ABSTRACT
Background: Istradefylline is a first-in-class, non-dopaminergic, selective adenosine A2A receptor antagonist for the treatment of Parkinson’s disease (PD) in patients experiencing the wearing-off phenomenon with levodopa (L-DOPA). The authors present an interim report from a post-marketing surveillance (PMS) evaluating the safety and effectiveness of long-term istradefylline in a real-world setting.
Research design and methods: Istradefylline safety was assessed by the incidence of adverse events (AE) and adverse drug reactions (ADRs). Effectiveness was assessed using the physician’s assessment of off-time, off-time symptoms and motor dysfunction, unified PD rating scale (UPDRS) Part III score, and the physician’s global assessment.
Results: This analysis evaluated 476 patients. Istradefylline was generally well tolerated, despite dyskinesia and hallucination being the most common ADRs. Reduction in off-time was observed in 38.2% of patients, off-time symptoms were improved or markedly improved in 44.7%, and motor dysfunction was improved or markedly improved in 48.5%. The mean UPDRS Part III score decreased from 33.7 to 30.3 at the end of the study. The physician’s global assessment rated the drug as effective in 61.3% of patients.
Conclusions: This PMS provides useful safety and effectiveness data for long-term treatment with istradefylline in a real-world setting for patients with PD exhibiting the wearing-off phenomenon with L-DOPA.
1. Introduction
Parkinson’s disease (PD) is the second-most common neurodegenerative disorder after Alzheimer’s disease, and the estimated prevalence worldwide is expected to be 14.2 million within the next two decades [Citation1]. PD is primarily characterized by motor symptoms including resting tremor, rigidity, bradykinesia, and postural instability. Most motor symptoms of PD are caused by the progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc), which eventually leads to reduced stimulation of the striatal dopamine receptors associated with the paucity of dopamine release caused by the degeneration of nigrostriatal dopaminergic neurons [Citation2,Citation3]. Although the pathophysiology of motor symptoms in PD is relatively established, the causes and progression of PD pathogenesis are not yet fully understood, but they might involve both genetic and environmental factors. Moreover, PD is characterized by some non-motor symptoms, e.g. neuropsychiatric symptoms including depression and anxiety, cognitive impairment, autonomic dysfunction including orthostatic hypotension, and rapid eye movement sleep behavioral disorder [Citation4,Citation5], and the pathophysiology of such symptoms is poorly understood.
The dopamine precursor levodopa (L-DOPA) as a dopamine replacement therapy has been the gold standard pharmacotherapy to ameliorate symptoms of PD since the 1960s. Other dopaminergic agents for the treatment of PD include dopamine receptor agonists that directly activate dopamine receptors, and monoamine oxidase-B inhibitors and catechol-O-methyltransferase inhibitors that inhibit the metabolic enzymes of dopamine or dopamine precursors. Other treatment options such as amantadine, zonisamide (only available in Japan), or deep brain stimulation are clinically available [Citation6]. Among them, L-DOPA therapy is the most effective treatment for the symptoms of PD. However, long-term therapy with L-DOPA induces motor complications including wearing-off and dyskinesia, which represents an unmet medical need that is poorly managed by current dopaminergic therapies for patients in the advanced stage of disease [Citation6]. Furthermore, dopaminergic medications cause cardiovascular, gastrointestinal, and psychiatric disorders including impulse control disorders, hallucinations, and dopamine dysregulation syndrome [Citation7] by stimulating dopamine receptors that are abundantly expressed in the central nervous system and/or peripheral tissues. Based on these complications induced by dopaminergic agents, non-dopaminergic therapies that are independent of the dopamine receptor system might be new treatment options for PD.
Istradefylline, a selective adenosine A2A receptor antagonist that lacks affinity/activity on dopamine receptors/enzymes [Citation8], was developed to address the unmet need of the wearing-off phenomenon in PD patients treated with L-DOPA. Since 2013, it has only been available in Japan.
