ABSTRACT
Background: This subgroup analysis of STELLA-LONG TERM interim data explored the long-term safety and efficacy of ipragliflozin in non-elderly vs. elderly Japanese type 2 diabetes mellitus (T2DM) patients.
Research design and methods: STELLA-LONG TERM is an ongoing 3-year prospective surveillance study of Japanese T2DM patients receiving ipragliflozin 50 mg once daily. In this subgroup analysis, patient characteristics, laboratory variables, and adverse drug reactions (ADRs) were compared between non-elderly (<65 years) and elderly (≥65 years) patients.
Results: Non-elderly patients had significantly higher body mass index and low-density lipoprotein cholesterol than elderly patients (P < 0.001). The proportion of patients with hemoglobin A1c (HbA1c) <8.0% was significantly higher among elderly patients (P < 0.001). HbA1c, fasting plasma glucose, and body weight significantly decreased from baseline to 3 and 12 months in both groups (all P < 0.05 vs. baseline). The ADR incidence was 10.83% vs. 10.42% in non-elderly and elderly patients. The incidence of skin complications was 0.98% vs. 1.65% and that of renal disorder was 0.47% vs. 0.95% in non-elderly and elderly patients (both P = 0.003).
Conclusions: Ipragliflozin was effective in non-elderly and elderly Japanese T2DM patients in a real-world clinical setting. The incidence of renal disorder and skin complications was significantly higher in elderly vs. non-elderly patients.
1. Introduction
Sodium–glucose cotransporter 2 (SGLT2) inhibitors promote urinary glucose excretion, leading to a reduction in blood glucose independent from insulin activity [Citation1,Citation2]. Ipragliflozin, an SGLT2 inhibitor, was approved in Japan for the treatment of type 2 diabetes mellitus (T2DM) based on the results of several randomized controlled trials [Citation3–Citation8]. However, these trials included a relatively low number of elderly patients. In a pooled analysis of six pre-approval trials, 325 of 996 (32.6%) patients in the pooled study population were aged ≥65 years [Citation9]. Thus, concerns were raised regarding the use of ipragliflozin in the elderly population.
To address this concern, the Specified drug use resulTs survEy of IpragLifLozin treAtment in ELDERly type 2 diabetic patients (STELLA-ELDER) study focused on the 1-year surveillance of adverse drug reactions (ADRs) related to ipragliflozin in elderly Japanese patients aged ≥65 years. Both the interim and final reports of this study have been published [Citation10,Citation11]. In the STELLA-ELDER study, ADRs and serious ADRs occurred in 721 (10.06%) and 44 (0.61%) patients in the interim report and 1438 (16.91%) and 127 (1.49%) patients in the final report, respectively. Skin complications, volume depletion, genital infection, urinary tract infection, polyuria/pollakiuria, hypoglycemia, cerebrovascular disease, cardiovascular disease, malignant tumor, fracture, and ketone body-related events were observed in the interim and final reports as ADRs of special interest. Overall, the incidences of both ADRs and serious ADRs remained low throughout the 1-year surveillance. No previously unidentified safety concerns were raised. In terms of efficacy, ipragliflozin treatment was associated with improvements in hemoglobin A1c (HbA1c), fasting plasma glucose (FPG), body weight, and systolic blood pressure.
The Specified drug use resulTs survEy of IpragLifLozin treAtment in type 2 diabetic patients: LONG-TERM use (STELLA-LONG TERM) study is an ongoing 3-year prospective post-marketing surveillance study to evaluate the long-term efficacy and safety of ipragliflozin under real-world clinical settings at medical institutions in Japan. Interim reports of baseline characteristics [Citation12] and efficacy and safety data collected up to 24 months [Citation13] from the STELLA-LONG TERM study have been published.
The present study is a subgroup analysis of the STELLA-LONG TERM interim report to further explore the long-term safety and efficacy of ipragliflozin in non-elderly versus elderly Japanese patients with T2DM. Although the present subgroup analysis includes patients who received treatment up to 24 months, the study focused on 3- and 12-month data. Findings were compared with those of the STELLA-ELDER study.
