KEYWORDS:
1. Introduction
In a recent issue of Expert Opinion on Pharmacotherapy, Maraolo et al. presented an outstanding review on the pharmacological treatment of bacterial peritonitis [Citation1]. The authors focused on spontaneous bacterial peritonitis (SBP), a form of bacterial peritonitis occurring almost exclusively in patients with liver cirrhosis. They highlight that selection of the appropriate empiric antibiotic drug has become a challenge. In 2010, the guidelines of the European Association for the Study of the Liver (EASL) stated that treatment with third-generation cephalosporines led to resolution of SBP in 77–98% of patients [Citation2]. Nowadays, resistance to third-generation cephalosporines is reported in up to 58% [Citation3], with no single antibiotic drug to replace third-generation cephalosporines with the same efficacy known from the past. The decreasing efficacy of third-generation cephalosporins is not only due to the general rise of multiresistant bacteria, but also caused by a shift toward infections with Gram-positive bacteria in SBP [Citation4]. Both developments correspond to the widespread use of quinolones in the primary and secondary prophylaxis of SBP [Citation2].
In the light of these developments, how does one decide on empirical treatment of bacterial peritonitis in clinical day to day work?
The first crucial step is to distinguish with which form of bacterial peritonitis the patient presents: SBP occurs in general only in patients suffering from advanced liver disease. In patients without liver cirrhosis, secondary bacterial peritonitis due to intestinal perforation or intra-abdominal abscess formation is much more frequent than SBP and requires surgical or interventional treatment in conjunction with antibiotic drug therapy. In case of polymicrobial peritonitis or clinical signs of intestinal perforation, a CT scan should be performed also in patients with liver cirrhosis and suspected SBP to rule out secondary peritonitis [Citation2]. Peritonitis in patients on continuous ambulatory peritoneal dialysis is characterized by a different pattern of causative bacteria, mostly Gram-positive bacteria [Citation5]. Rare forms of bacterial peritonitis are tuberculous or chlamydial peritonitis.
SBP is confirmed in patients with liver cirrhosis if the neutrophil count in the ascites exceeds 250 cells/µL [Citation2]. Rapid start of an effective antibiotic treatment improves survival [Citation6,Citation7]. However, selection of the appropriate empiric antibiotic drug is complicated by the rise of antimicrobial resistance and the variety of bacteria possibly causing SBP, with Escherichia coli, the most frequent pathogen, being identified in only 33% of cases [Citation8]. Detection of bacteria relies on conventional culture of ascites transferred at the bedside in blood culture bottles and takes at least 2 days. However, in a significant proportion of patients, ascites culture will remain negative [Citation9], most probably because of low bacterial loads in the ascites. Thus, we face uncertainty on which antibiotic to choose while knowing that failing to select the right drug may worsen the prognosis of the patient [Citation10]. How can we overcome this situation?
We may choose a combination of broad spectrum antibiotics which will most likely cover all possibilities. Piano and coauthors reported that the combination of meropenem plus daptomycin led to resolution of SBP in 13/15 (87%) versus 4/16 (25%) patients treated with ceftazidime in nosocomial SBP (p < 0.001) [Citation11]. Because frequent use of carbapenems has been linked to a rise in carbapenem-resistant infections, a significant downside of such an approach is the induction of colonization and infection with carbapenem-resistant Enterobacteriaceae [Citation12]. Given the limited number of new antibiotic drugs in development, the widespread use of carbapenems carries the risk of inducing resistance which will prevent effective treatment in the future. In addition, the combination therapy did not lead to lower in-hospital mortality or higher 30-day or 90-day transplant free survival [Citation11]. Moreover, the optimal dosage of daptomycin to treat enterococci, a frequent cause of SBP, is still debated [Citation13]. Thus, a broad combination therapy might be helpful for the resolution of infection, but the impact on mortality in SBP is not clear and we risk future treatment failure. This is particularly important because SBP is an infection with a high rate of recurrence [Citation2].