Adenosine A2A receptors are highly localized to the striatum, globus pallidus external segment (GPe), and accumbens [Citation9]. The basal ganglia-thalamo-cortical circuit was proposed to help understand the mechanisms involved in the motor symptoms in PD. A key feature of this neuronal circuit is the presence of two output pathways from the striatum: i) the striato-nigral pathway (direct pathway) regulated by dopamine D1 receptors; and ii) the striato-pallidal pathway controlled by dopamine D2 receptors (indirect pathway). Both pathways should be well balanced via dopaminergic input from the SNpc to ensure good motor control. A lack of dopaminergic inputs to those pathways occurs in PD, leading to an imbalance of the two pathways resulting in the decreased excitatory activity of the direct pathway and increased inhibitory activity of the indirect pathway, which causes motor dysfunction via the uncoordinated balance of the basal ganglia circuit [Citation10]. To date, studies have revealed that A2A receptor activation selectively increases the excitability of the indirect pathway via receptors in the striatum and GPe, mainly expressed by GABAergic interneurons (medium spiny neurons) [Citation11,Citation12]. Adenosine A2A receptor signals counteract dopaminergic D2 receptor function in the same indirect pathway, suggesting it is involved in the motor abnormalities observed in PD [Citation13,Citation14]. Indeed, selective adenosine A2A receptor agonists produce motor deficits in rodents and non-human primates, which are ameliorated by adenosine A2A receptor antagonists and vice versa [Citation15,Citation16].
There is growing evidence that adenosine A2A receptor antagonists might represent a new non-dopaminergic treatment option for PD. The selective adenosine A2A receptor antagonist istradefylline showed motor improvement in several rodent and non-human primate PD models [Citation17–Citation20]. Furthermore, excessive GABA release from the GPe in the indirect pathway of a PD animal model was inhibited by istradefylline. Therefore, blockade of the adenosine A2A receptor by istradefylline might reduce excitability of the indirect pathway and restore motor function under conditions of dopamine deficit [Citation21,Citation22].
Previous Phase IIB and III studies in Japan found that istradefylline reduced off-time, improved motor function, and was well tolerated in patients with PD [Citation23–Citation25]. The present post-marketing surveillance (PMS) was conducted in accordance with Good Post-marketing Study Practice and the Japanese regulations for PMS, in which the Pharmaceuticals and Medical Devices Agency requires the evaluation of the safety and effectiveness of a drug in a real-world clinical setting. The purpose of this interim analysis was to evaluate the incidence of adverse drug reactions (ADRs) and to identify unexpected ADRs, as well as factors potentially affecting the safety and effectiveness of the long-term use of istradefylline as an adjunct to L-DOPA in actual clinical practice.
2. Patients and methods
2.1. Study design
2.1.1. Study period
The planned period of the present survey is from 30 May 2013 to 31 May 2019 with the aim of collecting data from registered patients up to 1 year. For patients who ended or withdrew from treatment within 1 year, the observation period lasted until the time of treatment withdrawal. Eligible patients for the present interim analysis are as follows: 1) patients who were registered by 8 July 2015 (within the first 2-year period of the entire survey, which commenced when the first patient was enrolled) and 2) patients for whom locked data were available in case report forms.
2.1.2. Patient eligibility, registration, and treatment
Patients were included in this study if they were deemed fit by the physician to receive istradefylline treatment according to the indications and defined as those who experienced the wearing-off phenomenon with L-DOPA treatment. Patients selected for this surveillance study included those who were newly initiated with istradefylline treatment at 277 participating institutions. Istradefylline was administered as an oral tablet once daily at a dose of 20 or 40 mg.
Patients were registered using a central registration method. Data of survey items from patients were collected using an electronic data capture (EDC) system. At the time of starting treatment with istradefylline, the investigators registered the information into the EDC system and transmitted the data within 14 days.