2. Methods
The study design, patient selection criteria, and methods have been described in detail in the previous interim report [Citation12]. Briefly, Japanese patients with T2DM who were first prescribed ipragliflozin between 17 July 2014 and 16 October 2015 at a participating medical center in Japan were to be registered in STELLA-LONG TERM. A daily dose of ipragliflozin 50 mg was administered before or after breakfast in accordance with the package insert. A lower dose was permitted at the attending physician’s discretion in patients with severe hepatic impairment but was to be used with caution. A dose increase to 100 mg/day was allowed if the treating physician judged the treatment efficacy as insufficient; however, the patient’s clinical course had to be carefully monitored.
2.1. Study design
This surveillance study complied with Good Post-marketing Study Practice. The survey was performed as described in the previous interim report [Citation12]. Items in the survey included patients’ demographic and clinical characteristics, laboratory variables, vital signs (blood pressure and heart rate), and adverse events (AEs).
In this subgroup analysis, the cutoff date for the receipt of survey forms and data entry was 16 January 2017. Only patients whose data were locked at this time were included in this subgroup analysis.
Data were compared between non-elderly (<65 years) and elderly (≥65 years) patients.
Safety was evaluated based on AEs and ADRs that occurred during treatment with ipragliflozin. All ADRs were categorized according to system organ class and preferred term using MedDRA/J Version 19.1. Efficacy outcome measures were the changes in glycemic control, body weight, and laboratory variables from baseline up to 24 months.
2.2. Statistical analysis
Details of sample size calculations and rationale for the length of the study are described in the previous interim report [Citation12]. Efficacy variables, vital signs, and laboratory variables are shown as means ± standard deviation (SD), and changes from baseline were determined using paired t-tests. Categorical variables, including baseline characteristics and ADRs, are shown as the number (n) (%) of patients. The chi-squared test or two-sample t-test was used to compare patient demographic and clinical data and treatments used between non-elderly and elderly patients. Adjustments for type I error based on multiple hypothesis testing were not performed in this study. All statistical analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Patient disposition
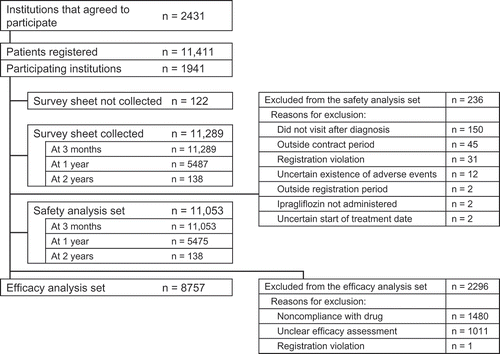
shows the disposition of patients registered in this surveillance study. Of 2431 contracted institutions, 1941 participated and initially registered 11,411 patients. Survey forms were collected for 11,289 patients at 3 months, 5487 patients at 12 months, and 138 patients at 24 months. Out of 11,289 patients included in the locked database, the safety analysis set comprised 11,053 patients at 3 months, 5475 at 12 months, and 138 patients at 24 months. The efficacy analysis set comprised 8757 patients. The reasons for excluding patients from the safety and efficacy analysis sets are shown in .
3.2. Patient characteristics
shows the baseline characteristics of patients in the safety analysis set (11,053 patients). Elderly patients (≥65 years) accounted for 28.6% (n = 3157) of the study population, and had a more favorable profile than non-elderly patients. Specifically, elderly patients had significantly lower body mass index (BMI), low-density lipoprotein cholesterol (LDL-C), diastolic blood pressure, non-high-density lipoprotein cholesterol, triglyceride, uric acid, hematocrit, aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyltransferase values at baseline compared with non-elderly patients (<65 years). The proportions of patients with complications, high systolic blood pressure, low estimated glomerular filtration rate, and HbA1c <8.0% were significantly higher in elderly patients than in non-elderly patients.
Table 1. Patient characteristics at baseline.
Some baseline characteristics, such as BMI, duration of diabetes, hepatic and renal function status, and estimated glomerular filtration rate, were similar between the elderly patients in the present study and those in STELLA-ELDER. In the present study, the most common complications among the elderly patients were hypertension (65.5%), dyslipidemia (63.4%), diabetic nephropathy (18.3%), and cardiovascular/cerebrovascular disease (17.7%). Similarly, in STELLA-ELDER, the most common complications were hypertension (59.3%), dyslipidemia (50.5%), macrovascular disease (20.0%), and diabetic nephropathy (11.6%).
