1. Introduction
The introduction of the proteasome inhibitor (PI) bortezomib (V) and immunomodulatory drugs (IMiDs) thalidomide (T) and lenalidomide (R/Revlimide) first in salvage, later in front-line multiple myeloma (MM) regimens has dramatically improved overall survival rates from 3–4 to ≥ 8 years, especially in standard-risk patients. Nevertheless, MM relapses in almost all patients, with the duration of subsequent responses decreasing with each line of therapy due to the emergence of resistant clones. Continuous treatment advances to further improve patient outcome are therefore needed. Excitingly, the armamentarium for salvage therapies has been expanded significantly during the past 5 years, now also including next-generation proteasome inhibitors carfilzomib (K) and ixazomib (I), next-generation IMiD pomalidomide (P), as well as first-in-class monoclonal antibodies elotuzumab (Elo) and daratumumab (Dara), and the HDAC inhibitor panobinostat. With several promising additional novel agents in the preclinical and early clinical pipeline, current treatment options in relapsed/refractory MM are anticipated to even further expand in the near future.
This editorial summarizes and cautiously interprets updated analyses of recent clinical trials; moreover it proposes potential algorithms for salvage treatment in MM patients.
2. General considerations for the choice of salvage treatment regimens
After front-line therapy close monitoring of the patient is warranted every three months. If progression is suspected based on clinical symptoms or on routine blood analysis (CBC, chemistry panel, as well as SPEP, SFLC, and quantitative immunoglobulins), imaging (whole-body-MRI, low-dose whole-body-CT) and bone marrow biopsy (histology, cytogenetics) should be performed. Accumulating data support a role of both PET/CT and MRD analysis in prognostication. Their impact on therapeutic decision-making (with the exception of identifying extramedullary disease with a PET/CT) is mostly unknown and, therefore, under active investigation. The use of a PET/CT is currently restricted to specialized centers, but may increasingly be used to identify MM not apparent on other imaging studies. Minimal residual disease testing of bone marrow samples by either next-generation sequencing (NGS) or next-generation fluorescence (NGF) analysis is limited to clinical trials.
The initiation of salvage treatment is indicated at a significant biochemical or symptomatic relapse, but not at oligoclonal reconstitution, which occasionally follows a deep response and lasts for several weeks to up to one year [Citation1,Citation2] ().
Table 1. Biochemical relapse, symptomatic relapse, and oligoclonal reconstitution.
The choice of treatment depends on patient-related features; therapy-related features; and disease-related features [Citation3,Citation4]. While the ultimate goal of salvage therapy is to achieve a long lasting disease remission, to prevent organ damage and relieve symptoms, the occurrence of adverse side effects always needs to be balanced against the potential benefits. The International Myeloma Working Group (IMWG) has developed a frailty score (fit, intermediate fit, frail), which not only includes age but also the functional status, comorbidities and feasibility of treatment. Fit patients are generally eligible for full-dose triplet therapies. In contrast, sequence modifications, dose reduction, doublet or mono therapies; or watch-and-wait strategies should be considered for unfit and frail patients in order to prevent cumulative toxicities of grades 3 or 4, which constrict quality of life and lead to premature treatment discontinuation, thereby limiting patient outcome. Importantly, supportive care is required to prevent increased risk of infections, bone disease, and venous thromboembolism (VTE), which come with the disease, targeted and chemo-therapy, as well as organ impairment [Citation5] ().
Figure 1. Treatment recommendations for patients with RRMM. V, Velcade/bortezomib; C, cyclophosphamide; T, thalidomide; M, melphalan; P, prednisone; R, Revlimid/lenalidomide; d or Dex, dexamethasone; DCEP/D-PACE, dexamethasone continous cyclophosphamid etoposid cisplatin; DT-PACE dexamethasone, thalidomide, continuous iv cisplatin doxorubicin cyclophosphamid etoposid; K, carfilzomib; Pom, pomalidomide; I, ixazomib; Elo, elotuzumab; Dara, daratumumab; in yellow: approved treatment regimens.