2.1.3. Planned sample size
Based on previous Japanese clinical trials in which two ADRs occurred in 649 patients (ADRs occurring at an incidence of 0.3%), 1000 istradefylline-treated eligible patients were to be enrolled so that psychiatric symptoms as priority survey items could be detected in at least one patient with more than 95% predictive accuracy.
2.1.4. Survey items
Patient demographic data included age, gender, duration of PD, duration of L-DOPA exposure, duration of motor complications, presence or absence of dyskinesia, modified Hoehn & Yahr severity of illness stage (during off-time and on-time), and history of surgical treatment. The survey forms also included medication data: daily dose, administration period, reason for discontinuation of istradefylline, and the type and dose of concomitant anti-parkinsonian medications. Safety was assessed by adverse events (AEs), ADRs, and laboratory values showing abnormal changes. AEs, serious AEs (SAEs), and serious ADRs (SADRs) were recorded and coded according to the Medical Dictionary for Regulatory Activities/Japanese version (MedDRA/J) terminology, version 19.1. They were classified as serious or non-serious, following the International Conference on Harmonization guideline E2D.
Effectiveness was assessed using the physician’s assessment of off-time (increased, reduced, unchanged, or undetermined), off-time symptoms and motor function (improved, markedly improved, worsened, unchanged, or undetermined), unified PD rating scale (UPDRS) Part III score, and the physician’s global assessment (effective, ineffective, or undetermined).
The priority survey item was the status of occurrences of psychiatric symptoms (considered clinically significant ADRs in Japanese clinical studies).
2.1.5. Oversight
The case registration, data management, tabulation and analysis, collection of safety management information, and the setup and maintenance of the EDC system were performed by the CAC Croit Corporation (Tokyo, Japan).
2.2. Statistical analysis
To compare patient characteristics, descriptive statistics were calculated. The safety analysis was conducted by calculating the incidences of AEs and ADRs. For the effectiveness analyses, descriptive statistics or proportions of patients were calculated according to the outcome measurement. To assess factors associated with the incidence of ADRs and the effectiveness of istradefylline, a Fisher’s (2 × 2) or Cochran–Armitage (2 × n) test was used with a significance level of p < 0.05 (two-tailed). The change in the UPDRS Part III score from baseline was analyzed using a Wilcoxon signed-rank test among individual subjects as pairwise testing, and the threshold for statistical significance was defined as p < 0.0167 to account for multiple hypothesis testing.
The safety analysis set was defined as eligible PD patients receiving istradefylline who were registered by 8 July 2015 and for whom locked data were available. The efficacy analysis set was defined as the safety analysis set excluding patients receiving istradefylline for off-label use. All statistical analyses were conducted by the contract research organization CAC Croit Co., Ltd. (Tokyo, Japan), using SAS software, version 9.3 (SAS Institute, Inc., Cary, NC, U.S.A.).
2.3. Ethics statement
The study was conducted in accordance with the Good Post-marketing Study Practice protocol and applicable Japanese regulations. Informed consent and institutional review board approval were not required, compliant with Good Post-marketing Study Practice.
3. Results
3.1. Patient disposition
3.1.1. Registered patients of the present survey
Registration of the surveillance was closed at the end of May 2017. The current number of eligible registered patients is 1359.
3.1.2. Eligible patients for the interim analysis
This interim analysis included data from patients who were registered within the first 2-year period. Among 706 patients who were registered during the period, locked data were available for 476 PD patients. The maximum duration of observation period was 1 year. No patients were excluded from the safety analysis or the full efficacy analysis sets, which, therefore, included all 476 PD patients.