Treatments used at baseline and/or during the survey period are summarized in . Most patients in both the non-elderly and elderly groups received an initial ipragliflozin dose of 50 mg (88.7 vs. 82.8%, respectively), followed by 25 mg (11.1 vs. 16.9%). Very few patients in either group received 100 mg of ipragliflozin as an initial dose (0.2 vs. 0.1%) and no patient received 75 mg as an initial dose. Although the proportion of patients taking concomitant antidiabetic drugs was statistically significantly higher in non-elderly than in elderly patients (81.4 vs. 79.7%, P = 0.018), the magnitude of the difference was small. The proportion of patients taking concomitant diuretics was statistically significantly lower among non-elderly vs. elderly patients (6.0 vs. 10.9%, P < 0.001). The treatments used at baseline and/or during the survey period were similar between the elderly patients in the present study and those in the STELLA-ELDER study.
Table 2. Treatments used at baseline and/or during the survey period.
The baseline characteristics of patients and treatments used at baseline and/or during the survey period stratified by three age categories (<65 years, ≥65 years to <75 years, ≥75 years) are shown in Supplementary Tables 1 and 2. The baseline characteristics and treatments used that were shown to be statistically significantly different between the two main age categories (<65 years and ≥65 years) were also found among the three age categories.
3.3. Efficacy
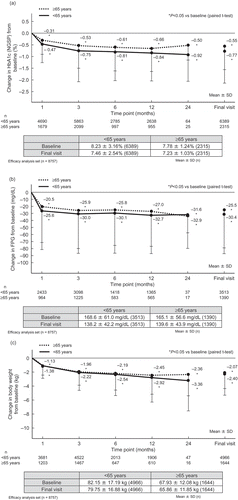
The efficacy of ipragliflozin was assessed in the efficacy analysis set up to 24 months (8757 patients). HbA1c (, FPG (, and body weight ( significantly decreased from baseline to 3 and 12 months in both age categories (all P < 0.05 vs. baseline). In non-elderly and elderly patients, the mean ± SD HbA1c was 8.23 ± 3.16% and 7.78 ± 1.24% at baseline, with a mean change of −0.75% and −0.53% at 3 months and −0.84% and −0.66% at 12 months, respectively; the mean ± SD FPG was 168.6 ± 61.0 mg/dL and 165.1 ± 56.6 mg/dL at baseline, with a mean change of −30.0 mg/dL and −25.9 mg/dL at 3 months and −32.7 mg/dL and −27.0 mg/dL at 12 months, respectively; and the mean ± SD body weight was 82.15 ± 17.19 kg and 67.93 ± 12.08 kg at baseline, with a mean change of −2.22 kg and −1.96 kg at 3 months and −2.92 kg and −2.45 kg at 12 months, respectively. Changes in HbA1c, FPG, and body weight at 3 and 12 months were similar to those in elderly patients in the STELLA-ELDER study (HbA1c: 7.78 vs. 7.84% [baseline], −0.53 vs. −0.54% [3 months], −0.66 vs. −0.67% [12 months]; FPG: 165.1 vs. 164.3 mg/dL [baseline], −25.9 vs. −22.5 mg/dL [3 months], −27.0 vs. −28.1 mg/dL [12 months]; and body weight: 67.64 vs. 67.64 kg [baseline], −1.96 vs. −2.20 kg [3 months], −2.45 vs. −2.91 kg [12 months]).
3.4. Safety
ADRs were assessed in the safety analysis set (11,053 patients). In the present study, the incidence of ADRs was similar between the non-elderly and elderly patients (10.83 vs. 10.42%) (). However, the incidence of serious ADRs was significantly lower in non-elderly than elderly patients (0.46 vs. 0.82%, P = 0.019). The incidence of ADRs in elderly patients in this survey was lower than that reported in the STELLA-ELDER study (10.42 vs. 16.91%). ADRs and serious ADRs stratified by three age categories (<65 years, ≥65 years to <75 years, ≥75 years) are shown in Supplementary Table 3.
Table 3. ADRs and serious ADRs.
