1. Introduction
Tobacco smoking remains a major cause of preventable death and disability. Quitting smoking is associated with considerable improvements in mental and physical health and life expectancy. All current smokers should be encouraged to quit smoking and offered therapeutic options for smoking cessation. Current pharmacotherapies have limited effectiveness, with 75% of smokers relapsing within 12 months of treatment and only 4% to 7% successfully quitting permanently after a quit attempt [Citation1]. Reasons for relapse or failure to quit are highly individualized. There is an urgent need for better therapies for smoking cessation, either through more effective pharmacotherapies for population based approaches and/or through the development of treatments that are individualized and tailored to the specific needs and preferences of the individual smoker. Further research is required to evaluate novel pharmacotherapies and individualised approaches that may have greater efficacy or advantages in special populations.
Historically, major inroads in the reduction of tobacco use have been achieved through population-based approaches, including; public health messages, regulation of the tobacco industry and tobacco products, restrictions on advertising, tobacco sales, and smoking in public places, and the provision of services to assist smokers to quit. Pharmacotherapies have conformed to this same model, developing treatment strategies that can be used as broadly as possible. Efficacy studies of smoking cessation pharmacotherapies have generally focused on smokers in the general community, although the focus has shifted to high prevalence smokers such as those with psychiatric comorbidities [Citation2]. More recently, individualized therapies have been attracting attention, and both individualized and population-based strategies are likely to continue to play important roles in the future.
Therapies can also be refined to integrate them into windows of opportunity for specific individuals. Opportunities for intervention may arise during periods of medical treatment or hospitalization or during reproductive events such as pregnancy. Advice from a doctor to quit smoking has been shown to result in higher rates of quitting, as is the motivational impact of a potentially smoking-related disorder [Citation3]. Many hospitalized patients are not able to smoke due to restrictions on smoking in the hospital setting. A study of psychiatric in-patients in a smoke-free hospital setting demonstrated the importance of providing nicotine replacement therapy (NRT) to avoid withdrawal and discharge against medical advice [Citation4]. Abstinence from smoking while in hospital, as well as increased motivation to quit provides an ideal opportunity for intervention and there is evidence that a smoking cessation intervention after hospital discharge is associated with higher rates of sustained abstinence [Citation5]. Further research is required to establish if higher quit rates can be achieved through interventions managed by the same clinicians treating the physical illness, or in the same health-care setting result in higher quit rates compared to referring patients to smoking cessation treatments delivered elsewhere. Pregnancy is also a window of opportunity for intervention as smokers may be more motivated to quit if they are pregnant or considering pregnancy and a critical period due to concerns about the safety of pharmacotherapeutic interventions.
2. Pharmacotherapeutic options for treating nicotine dependence
Only three pharmacotherapeutic options are currently approved by major regulatory authorities, such as the U.S. Food and Drug administration and the European Medicines Agency as first-line treatments. These are NRT, which comes in a range of products, varenicline, and bupropion. Nortriptyline and clonidine are recognized as second-line treatments. In addition, more than 40 pharmacotherapies have been investigated for smoking cessation, with varying levels of evidence. Some of these therapies appear promising and merit further investigation.
NRT as an effective smoking cessation aide, whether formulated as gum, transdermal patch, nasal spray, inhaler or sublingual tablets/lozenges, is evidenced by over 150 randomized clinical trials totaling over 50,000 trial participants. NRTs increase the chances of successfully stopping smoking for a quit attempt by 50% to 70% compared to placebo. Highly dependent smokers showed greater benefit from 4 mg gum than from 2 mg gum, but not from higher doses of patch. Combining a patch with rapid delivery NRT was more effective than a single type of NRT. Treatment with NRT was shown to be as effective as bupropion, and combination NRT with bupropion was more effective than bupropion monotherapy [Citation6]. Meta-analyses suggest that varenicline and combined forms of NRT are associated with higher quit rates than bupropion or single-form NRT [Citation7]. Varenicline was associated with greater continuous abstinence from smoking during weeks 15 to 24 compared to placebo (37.8% for varenicline; 6.9% for placebo) in an RCT of smokers willing to reduce cigarette consumption and make a quit attempt within 3 months [Citation8].