3.2. Patient characteristics
The baseline characteristics of patients are shown in . The mean (± standard deviation [SD]) age of analyzed patients (n = 476) was 70.7 ± 9.4 years, and 45.8% (218/476) of patients were male. The mean duration of PD was 8.8 ± 5.8 years (n = 464). Dyskinesia was present in 37.8% (180/476) of patients. The most common stages of the modified Hoehn & Yahr scale at off-state and on-state were 4 (39.7%, 189/476) and 3 (34.5%, 164/476), respectively. The mean dose of L-DOPA taken by patients at baseline was 418.9 ± 185.0 mg/day (n = 451) and the mean levodopa-equivalent dose [Citation26] was 691.8 ± 371.6 mg/day (n = 461).
Table 1. Baseline patient characteristics.
3.3. Treatment characteristics
At the end of the observation period, most patients were treated with 20 mg istradefylline (81.5%, 388/476). The mean (± SD) dose of istradefylline throughout the observation period was 22.6 ± 5.8 mg/day, and the mean (± SD) period of istradefylline treatment was 283.8 ± 130.7 days.
The most common concomitant medications administered during the observation period were L-DOPA (96.6%, 460/476), dopamine agonists (73.3%, 349/476), entacapone (34.0%, 162/476), zonisamide (29.0%, 138/476), and selegiline (28.4%, 135/476). A less significant modification of L-DOPA dosage was observed during istradefylline administration, where the change in L-DOPA dosage from baseline was 1.3 ± 61.8 mg/day (mean ± SD).
A total of 155 patients (32.6%) discontinued treatment. The main reason for discontinuation was AEs (13.7%, 65/476), followed by lack of efficacy (9.5%, 45/476), hospital transfer (6.3%, 30/476), spontaneous progression of disease (2.1%, 10/476), and death (1.7%, 8/476). Some subjects discontinued treatment for multiple reasons.
3.4. Safety
3.4.1. ADRs and SADRs
ADRs occurred in 20.8% (99/476) of patients (). The most common ADRs were dyskinesia (5.0%, 24/476), hallucination (3.4%, 16/476), visual hallucination (1.3%, 6/476), and somnolence (1.1%, 5/476). Dyskinesia worsened in 10.6% (19/180) of patients who were already dyskinetic at baseline. The details of all ADRs are shown in Supplementary Table 1. ADRs that led to discontinuation of the study included delusion (n = 8), dyskinesia (n = 7), and somnolence (n = 4); all of which are not unexpected ADRs. Supplementary Table 2 shows SADRs that were considered possibly related to istradefylline treatment, among all SAEs (9.0%, 43/476). The most common SADRs were delusion (0.6%, 3/476) and hallucination (0.4%, 2/476).
Table 2. Adverse drug reactions.
3.4.2. Subgroup analysis of ADRs and patient background
The results of the analysis on the association between ADRs and patient background are shown in . The occurrence of ADRs tended to be associated with a long duration of PD symptoms, long period of L-DOPA treatment, and high levodopa-equivalent dose (p < 0.05). Furthermore, patients with dyskinesia at baseline and with a long duration of motor complications were associated with a high incidence of ADRs (p < 0.05).
Table 3. Adverse drug reactions by patient characteristics.
3.5. Effectiveness
3.5.1. Physician’s assessment of off-time, off-time symptoms, and motor dysfunction
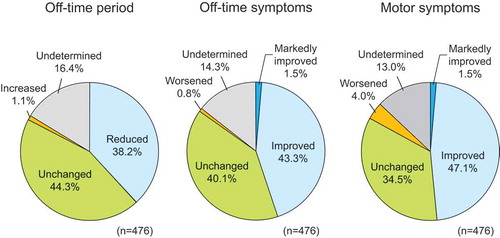
The results of the physician’s assessment of off-time, off-time symptoms, and motor dysfunction are shown in . Off-time was reduced in 38.2% (182/476) of patients. Off-time symptoms and motor dysfunction were ‘markedly improved’ and ‘improved’ in 44.7% (213/476) and 48.5% (231/476) of patients, respectively.
Figure 1. Effect of istradefylline on wearing-off phenomenon and motor dysfunction (physician’s assessment).