To limit the use of broad spectrum and last resort antibiotics, we need parameters to identify patients at risk for resistance to common antibiotics. First, substantial regional differences in the prevalence of antibiotic resistance should be considered. In nosocomial SBP, for example, resistance to third-generation cephalosporines was detected in 83% of patients from Italy [Citation11] compared to 30% of patients from Germany [Citation10]. Therefore, knowing the local resistance pattern is essential [Citation14]. However, it is a challenge to acquire this knowledge for different forms of infection and to keep it up to date. In addition, a general analysis of detected microorganisms from peritoneal fluid may not be indicative for pathogens relevant for SBP, because other forms of bacterial peritonitis present with different microbial patterns. Furthermore, bacteria identified in the ascites without elevation of the neutrophil count in patients with liver cirrhosis, the so-called bacterascites, do not require treatment in a significant proportion of cases [Citation14].
Second, risk factors for infection with multiresistant bacteria concerning the individual patient need to be assessed. In a Spanish study, previous infection with a multiresistant microorganism, nosocomial acquisition of infection, intake of quinolone prophylaxis, and previous treatment with beta-lactams were revealed as predictors [Citation15]. Patients with sepsis are deemed to be particularly at risk for increased mortality if the empiric antibiotic treatment fails, so that these patients are considered candidates for antibiotic broad spectrum combination therapy [Citation14]. After empiric treatment has been started, it is essential to observe the treatment response of the patient and to keep close contact with the microbiology laboratory. It has been proposed that a drop of less than 25% in the ascites neutrophil count after 48 h indicates treatment failure [Citation14]. The results of microbial culture concerning species identification and antibiotic resistance should be communicated quickly to the treating clinicians to facilitate prompt adjustment of antibiotic treatment.
To handle therapeutic uncertainty better, research on several issues is required. It will be crucial to better define a reliable set of parameters to predict risk for antimicrobial resistance. However, the set of parameters should be simple, so that application in clinical practice is not hampered by complexity. It has been proposed that defining healthcare-associated SBP as a third category next to community-acquired and nosocomial SBP might help to stratify patients for empiric antibiotic treatment [Citation14]. However, the validity of this approach for SBP has not yet been demonstrated unequivocally. In addition, variations in the definition of multiresistance might explain diverging findings from different studies and limit translation into clinical practice. Finally, a more rapid identification of causative pathogens and their antimicrobial resistance will be essential to guide antibiotic therapy and restrict treatment with broad spectrum antibiotics. PCR-based methods have been investigated intensively and constitute a promising approach. However, the clinical benefit of molecular methods has not been shown so far.
2. Expert opinion
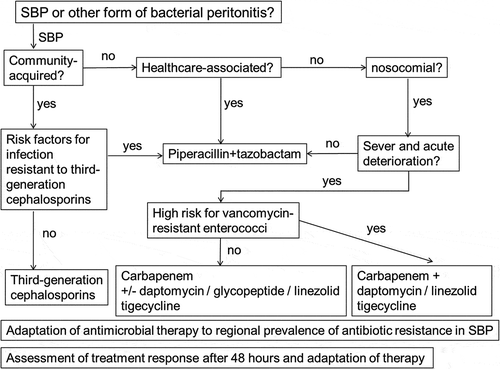
Proper diagnosis of the form of bacterial peritonitis is the first step to choose the best empiric therapy. Both ascites and blood should be inoculated into blood culture bottles at the bedside before the start of antibiotic treatment. Identification of the causative pathogen by microbiological analysis facilitates targeted therapy. Knowing local epidemiology of antibiotic resistance is very helpful. When presence of SBP is established by an ascites neutrophil count ≥250 cells/µL in the absence of a contiguous source of intraabdominal infection in patients with advanced liver disease, we recommend to follow the recent guidelines by EASL [Citation14]: third-generation cephalosporins or piperacillin/tazobactam for community-acquired SBP. In healthcare-associated SBP and non-severe nosocomial SBP, we use piperacillin/tazobactam, unless bacteria resistant to this drug have caused a previous episode of SBP in the affected patient. In contrast, in patients with acute and severe deterioration, a combination of a carbapenem plus vancomycin/linezolid/daptomycin is indicated. In addition to antibiotic treatment, administration of intravenous albumin is recommended for patients with SBP [Citation14]. Monitoring blood and ascites neutrophil counts and rapid communication of microbiological results will permit to switch antibiotic treatment, if indicated, without delay ().