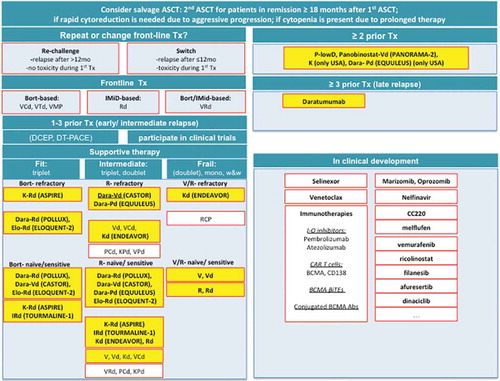
First relapse. Treatment options for relapsed/refractory (RR) MM at first relapse include a re-challenge with previous therapy regimens; a switch of therapy regimens; stem cell transplantation; or inclusion into a clinical trial. Patients who are transplant-ineligible and who relapse ≥ (6-) 12 months after initial therapy are likely to respond to a re-challenge of the previous therapy. Switching agents is preferred if an effective alternative treatment is available. Initially used agents may be given again at a later relapse. Alternative treatment regimens may even select for sensitive tumor clones enabling the use of initial drugs in previously refractory patients during subsequent relapses. Patients with an indolent relapse may be treated with a single agent or two-drug combinations followed by maintenance therapy; or watch-and-wait strategies. In general, triplet therapies should always be preferred to single and doublet agent use. Indeed, several recent phase 3 trials testing novel agents in combination with Rd or Vd demonstrated superior response rates when compared to doublet combinations. Importantly, triplet combinations did not only improve progression free survival (PFS) in standard-risk but also in high-risk patients ((del17p, t(4;14), t(14;16), high-risk gene expression profile, GEP). Although poor prognosis is not completely abrogated, immediate and intensive treatment with triplet therapies is required, especially in this patient subgroup, to achieve disease control and to improve survival. Besides triplet regimens, dexamethasone/continous cyclophosphamide/etoposid/cisplatin (DCEP), and dexamethasone/thalidomide/continuous iv cisplatin/doxorubicin/cyclophosphamide/etoposid (DT-PACE) may be considered in fit patients with aggressive relapse. A second high-dose chemotherapy/autologous stem cell transplantation (ASCT) should be considered if PFS lasted ≥ 18 months after the initial transplantation followed by a combination maintenance regimen. Due to ASCT-related toxicity without clinical benefit re-ASCT after < 18 months is not recommended [Citation6]. The role of allogeneic stem cell transplantation remains unclear and is associated with mortality rates up to 26% in heavily pretreated patients; this option should therefore only be considered in the clinical trial setting. In the future, chimeric antigen receptor (CAR)-T cell therapy may be an alternative to allogeneic stem cell transplantation.
Second and later relapse. Selection of resistant clones during disease progression decreases treatment responses with each successive treatment regimen. Indeed, patients double-refractory to lenalidomide and bortezomib have a median overall survival (OS) and event-free survival (EFS) of only 9 months and 5 months, respectively. MM patients relapsed and/or refractory to lenalidomide or bortezomib or both are generally treated with next-generation IMiDs (pomalidomide, P), next-generation PIs (ixazomib, I; carfilzomib, K), or monoclonal antibodies, daratumumab (Dara) in particular. Importantly, pomalidomide is currently the only approved drug with anti-MM activity in patients carrying del17p.
Based on promising preliminary results, evolving agents with novel anti-MM mechanisms including selinexor, venetoclax and immunotherapies (e.g. monoclonal antibodies, immune checkpoint inhibitors, CAR T cells) are likely to once more change treatment paradigmas in MM in the near future ().