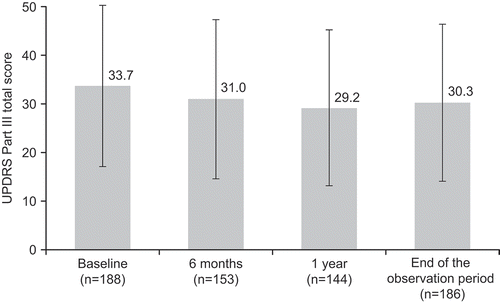
The UPDRS Part III total score was used for patients who had a complete evaluation of all sub-items of the subscale at the observation point. The mean (± SD) value of the UPDRS Part III total score was 33.7 ± 16.6 at baseline (n = 188), 31.0 ± 16.4 after 6 months of treatment (n = 153), 29.2 ± 16.0 after 1 year (n = 144), and 30.3 ± 16.1 at the end of the observation period (n = 186) (). Regarding the UPDRS Part III score pairwise tests, all assessment times were significantly reduced from baseline (p < 0.001, n = 153: baseline vs 6 months; p < 0.001, n = 144: baseline vs 1 year; p < 0.001, n = 186: baseline vs end of study observation). Additionally, all sub-items of the UPDRS Part III score improved.
Figure 2. Effect of istradefylline on unified Parkinson’s disease rating scale (UPDRS) Part III total score.
Results are presented as the mean, and the error bars indicate standard deviation. For the UPDRS Part III scores pairwise test, all assessment times were significantly reduced from baseline (p < 0.001, n = 153: baseline vs 6 months; p < 0.001, n = 144: baseline vs 1 year; p < 0.001, n = 186: baseline vs end of study observation).
3.5.2. Physician’s global assessment
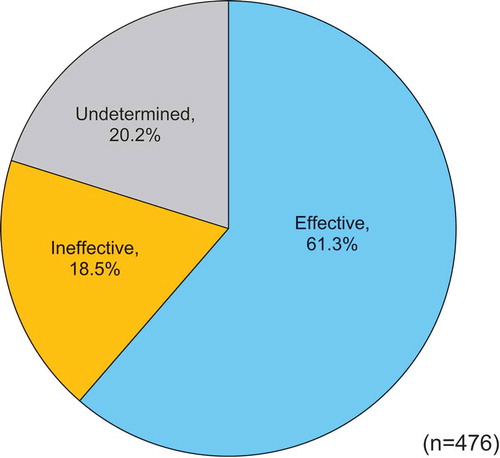
The physician’s global assessment showed that the treatment for a significant number of patients was rated as ‘effective’ (61.3%, 292/476) compared with ‘ineffective’ (18.5%, 88/476) and ‘undetermined’ (20.2%, 96/476) ().
3.5.3. Subgroup analysis of physician’s global assessment
shows the association between the physician’s global assessment results and patient background. Patients in whom istradefylline was rated as effective tended to have a < 10-year duration of PD, shorter period of occurrence of motor complications, or lower modified Hoehn & Yahr stage (on-state) (p < 0.05).
Table 4. Physician’s global assessment by patient characteristics.
3.5.4. Psychiatric disorders (priority survey item)
Given that the number of patients in the present interim analysis did not reach 1000, which is the target sample size needed to analyze psychiatric disorders, no analysis was performed for psychiatric disorders. Therefore, this will be performed in the final report when sufficient numbers of patients have been accumulated.