ADRs and serious ADRs are listed by system organ class and preferred term in Supplementary Tables 4 and 5, and the incidences reported in the pre-approval clinical trials and in the STELLA-ELDER study are also shown. In the pre-approval clinical trials conducted of ipragliflozin, the incidence of ADRs was 32.89% and the most frequently reported ADRs were those related to renal and urinary disorders (10.55%), investigations (7.97%), general disorders and administration site conditions (6.05%), gastrointestinal disorders (6.05%), infections and infestations (3.83%), skin and subcutaneous tissue disorders (2.88%), and nervous system disorders (2.52%) (Supplementary Table 4). No new unreported safety concerns were observed in this survey. The most common serious ADR in elderly patients was cerebral infarction (0.20%) (Supplementary Table 5).
Table 4. Adverse drug reactions (ADRs) of special interest.
shows the incidence of ADRs of special interest. The incidences of skin complications (0.98 vs. 1.65%, P = 0.003) and renal disorder (0.47 vs. 0.95%, P = 0.003) were higher in elderly patients, while the incidence of polyuria/pollakiuria was higher in non-elderly patients (5.23 vs. 3.10%, P < 0.001). The incidence rates of ADRs of special interest stratified by three age categories (<65 years, ≥65 years to <75 years, and ≥75 years) are shown in Supplementary Table 6. Similar to the comparison between the two age categories, the incidence of skin complications was significantly higher among the higher age groups vs. the lower age group (0.98 vs. 1.62 vs. 1.73%, P = 0.012) and that of polyuria/pollakiuria was significantly higher among the lower age group vs. the higher age groups (5.23 vs. 3.49 vs. 1.86%, P < 0.001). No significant differences in other ADRs were shown among the three age categories.
4. Discussion
We conducted a subgroup analysis of non-elderly vs. elderly patients from the STELLA-LONG TERM interim report [Citation13] to evaluate the long-term safety and efficacy of ipragliflozin in Japanese T2DM patients in the real-world clinical setting and compared our findings with those of the STELLA-ELDER study. The interim results of the STELLA-LONG TERM study [Citation13] showed that ipragliflozin 50 mg once daily significantly improved HbA1c (−0.79%), fasting plasma glucose (−31.0 mg/dL), body weight (−2.81 kg), systolic and diastolic blood pressure (−4.2 mmHg and −2.6 mmHg), heart rate (−0.9 beats/min), LDL-C (−3.8 mg/dL), and triglycerides (−25.4 mg/dL) in Japanese T2DM patients. The incidence of ADRs was 10.71%, and the most common ADRs were renal and urinary disorders (5.06%), infections and infestations (1.24%), and skin and subcutaneous tissue disorders (1.14%).
In this subgroup analysis, some background characteristics were less favorable in non-elderly patients than in elderly patients. For example, in the present subgroup analysis, non-elderly patients had significantly higher BMI, HbA1c, and LDL-C values at baseline than elderly patients. In addition, the proportion of patients with normal liver function was lower in non-elderly than elderly patients. A recommendation was issued by a panel of experts in June and August 2014 on the use of SGLT2 inhibitors in patients over 65 years of age [Citation14]. This may have raised awareness among physicians on the use of SGLT2 inhibitors in older patients, and thus, may have contributed to the more favorable characteristics among the elderly patients in the present subgroup analysis. The recommendation was further updated in May 2016 [Citation15], based on the data obtained from post-marketing surveillance studies of SGLT2 inhibitors, which showed that the incidence of ADRs was not higher than that in the pre-approval clinical trials of ipragliflozin. The updated recommendation states that SGLT2 inhibitors must be used with caution in patients over 75 years of age, as well as those over 65 years of age with geriatric syndrome.
Regarding safety, while the overall incidence of ADRs was similar among non-elderly and elderly patients, the overall incidence of serious ADRs was significantly lower in non-elderly patients than in elderly patients (0.46 vs. 0.82%, P = 0.019). It should be noted that the most common serious ADR in elderly patients was cerebral infarction (six events, 0.20%).