Figure 1. Flowchart for suggested empirical antibiotic therapy in patients with suspicion of spontaneous bacterial peritonitis (SBP). In patients with previous culture-positive SBP therapy should be chosen to cover previously detected bacteria.
The ultimate goal of future research is to identify simple treatment algorithms for targeted therapy of SBP that reduce mortality of affected patients. More clinical interventional studies are needed for this purpose. However, regional differences in antimicrobial resistance patterns limit the generalizability of single studies. In addition, mortality of patients with SBP is not only determined by the infection itself, but also by the severity of underlying liver insufficiency. Therefore, the most promising approach for day-to-day clinical work is to establish rapid molecular tests for the identification of bacterial species and antimicrobial resistance. This is an outstanding challenge due to the broad spectrum of bacteria causing SBP; therefore, conventional culture will be needed also in the future to detect bacteria and antimicrobial resistance which are not covered by the molecular panels. While the goal of new molecular approaches was in the past to identify bacteria causing culture-negative SBP, a more rapid diagnosis of pathogens and resistance should be the primary aim now. Finally, preventing SBP is the best option to reduce mortality. Traditional approaches using antibiotics are hampered by the induction of antimicrobial resistance. New approaches should aim at decreasing intestinal bacterial translocation and modulating the immune response of patients with liver cirrhosis favorably.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Maraolo AE , Gentile I , Pinchera B , et al. Current and emerging pharmacotherapy for the treatment of bacterial peritonitis. Expert Opin Pharmacother. 2018;19:1317–1325.
- European Association for the Study of the Liver . EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417.
- Fiore M , Gentile I , Maraolo AE , et al. Are third-generation cephalosporins still the empirical antibiotic treatment of community-acquired spontaneous bacterial peritonitis? A systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:329–336.
- Fiore M , Maraolo AE , Gentile I , et al. Current concepts and future strategies in the antimicrobial therapy of emerging Gram-positive spontaneous bacterial peritonitis. World J Hepatol. 2017;9:1166–1175.
- Akoh JA. Peritoneal dialysis associated infections: an update on diagnosis and management. World J Nephrol. 2012;1:106.
- Kim JJ , Tsukamoto MM , Mathur AK , et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109:1436–1442.
- Karvellas CJ , Abraldes JG , Arabi YM , et al. Appropriate and timely antimicrobial therapy in cirrhotic patients with spontaneous bacterial peritonitis-associated septic shock: a retrospective cohort study. Aliment Pharmacol Ther. 2015;41:747–757.
- Dever JB , Sheikh MY. Review article: spontaneous bacterial peritonitis–bacteriology, diagnosis, treatment, risk factors and prevention. Aliment Pharmacol Ther. 2015;41:1116–1131.
- Wiest R , Krag A , Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut. 2012;61:297–310.
- Lutz P , Nischalke HD , Krämer B , et al. Antibiotic resistance in healthcare-related and nosocomial spontaneous bacterial peritonitis. Eur J Clin Invest. 2017;47:44–52.
- Piano S , Fasolato S , Salinas F , et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: results of a randomized, controlled clinical trial. Hepatol Baltim Md. 2016;63:1299–1309.
- McLaughlin M , Advincula MR , Malczynski M , et al. Correlations of antibiotic use and carbapenem resistance in Enterobacteriaceae. Antimicrob Agents Chemother. 2013;57:5131–5133.
- Chuang Y-C , Lin H-Y , Chen P-Y , et al. Daptomycin versus linezolid for the treatment of vancomycin-resistant enterococcal bacteraemia: implications of daptomycin dose. Clin Microbiol Infect. 2016;22:890.e1–890.e7.
- Angeli P , Bernardi M , Villanueva C , et al. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. [Internet]. 2018 [cited 2018 Jul 16 ]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168827818319664.
- Fernández J , Acevedo J , Castro M , et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatol Baltim Md. 2012;55:1551–1561.