4. Discussion
Here, we report on the findings from a survey in real-world clinical practice to evaluate the safety and effectiveness of the long-term use of the adenosine A2A receptor antagonist istradefylline in PD patients with the wearing-off phenomenon. The safety analysis indicated that istradefylline was well tolerated, despite dyskinesia, hallucination, visual hallucination, and somnolence being the most common ADRs. The overall incidence of ADRs was lower than in a previous phase III, open-label, 52-week, long-term study (LTS) (20.8% vs 57.0%), although in this PMS, istradefylline was used in patients with a higher mean age (70.7 years vs 65.5 years) and longer duration of illness (8.8 years vs 7.7 years) compared with the previous LTS [Citation23]. This finding might be explained by the physicians’ greater flexibility in administering drugs in a real-world setting, where they are likely to optimize the dose and treatment period as needed. Conversely, there are more restrictions on istradefylline and concomitant medications under clinical trial settings such as the LTS. For example, the dosage and regimen of concomitant anti-parkinsonian drugs were maintained at a constant level for as long as possible until week 8, and changes in istradefylline dose were not permitted for the remainder of the study period from weeks 8 to 52.
The most common ADR was dyskinesia (5.0%), although the dosage of L-DOPA was not significantly changed during this study. During the clinical development of istradefylline, dyskinesia was reported to be the most frequent drug-related treatment emergent AE [Citation23–Citation25]. Therefore, results of this study indicate a similar tendency to individual clinical studies. Furthermore, we carried out a sub-analysis to stratify patients who had or had not experienced dyskinesia at baseline. The analysis showed a higher frequency of dyskinesia in patients with pre-existing dyskinesia rather than in patients without dyskinesia (10.6% vs 1.7%). It is likely that istradefylline treatment (adjuvant to L-DOPA) in PD patients does not newly induce dyskinesia, although it might mildly enhance the occurrence of pre-existing dyskinesia.
No new significant safety signals were detected and the risk/benefit balance of istradefylline was considered favorable compared with its status at launch, suggesting that istradefylline is a generally well-tolerated treatment option for PD patients experiencing the wearing-off phenomenon.
Effectiveness as rated by the physician’s global assessment in this study can be considered to be a summation of the three categories of the Clinical Global Impressions-Global Improvement Scale (treatment is deemed effective if the patient’s condition is classified as ‘very much improved’, ‘much improved’, or ‘minimally improved’). The effectiveness of istradefylline in this survey (61.3%) was close to that of the previous LTS (60.5%) [Citation23]. However, in our study, we observed a discrepancy between the physician’s assessment of motor dysfunction (48.5% of patients were assessed as improved/markedly improved) and the physician’s global assessment (61.3% assessed as effective), which suggests that the physician’s global assessment may also capture improvements in non-motor symptoms in patients. In addition, several clinical research and animal model studies reported that A2A antagonists were expected to improve some non-motor symptoms [Citation27–Citation30].
The proportion of patients with reduced off-time was 38.2%, as evaluated by the physician’s global assessment (rather than a 24-h patient diary). Improvements in off-time symptoms and motor dysfunction were observed for approximately half of the patients.
In the present study, the UPDRS Part III total score was only analyzed in patients who completed a full assessment of the sub-items of UPDRS Part III; approximately 60% of patients were not analyzed because of a lack of complete assessment of UPDRS Part III. With this limitation, available patients with complete assessment showed an improvement in UPDRS Part III score. The mean score change was −3.4 (baseline−end point: 33.7−30.3) (), which was comparable to the result in the LTS where the mean score change was −4.1 (18.1−14.0) [Citation23]. However, there were obvious differences in the baseline score of UPDRS Part III between our study and the previous LTS. The differences in baseline score may be attributed to different enrollment criteria related to patient severity. For example, the LTS reported by Kondo et al. [Citation23] enrolled patients rated at 2 to 4 by the modified Hoehn & Yahr stage (off-state), whereas the present study included patients up to stage 5, and the ratio of stage 5 patients was 18.1% of total patients (). Furthermore, the LTS excluded patients who had undergone surgery such as deep brain stimulation, whereas in the present study 6.5% of total patients underwent deep brain stimulation. More advanced PD patients were enrolled in this study compared with the LTS, which might reflect differences in the duration of PD. Moreover, concomitant dopamine agonists were used less often in the current study (73.3%) compared with the LTS (91.1%).