Electronic nicotine delivery systems (ENDS) have become widely available and have rapidly increased in popularity. Users reports suggest that they are used for smoking cessation or reduction, harm minimisation, or recreational use [Citation9]. There is evidence to demonstrate the efficacy of ENDS for cessation of tobacco smoking [Citation10] suggesting that it may have a role as a harm minimization strategy, possibly for heavy smokers who are not contemplating quitting. There are concern and limited evidence that ENDS use may be a gateway to tobacco use [Citation11]. It is not possible to assess if there will be any future therapeutic role for ENDS due to a paucity of long-term data and an unclear regulatory framework.
In addition to NRT, varenicline, and bupropion, several conventional pharmaceuticals with indications for other conditions have been investigated as smoking cessation aides. The tricyclic antidepressant nortriptyline and the antihypertensive agent clonidine have both demonstrated superiority to placebo for smoking cessation randomized controlled trials. Their use in treatment can be considered depending on patient preference, consideration of the risks of adverse events, and comorbid medical conditions [Citation12]. Trials of Selective Serotonin Reuptake Inhibitors for smoking cessation have failed to demonstrate efficacy, suggesting that the mechanisms of action for nortriptyline and bupropion are not through their antidepressant activity [Citation1].
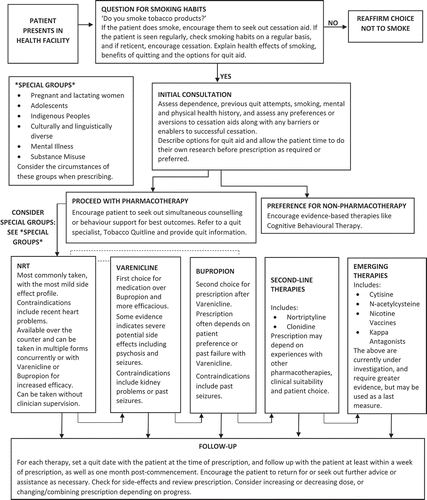
Emerging data suggests that some novel therapies merit further investigation. These include; a pilot study of N-acetylcysteine that showed superiority to placebo for quit rates and reduced number of cigarettes smoked [Citation13], and cytisine in numerous small studies, meta-analyses, and a non-inferiority trials versus NRT all suggesting efficacy [Citation1]. Numerous other agents, including other conventional pharmaceuticals and alternative or natural therapies, have been investigated but were found not to be effective, or had unacceptable risks, or do not have sufficient evidence to suggest usefulness [Citation1]. Other agents are still being trialed. There is some data from rodent studies of kappa-opioid receptor antagonists [Citation14]. Non-pharmacological interventions can also be effective, with offering financial incentive demonstrating great efficacy for smoking cessation than pharmacotherapies [Citation15]. Adjunctive psychological approaches, including telephone, internet or clinician support, are beneficial for people attempting to quit. Clinical trials of nicotine vaccines have so far failed to demonstrate efficacy for smoking cessation; however, further vaccine development is continuing [Citation16]. A decision tree for smoking cessation treatment allocation is provided as .
3. Targeted and individualized therapies
Individualized therapies are tailored to the characteristics and preferences of the individual smoker. This can be characteristics of smoking behavior, motivation to quit, or characteristics of special populations. There is some data to suggest that heavy or highly dependent smokers can benefit from higher dose of NRT [Citation6] or more intensive interventions [Citation17] as suggested by 3-week inpatient intervention for heavy smokers. Nicotine metabolism rate varies between individuals is influenced by genomic and hormonal factors and may result in variation in smoking behavior and response to smoking cessation therapy. In general, rapid metabolizers of nicotine are more dependent and have poorer outcomes for cessation attempts compared to slow metabolizers [Citation18]. A trial of varenicline in heavy smokers with mild-to-moderate chronic obstructive pulmonary disease found that extending the treatment period for >12 weeks was beneficial [Citation19].