Among ADRs of special interest, the incidence of polyuria/pollakiuria was significantly higher in non-elderly than elderly patients, while the incidence of renal disorder and skin complications was significantly higher in elderly patients than in non-elderly patients. A possible reason for the higher incidence of polyuria/pollakiuria among non-elderly patients is lower water intake among the elderly population [Citation16]. The progressive functional deterioration of the kidneys with aging has been previously reported [Citation17], which may explain the higher incidence of renal disorders among the elderly group. Regarding skin complications, dry skin or xerosis is a common problem in older adults [Citation18]. This condition may be associated with a lower water intake and higher use of diuretics among elderly than non-elderly patients, the latter of which was observed in the present study (concomitant use of diuretics: 6.0 vs. 10.9%, P < 0.001).
Compared with the STELLA-ELDER study, the overall incidences of ADRs and serious ADRs were lower in elderly patients in this survey (ADRs 16.91 vs. 10.71% and serious ADRs 1.49 vs. 0.56%). The reason for this difference between the two studies could be that the present study only analyzed interim data. This difference might become smaller once the data at 12 months are all collected and analyzed.
Greater improvements in some efficacy parameters in non-elderly vs. elderly patients may be attributed to the less favorable baseline characteristics in the former population. For example, higher HbA1c and body weight in the non-elderly population may allow for a greater reduction with treatment. Our findings suggest that the efficacy of ipragliflozin is not affected by age itself.
The strength of this study was that a safety analysis could be performed on a large sample of patients, ensuring the robustness of the results. The limitations of the study included potential bias from incorrect completion of the survey report forms. Additionally, although the data at 3, 12, and 24 months were combined and analyzed together, there is still a large amount of unlocked data corresponding to the 12-month period and beyond that was not included in the current analysis. There was no control group receiving placebo or other treatment for comparisons. The present post-marketing surveillance study collected data in real-world clinical practice and was not designed to specifically target patients with high cardiovascular risk. Therefore, enrolled patients had a low cardiovascular risk as evidenced by the relatively low incidence of cardiovascular complications at baseline and during treatment. Further studies on ipragliflozin in T2DM patients with higher cardiovascular risk are warranted. Finally, while the safety analysis set comprised 11,053 patients at 3 months, at 12 months, safety data were only available for approximately half of the population.
5. Conclusion
Ipragliflozin was well tolerated and effective in both non-elderly and elderly T2DM patients in the real-world clinical setting in Japan. Efficacy parameters in elderly patients were similar to those reported in the STELLA-ELDER study. Greater improvements in some efficacy parameters in non-elderly vs. elderly patients may be attributed to the less favorable baseline characteristics in the former population. The overall incidence rates of ADRs and serious ADRs in elderly patients were lower than those reported in the STELLA-ELDER study. The incidence of renal disorder and skin complications was significantly higher in elderly versus non-elderly patients.
Declaration of interest
H Maegawa has received lecture fees from Astellas Pharma Inc., AstraZeneca K.K., Taisho Toyama Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., MSD K.K., Daiichi Sankyo Company Limited, Nippon Boehringer Ingelheim Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Sanofi K.K., Kowa Pharmaceutical Company Ltd., and Takeda Pharmaceutical Company Limited; research support from Astellas Pharma Inc., AstraZeneca K.K., and Nippon Boehringer Ingelheim Co., Ltd.; and grants from Takeda Pharmaceutical Company Limited, Astellas Pharma Inc., MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Kowa Pharmaceutical Company Ltd., Ono Pharmaceutical Co., Ltd., Daiichi Sankyo Company Limited, Sanofi K.K., Mitsubishi Tanabe Pharma Corporation, Sanwa Kagaku Kenkyusho Co., Ltd., Sunstar Inc., Sumitomo Dainippon Pharma Co., Ltd., Eli Lilly Japan K.K., AstraZeneca K.K., and Novo Nordisk Pharma Ltd. K Tobe has received lecture fees from Astellas Pharma Inc., AstraZeneca K.K., Kowa Pharmaceutical Company Ltd., Takeda Pharmaceutical Company Limited, and Novo Nordisk Pharma Ltd.; and grants from Kowa Pharmaceutical Company Ltd., Astellas Pharma Inc., Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Company Limited, Nippon Boehringer Ingelheim Co., Ltd., Takeda Pharmaceutical Company Limited, Sanofi K.K., and Fuji Chemical Industries Co., Ltd. I Nakamura, H Tabuchi, and S Uno are employees of Astellas Pharma Inc. Writing assistance was utilized in the production of this manuscript and funded by Astellas Pharma Inc. One referee has done consultancy work including recent consultancy work for Sanofi and AstraZeneca.