More flexible adjustments of various medications occurred in the present study compared with the LTS, because patients’ clinical conditions in a real-world setting are different from those in clinical trials. These factors might have contributed to the higher baseline UPDRS Part III score in the current study.
L-DOPA dosage was not significantly changed during the observation period of this study. Although we cannot conclude that istradefylline treatment had an influence on L-DOPA therapy because of the study limitations (e.g. non-randomized clinical trial), the overall safety and efficacy in this study are indicated by the fact that less modification of L-DOPA therapy was conducted during the study. These findings indicate that long-term treatment with istradefylline may have a sustained therapeutic effect in treating the wearing-off phenomenon and motor dysfunction of PD without needing to change the dosage of L-DOPA.
Recently, Sako et al. conducted a meta-analysis for the efficacy and safety of istradefylline treatment from randomized placebo-controlled clinical studies of advanced PD patients [Citation31]. The present study provided similar results to the meta-analysis by Sako et al. regarding the efficacy and safety of istradefylline, in which the drug reduced off-time and improved motor symptoms evaluated by UPDRS Part III score, and increased the risk of dyskinesia.
The overall findings of the present interim analysis are comparable with previous pre-approval clinical trials in Japan, as well as the aforementioned LTS [Citation23]. However, the current interim analysis included only 35% of all registered patients of the survey, who were enrolled within the first 2-year period of the entire 6-year survey (which is ongoing). The final analysis with all registered patients will be conducted after completion of the survey to provide overall conclusions.
5. Conclusions
The interim results of the current PMS demonstrate the safety and effectiveness of istradefylline in PD patients with the wearing-off phenomenon treated with L-DOPA. Istradefylline was well tolerated with acceptable effectiveness demonstrated in the real-world clinical setting.
Declaration of interest
M Takahashi has received honoraria for manuscript writing from Kyowa Hakko Kirin Co., Ltd. M Fujita, N Asai, M Saki, and A Mori are all employees of Kyowa Hakko Kirin Co., Ltd., who funded this study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Writing assistance utilized in the production of this manuscript was funded by Kyowa Hakko Kirin Co., Ltd.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
M Takahashi contributed to data analysis, data interpretation, and drafting and editing of the manuscript. M Fujita and N Asai contributed to study design, study conduct, data collection, data analysis, data interpretation, and drafting of the manuscript and revising it critically for intellectual content. M Saki and A Mori contributed to data interpretation, drafting of the manuscript, and critical revision for intellectual content. All authors approved the final version and agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (48.5 KB)Acknowledgments
Writing and editorial support was provided by J.L. Croxford, PhD, and Rhonna Gurevich, PhD, of Edanz Medical Writing. The authors would like to thank the study investigators and participating patients in this surveillance study. This trial was registered in the UMIN clinical trials registry under the identifier: UMIN000030014.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Dorsey ER, Bloem BR. The Parkinson pandemic—A call to action. JAMA Neurol. 2018;75(1):9–10.
- Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann NY Acad Sci. 2003;991:1–14.
- Barzilai A, Melamed E. Molecular mechanisms of selective dopaminergic neuronal death in Parkinson’s disease. Trends Mol Med. 2003;9(3):126–132.
- Langston J. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–596.
- Blonder LX, Slevin JT. Emotional dysfunction in Parkinson’s disease. Behav Neurol. 2011;24:201–217.
- Cacabelos RP. Parkinson’s disease: from pathogenesis to pharmacogenomics. Int J Mol Sci. 2017;18:551–579.
- Connolly BS, Lang AE. Pharmacological treatment of Parkinson’s disease: a review. JAMA. 2014;16:1670–1683.
- Saki M, Yamada K, Koshimura E, et al. In vitro pharmacological profile of the A2A receptor antagonist istradefylline. Naunyn-Schmiedeberg’s Arch Pharmacol. 2013;386(11):963–972.
- Rosin DL, Robeva A, Woodard RL, et al. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol. 1998;401:163–186.
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271.