3. Detailed considerations for the choice of salvage treatment regimens
Rd (PFS ~ 15 months) is the standard-of-care salvage therapy for R-sensitive patients who have been treated with bortezomib-containing induction regimens. Four recent clinical trials of Rd in combination with novel agents have challenged this regimen. ORR and PFS for these triplet therapies were 87 vs 67%/26.3 vs 17.6 months for K-Rd vs Rd (ASPIRE trial [Citation7]); 78 vs 72%/20.6 vs 14.7 months for IRd vs Rd (TOURMALINE trial [Citation8]); 79 vs 66%/19.4 vs 14.9 months for Elo-Rd vs Rd (ELOQUENT-2 trial [Citation9]); and 93 vs 76%/NR vs 18.4 months for Dara-Rd vs Rd (POLLUX trial [Citation10]). Most strikingly, Dara-Rd vs Rd induced MRD negativity of 6% vs 0.4% (sensitivity cut-off: 10−6), which correlated well with survival. Of note, a significant benefit also for OS was reported for KRd vs Rd (48.3 vs 40.4 months), as well as for Elo-Rd vs Rd (43.7 vs 39.6 months) [Citation9,Citation11]. Other viable options for R-sensitive patients include VRd/VRd-lite, KPd, VPd, PCd, VCd, Vd, or Kd. In case of economic constraints the combination of RCd or VTd may also be considered. In R-refractory patients Vd (PFS 9.7 months) and Kd (PFS 18.7 months), but also Elo-Vd (PFS 9.7 months), Dara-Vd (12 months PFS), and Panobinostat-Vd (PFS 12 months), as well as KCd, VMP (83% at 1 year), VTd, VRd, VPd, KPd, PCd, Pd are reasonable treatment options. In case of economic constraints VCd may be additionally considered (ORR 68%, PFS 16 months) [Citation11]. Specifically, the ENDEAVOR [Citation12] trial was the first study to directly compare the first-in-class PI bortezomib with the next-generation PI carfilzomib. ORR (77% vs 63%) as well as PFS (18.7 months vs 9.4 m months) and OS (47.6 vs 40 months) were significantly prolonged for Kd vs Vd indicating that carfilzomib is more potent than bortezomib. However, results may be biased in favor for carfilzomib due to a large cohort of patients who were re-exposed to bortezomib vs new exposure to carfilzomib. Head-to-head comparisons between bortezomib, carfilzomib, and ixazomib are needed. Moreover, additional studies are also needed to define the optimal dosing and schedule of carfilzomib in distinct patient cohorts.
Similar to Kd, Dara-Vd vs Vd (CASTOR trial [Citation13]) showed an improvement of ORR and PFS (84% vs 63% and 16.7 months vs 7.1 months). In V-exposed but not-refractory patients, combination therapies of Dara with both lenalidomide as well as bortezomib have the highest efficacy among triplet regimens followed by KRd, IRd, Elo-Rd, or VRd/VRd lite; and the doublets Kd and Rd. In V-refractory patients K- or Elo-Rd would be a meaningful treatment option (ASPIRE trial, ELOQUENT-2 trial). Since the TOURMALINE-2 and the ENDEAVOR trial excluded these patients, no data are available on the efficacy of IRd or Kd, respectively. Further studies are, therefore, needed. Subgroup analysis of the POLLUX, CASTOR and ENDEAVOR trial indicates that Dara-Rd, Dara-Vd, and Kd, respectively, overcome the negative impact of age since no significant difference was observed in the ≥ 65 years vs ≤ 65 years cohort. In contrast, KRd seems to be more toxic in the elderly patient cohort and should, therefore, be avoided ().
Table 2. Selected clinical phase III trials and EQUULEUS phase 1b combination trial in the RRMM setting. V, Velcade/bortezomib; K, carfilzomib; d, dexamethasone; R, Revlimid/lenalidomide; I, ixazomib; Elo, elotuzumab; Dara, daratumumab; HiR, high risk; SR, standard risk; PFS, progression free survival; HR, hazard ratio; OS, overall survival; NM, not mature.