3_20180221_supplementary_tables.docx
Download MS Word (120.8 KB)Acknowledgments
The authors would like to thank the study investigators and patients at the 1941 participating institutions. The authors acknowledge medical writing support from Dr. Keyra Martinez Dunn (Edanz Medical Writing) and ELMCOM.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Isaji M. SGLT2 inhibitors: molecular design and potential differences in effect. Kidney Int Suppl. 2011;79(120):S14–S19.
- Abdul-Ghani MA, Norton L, DeFronzo RA. Efficacy and safety of SGLT2 inhibitors in the treatment of type 2 diabetes mellitus. Curr Diab Rep. 2012;12:230–238.
- Kashiwagi A, Akiyama N, Shiga T, et al. Efficacy and safety of ipragliflozin as an add-on to a sulfonylurea in Japanese patients with inadequately controlled type 2 diabetes: results of the randomized, placebo-controlled, double-blind, phase III EMIT study. Diabetol Int. 2015;6:125–138.
- Kashiwagi A, Kazuta K, Goto K, et al. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2015;17:304–308.
- Kashiwagi A, Kazuta K, Takinami Y, et al. Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. Diabetol Int. 2015;6:8–18.
- Kashiwagi A, Kazuta K, Yoshida S, et al. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5:382–391.
- Kashiwagi A, Shiga T, Akiyama N, et al. Efficacy and safety of ipragliflozin as an add-on to pioglitazone in Japanese patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study (the SPOTLIGHT study). Diabetol Int. 2015;6:104–116.
- Kashiwagi A, Takahashi H, Ishikawa H, et al. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17:152–160.
- Kashiwagi A, Yoshida S, Kawamuki K, et al. Effects of ipragliflozin, a selective sodium–glucose co-transporter 2 inhibitor, on blood pressure in Japanese patients with type 2 diabetes mellitus: a pooled analysis of six randomized, placebo-controlled clinical trials. Diabetol Int. 2017;8:76–86.
- Terauchi Y, Yokote K, Nakamura I, et al. Safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA-ELDER): interim results of a post-marketing surveillance study. Expert Opin Pharmacother. 2016;17:463–471.
- Yokote K, Terauchi Y, Nakamura I, et al. Real-world evidence for the safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA-ELDER): final results of a post-marketing surveillance study. Expert Opin Pharmacother. 2016;17:1995–2003.
- Maegawa H, Tobe K, Tabuchi H, et al. Baseline characteristics and interim (3-month) efficacy and safety data from STELLA-LONG TERM, a long-term post-marketing surveillance study of ipragliflozin in Japanese patients with type 2 diabetes in real-world clinical practice. Expert Opin Pharmacother. 2016;17:1985–1994.
- Nakamura I, Maegawa H, Tobe K, et al. Safety and efficacy of ipragliflozin in Japanese patients with type 2 diabetes in real-world clinical practice: interim results of the STELLA-LONG TERM post-marketing surveillance study. Expert Opin Pharmacother. 2017 November 29:1–13. DOI:10.1080/14656566.2017.140879
- Yabe D, Nishikino R, Kaneko M, et al. Short-term impacts of sodium/glucose co-transporter 2 inhibitors in Japanese clinical practice: considerations for their appropriate use to avoid serious adverse events. Expert Opin Drug Saf. 2015 Jun;14(6):795–800.
- Inagaki N, Ueki K, Kaku K, et al. Recommendations for appropriate use of SGLT2i. (Written on 13 June 2014; revised on 28 August 2014; revised on 12 May 2016). [cited 2017 Jul 31]. Available from: http://www.jds.or.jp/modules/important/index.php?page=article&storyid=48
- Sui Z, Zheng M, Zhang M, et al. Water and beverage consumption: analysis of the Australian 2011-2012 National Nutrition and Physical Activity Survey. Nutrients. 2016;8:678.
- Yoon HE, Choi BS. The renin-angiotensin system and aging in the kidney. Korean J Intern Med. 2014;29:291–295.
- White-Chu EF, Reddy M. Dry skin in the elderly: complexities of a common problem. Clin Dermatol. 2011;29:37–42.