- Mori A, Shindou T. Modulation of GABAergic transmission in the striatopallidal system by adenosine A2A receptors: a potential mechanism for the antiparkinsonian effects of A2A antagonists. Neurology. 2003;61(11 Suppl 6):S44–48.
- Mori A. Mode of action of adenosine A2A receptor antagonists as symptomatic treatment for Parkinson’s disease. Int Rev Neurobiol. 2014;119:87–116.
- Fredholm BB, Chen JF, Cunha RA, et al. Adenosine and brain function. In: Bradley RJ, Harris RA, Jenner P, editors. International review of neurobiology. London: Elsevier; 2005. p. 191–270.
- Reyhani-Rad S, Mahmoudi J. Effect of adenosine A2A receptor antagonists on motor disorders induced by 6-hydroxydopamine in rat. Acta Cir Bras. 2016;31:133–137.
- Ferré S, Rubio A, Fuxe K. Stimulation of adenosine A2 receptors induces catalepsy. Neurosci Lett. 1991;130:162–164.
- Kanda T, Tashiro T, Kuwana Y, et al. Adenosine A2A receptors modify motor function in MPTP-treated common marmosets. Neuroreport. 1998;24:2857–2860.
- Shiozaki S, Ichikawa S, Nakamura J, et al. Actions of adenosine A2A receptor antagonist KW-6002 on drug-induced catalepsy and hypokinesia caused by reserpine or MPTP. Psychopharmacology. 1999;147:90–95.
- Koga K, Kurokawa M, Ochi M, et al. Adenosine A2A receptor antagonists KF17837 and KW-6002 potentiate rotation induced by dopaminergic drugs in hemi-parkinsonian rats. Eur J Pharmacol. 2000;408:249–255.
- Kanda T, Jackson MJ, Smith LA, et al. Adenosine A2A antagonist: a novel antiparkinsonian agent that does not provoke dyskinesia in parkinsonian monkeys. Ann Neurol. 1998;43:507–513.
- Grondin R, Bedard PJ, Hadj Tahar A, et al. Antiparkinsonian effect of a new selective adenosine A2A receptor antagonist in MPTP-treated monkeys. Neurology. 1999;52:1673–1677.
- Jenner P, Mori A, Hauser R, et al. Adenosine, adenosine A2A antagonists, and Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:406–413.
- Schwarzschild MA, Agnati L, Fuxe K, et al. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29:647–654.
- Kondo T, Mizuno Y. Japanese istradefylline study group. A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin Neuropharmacol. 2015;38:41–46.
- Mizuno Y, Kondo T. Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Mov Disord. 2013;28:1138–1141.
- Mizuno Y, Hasegawa K, Kondo T, et al. Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease: a randomized, controlled study. Mov Disord. 2010;25:1437–1443.
- Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–2653.
- Yamada K, Kobayashi M, Shiozaki S, et al. Antidepressant activity of the adenosine A2A receptor antagonist, istradefylline (KW-6002) on learned helplessness in rats. Psychopharmacology (Berl). 2014;231(14):2839–2849.
- Kadowaki Horita T, Kobayashi M, Mori A, et al. Effects of the adenosine A2A antagonist istradefylline on cognitive performance in rats with 6-OHDA lesion in prefrontal cortex. Psychopharmacology. 2013;230(3):345–352.
- Matsuura K, Kajikawa H, Tabei K, et al. The effectiveness of istradefylline for the treatment of gait deficits and sleepiness in patients with Parkinson’s disease. Neurosci Lett. 2018;662:158–161.
- Suzuki K, Miyamoto M, Miyamoto T, et al. Istradefylline improves daytime sleepiness in patients with Parkinson’s disease: an open-label, 3-month study. J Neurol Sci. 2017;380:230–233.
- Sako W, Murakami N, Motohama K, et al. The effect of istradefylline for Parkinson’s disease: a meta-analysis. Sci Rep. 2017;7(1):18018.