Of note, the genetic status should be re-assessed at any relapse in order to identify new high-risk features that may guide the selection of doublet or triplet regimens. Although the hazard ratio (HR) of recent clinical phase 3 trials is always in favor of the triplet therapy, patients with high-risk cytogenetic abnormalities (t(4;14), t(14;16), and del(17p)) usually have a worse outcome than patients with standard-risk features. Only IRd with a HR of 0.64 vs 0.54 seems to overcome the adverse prognosis of high-risk vs standard-risk patients. Finally, patients having received 1 vs ≥ 2 prior therapies in the investigative arm of the ASPIRE, TOURMALINE-MM1, and ELOQUENT-2 trial had a similar HR. In contrast, the HR in patients treated with Dara-Rd was higher after 2 lines of prior therapy, while patients treated with Dara-Vd or Kd benefited most after having received only one line of prior therapy.
Regarding toxicity, triplet therapies are generally well tolerated, with a very low incidence of peripheral polyneuropathy (PNP) for the new PIs when compared to bortezomib, thalidomide, and vinca alkaloids. Cardiotoxicity and arterial hypertension are associated with carfilzomib and anthracyclines, with around 5% severe cardiac events. While the cardiotoxic effect of anthracyclines is direct (mediated via ROS and TOPB2), the cardiotoxic effect of carfilzomib seem to be indirect, triggered via endothelial injury. Close observation of the blood pressure and heart function is, therefore, recommended. Base-line echocardiograms do not appear to be predictive for the development of cardiovascular toxicities by carfilzomib. Moreover, IMiDs and corticosteroids, but also anthracyclines are associated with an elevated risk of thrombosis. While prophylaxis with aspirin is sufficient in patients with ≤ 1 risk factor in their prior history, prophylactic low molecular weight heparine (LMWH), or full dose warfarin should be given to patients with ≥ 2 risk factors. Promising data indicate that LMWH or warfarin may be replaced by oral apixiban or edoxaban in the near future. Other toxicities include fatigue and wasting (PIs and IMiDs), diarrhea (IMiDs), depression and myopathy (corticosteroids), and myelosuppression. In the case of renal dysfunction, combinations of Vd with/out thalidomide, doxorubicin, or cyclophosphamide are the treatment of choice. PIs, thalidomide, cyclophosphamide, and pomalidomide do not require dose adjustment. In contrast, dose adaptation is needed for lenalidomide. The anti-MM activity of next-generation PIs in patients with renal dysfunction is under investigation. Monoclonal antibody-related infusion reactions occurred mainly during the first infusion, but were mostly minor and well manageable. Due to their exclusion from above discussed clinical trials the benefit of triplet vs doublet or mono therapies in frail elderly patients is less evident. Additional studies need to determine whether full-dose intensity regimens are applicable in this patient subgroup or whether lower-dose intensity regimens, sequencing of two-drug instead of three-drug combinations should be recommended for this patient subgroup.
Finally, options for double V- and R-refractory patients include daratumumab-, pomalidomide-, and carfilzomib-based combinations (i.e. Pd, Dara-Pd, or Dara monotherapy). Of note Dara-Pd was FDA approved in June 2017 based on a remarkable 71% and 67% ORR in total or V/R-double refractory patients (Phase 1b EQUULEUS MMY1001 trial) [Citation14]. Another option is the all-oral combination of lenalidomide, cyclophosphamide, and prednisolone (ORR 67%, median PFS 12.1 months, OS 29 months).
However, if available, patients should always be enrolled into clinical trials.
