efficacy of glycemic control with saxagliptin in older patients (aged >/= 65 years) with inadequately controlled type 2 diabetes mellitus. Clin Interv Aging. 2013;8:419–430.
- Rosenstock J, Wilson C, Fleck P. Alogliptin versus glipizide monotherapy in elderly type 2 diabetes mellitus patients with mild hyperglycaemia: a prospective, double-blind, randomized, 1-year study. Diabetes Obes Metab. 2013;15:906–914.
- Strain WD, Lukashevich V, Kothny W, et al. Individualised treatment targets for elderly patients with type 2 diabetes using vildagliptin add-on or lone therapy (INTERVAL): a 24 week, randomised, double-blind, placebo-controlled study. Lancet. 2013;382:409–416.
- Monami M, Cremasco F, Lamanna C, et al. Predictors of response to dipeptidyl peptidase-4 inhibitors: evidence from randomized clinical trials. Diabetes Metab Res Rev. 2011;27:362–372.
- Goda M, Kadowaki T. Teneligliptin for the treatment of type 2 diabetes. Drugs Today (Barc). 2013;49:615–629.

![Figure 3. LS-mean change in HbA1c from baseline to 2 years according to age group in the efficacy analysis set. Data are expressed as LS-Mean ± SE. The number of patients [N] at each point is shown in parentheses.](/cms/asset/742dd1b2-bfaf-4fb0-babb-a9d15cae53e6/ieop_a_1420165_f0003_oc.jpg)