4. Potential future treatment options
In addition to daratumumab, the alternative CD38 inhibitor isatuximab remains under clinical evaluation. In contrast, the clinical development of MOR202, yet another CD38 inhibitor was recently discontinued due to the lack of a partner and its slow progress. In multiple refractory patients preliminary data on agents with novel anti-MM mechanisms including selinexor, the first-in-class selective inhibitor of nuclear export (SINE), which binds and inhibits protein Exportin-1 (XPO-1) (ORR 20% in quad- and penta-refractory patients as single agent; and ORR 77% when combined with Vd); venetoclax, an oral BH3-mimetic which inhibits Bcl-2 (ORR 40% in t(11;14) MM patients as single agent; and ORR 67% when combined with Vd); nelfinavir, an oral HIV protease inhibitor (ORR 65%); the immune checkpoint inhibitors pembrolizumab, nivolumab, durvalumab, atezolizumab; as well as B cell maturation antigen (BCMA)- targeting approaches including BCMA- (bb2121, LCAR-B38M) CAR-T cells, BCMA-BiTEs (EM801, BI836909), BCMA-monomethyl auristatin F (GSK 2,857,916) are promising. Of note, despite excellent ORR, clinical trials evaluating all immune-checkpoint inhibitors are on hold due to an increased death rate in patients in the pembrolizumab-containing arm of the Keynote-183, 185, and 023 trials. A clinical trial to investigate the clinical impact of immune-checkpoint inhibitors in patients at high-risk of post-transplant recurrence is currently ongoing (https://clinicaltrials.gov/ct2/show/NCT02681302).
Other potential targets with preclinical and early clinical activity in MM include melflufen (melphalan flufenamide), the next-generation PIs oprozomib and marizomib, the BRaf inhibitor vemurafenib, the HDAC6 inhibitor ricolinostat (ACY-1215), the KSP-1 inhibitor filanesib, the AKT3 inhibitor afuresertib, the cereblon inhibitor CC220, the PIM kinase inhibitor LGH447, the CDK inhibitor dinaciclib, as well as CAR T-cells directed against CD138 (anti-CD138–28zeta) and CD19, and kappa-28 zeta; as well as vaccines. Several excellent recent review articles summarize data on these agents and immunotherapies ().
5. Expert opinion
With many different treatment options in the relapsed/refractory setting at hand, this article discussed several considerations of how to tailor rational algorithms and combination therapies for biologically defined distinct patient subgroups.
In general, triplet therapies should always be preferred to single and doublet agent use, at early relapse in particular. Carfilzomib and daratumumab are viable options as backbones of such triplet regimens. Due to their impressive hazard ratios, the best reported in the relapsed setting, minimal long-term toxicity, and greater convenience daratumumab-based combination are favored over carfilzomib-based combination therapies. Indeed, a recent network meta-analysis of all published phase 3 trials in RRMM indicated that daratumumab is the best combination partner for PFS across all patient groups [Citation15]. The currently ongoing development of a subcutaneous formulation of daratumumab is likely to further facilitate its applicability [Citation16]. However, additional randomized prospective phase 3 trials are needed to confirm these data. In case the pace of the disease is not very rapid, the all-oral IRd regimen is another valid option. For frail patients or patients older than 80 years daratumumab monotherapy may be considered.
The extensive use of bortezomib and lenalidomide in upfront MM therapy will likely challenge their role in relapsed/refractory MM in the near future. The eminent addition of daratumumab as a third backbone in upfront therapy will further increase the complexity of patient management in the relapsed/refractory setting. Moreover, a weakness of recent phase 3 trials is, that they generally did not include patients on lenalidomide maintenance. Therefore, most patients treated according to the current standard-of-care are likely already refractory to lenalidomide and/or bortezomib at the time of first relapse. In these cases pomalidomide-containing regimens, i.e. VPd (OPTIMISSM trial [Citation17], or KPd [Citation18] may be considered. As another emerging approach, daratumumab may be partnered with carfilzomib (Dara-Kd), in a high-risk, rapidly progressing patient in particular ([Citation19]; phase 3 CANDOR trial, https://clinicaltrials.gov/ct2/show/NCT03158688).
Besides aiming to optimize dosing and scheduling of the existing anti-MM armamentarium, ongoing efforts continue: (1) to address the complexity of MM pathogenesis in order to further expand the rationally derived therapeutic arsenal; (2) to utilize the immune system to overcome drug resistance; (3) to therapeutically manipulate minimal residual disease (MRD) and the emergence of resistant tumor clones; and maybe most importantly (4) to identify reliable biomarkers to guide personalized therapy, maximize treatment efficacy, reduce unnecessary toxicity, minimize a delay to treatment initiation, and enhance cost-effectiveness.
Based on these worldwide efforts I am convinced that evolving treatment strategies in MM in general, and relapsed/refractory MM in particular, will further improve outcome with an even faster pace than during the last two decades; and will thereby allow MM to be turned into a chronic disease and even to be cured in some patients in the near future.
Declaration of interest
K Podar has received speaker’s honoraria from Celgene, Amgen Inc, and Janssen Pharmaceuticals. He has also received consultancy fees from Celgene and Janssen Pharmaceuticals. He has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One referee declares that they’ve received honoraria from Janssen-Cilag, Celgene, Amgen, Takeda and Bristol-Myers Squibb.
Additional information
Funding
References
- Kumar SK, Callander NS, Alsina M, et al. NCCN guidelines insights: multiple myeloma, version 3.2018. J Natl Compr Canc Netw. 2018;16:11–20.
- Tovar N, De Larrea CF, Arostegui JI, et al. Natural history and prognostic impact of oligoclonal humoral response in patients with multiple myeloma after autologous stem cell transplantation: long-term results from a single institution. Haematologica. 2013;98:1142–1146.
- Palumbo A, Bringhen S, Mateos M-V, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an international myeloma working group report. Blood. 2015;125:2068–2074.
- Engelhardt M, Domm A-S, Dold SM, et al. A concise revised myeloma comorbidity index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. 2017;102:910–921.
- Ludwig H, Miguel JS, Dimopoulos MA, et al. International myeloma working group recommendations for global myeloma care. Leukemia. 2014;28:981–992.
- Giralt S, Garderet L, Durie B, et al. American society of blood and marrow transplantation, european society of blood and marrow transplantation, blood and marrow transplant clinical trials network, and international myeloma working group consensus conference on salvage hematopoietic cell transplantation in patients with relapsed multiple myeloma. Biol Blood Marrow Transpl. 2015;21:2039–2051.
- Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–152.
- Moreau P, Masszi T, Grzasko N, et al. Oral Ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–1634.
- Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–631.
- Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–1331.
- Sonneveld P. Management of multiple myeloma in the relapsed/refractory patient. Hematol Am Soc Hematol Educ Progr. 2017;2017:508–517.
- Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38.
- Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–766.
- Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130:974–981.
- Chy VB-T, Franken MG, Blommestein HM, et al. Systematic literature review and network meta-analysis of treatment outcomes in relapsed and/or refractory multiple myeloma. J Clin Oncol. 2017;35:1312–1319.
- Chari A, Usmani SZ, Mateos MV, et al. Subcutaneous daratumumab in patients with relapsed or refractory multiple myeloma: part 2 update of the open-label, multicenter, dose escalation phase 1b study (PAVO). J Clin Oncol. 2018;36(suppl; abstr 8013).
- Richardson PG, Rocafiguera AO, Beksac M, et al. Sonneveld, P on behalf of the OPTIMISMM trial investigators. Pomalidomide, bortezomib, and low‐dose dexamethasone (PVd) vs bortezomib and low-dose dexamethasone (Vd) in lenalidomide- exposed patients with relapsed or refractory multiple myeloma: phase 3 OPTIMISMM trial. J Clin Oncol. 2018;36(suppl; abstr 8001).
- Bringhen S, Mina R, Cafro AM, et al. Once-weekly carfilzomib, pomalidomide, and low-dose dexamethasone for relapsed/refractory myeloma: a phase I/II study. Leukemia. 2018;32(8):1803–1807.
- Chari A, Martinez-Lopez J, Mateos MV, et al. Daratumumab in combination with carfilzomib and dexamethasone (D-Kd) in lenalidomide-refractory patients with relapsed multiple myeloma: subgroup analysis of MMY1001. J Clin Oncol. 2018;36(suppl; abstr 8002).