Mental illness is associated with high rates of smoking and these rates have remained high while smoking rates in the rest of the population have fallen, suggesting a need for target intervention strategies in this group. Smoking is a major cause of premature death for people with mental illness [Citation20]. What is less clearly appreciated by patients and clinicians is that smoking cessation brings benefit in terms of improved mental health, a message that has a clear motivational capacity in this group [Citation21]. There are no clearly divergent recommendations regarding optimal strategies for smoking cessation for people with mental illness, with some evidence to suggest that conventional pharmacotherapies are effective. However, there are also reports of mood fluctuation, irritability, and anxiety associated with quit attempts, altered pharmacokinetics with smoking cessation and concerns regarding other common comorbidities including substance and alcohol dependence [Citation22]. Changes in CYP1A2 activity due to smoking cessation will change the pharmacokinetics of some psychotropic agents used in psychiatric treatment, such as clozapine. A planned treatment strategy negotiated with the patient may be useful. It may be necessary to change medication doses and monitor blood concentrations. Clearly, an individualized approach to smoking cessation is necessary within this population and mental health services and clinicians need to have clear strategies regarding how they will approach the issue of tobacco use with their patients. There is also a need to provide NRT in hospital settings and postdischarge support. There is a clear need for further research investigating the effectiveness and safety of smoking cessation therapies within this population.
Further special populations for consideration with smoking cessation include and broad range of socially excluded and socio-economically disadvantaged populations where smoking prevalence remains high. Examples are ethnic and indigenous minorities, low income, and homeless people, incarcerated people, people with health conditions, people living in rural and remote locations, people from the LGBTQIA community, and other groups. Arguably, women, young people, old people, and people currently or previously in the military can also be considered special populations. Special populations can benefit from individualized smoking cessation therapies for varied reasons, including; lower motivation to quit amongst some socially excluded populations where health literacy is poorer and public health messages may have had less penetrance, preferences for smoking cessation treatment in health settings that they attend for other reasons, pharmacological reasons due to co-medications or CYP450 phenotype. Many smokers may fit into more than one special population category.
In developed countries where tobacco smoking prevalence has declined, only a minority of smokers will not fit into a special population. Some individuals who do not identify with any additional factors contributing towards smoking may have a preference for conventional treatments, although there is evidence that over the counter NRT is less effective than prescription NRT [Citation23], suggesting that even these individuals would benefit from a more structured, individualized treatment. Nevertheless, a role for easily available therapies and population-based approaches to smoking cessation and tobacco control is likely to remain necessary and popular into the future due to personal preference, affordability, and availability, and ongoing need especially in countries where high rates of tobacco smoking persist.
Elements of the individualized therapies can include; treatments that are tailored for a special population, treatments delivered in a preferred health setting or amongst peers, and treatments that take into account patient characteristics and preferences. Some patients prefer to attempt to quit by themselves and others prefer to support, where the treatment alliance between the treating clinician and the patient becomes important and a treatment plan may be useful. Internet-based quit resources are valuable in this context. Planning and timing have been demonstrated to impact the effectiveness of a smoking cessation intervention, with planning ranging from an intention to quit, a quit strategy, and various forms of preparatory activities and anticipatory strategies [Citation24]. Similarly, clinician engagement and encouragement can drive motivation. Cross-cultural and multilingual access can also be an important feature of individualized treatment. Individualized approaches offer the ability to tailor pharmacological intervention, provide tailored additional support, and if desired to combine pharmacological and non-pharmacological approaches for smoking cessation. However, individualized approaches remain limited due to a paucity of high-quality smoking cessation effectiveness data for special populations.
4. Conclusion
Starting with the introduction of the nicotine patch in 1984, smoking cessation pharmacotherapy has made modest progress in the last three decades. NRT has been formulated in multiple ways and two further agents, varenicline and bupropion, have gained regulatory approval. Although most smokers have some degree of motivation to quit, most quit attempts end in relapse within 12 months of quitting. Reduction in tobacco prevalence is largely attributable to public health interventions and regulatory interventions rather than smoking cessation interventions. Efforts to prevent smoking onset in young people are important. More effective smoking cessation interventions are also required. Individualized tailored approaches are likely to become more prominent in the future because as tobacco prevalence declines the remaining smokers often have complex problems, new interventions that can better target individuals are being developed, new pharmacotherapeutic strategies are emerging that may have advantages in special populations, and personal preference will sometimes favor an individualized approach. However, further research is required to develop interventions that target special populations and assess their effectiveness and safety. Conventional, non-individualized pharmacotherapeutic interventions will retain an important role in countries with high tobacco prevalence and will continue to have a role elsewhere. Public health strategies and regulatory interventions are likely to continue to be strong drivers of the further reduction in tobacco prevalence.
5. Expert opinion
Since the introduction of nicotine patches in 1984, there have been new formulations of NRT and the emergence of two new agents, varenicline and bupropion, that have obtained regulatory approval. Treatment effectiveness has not measurably changed in this period. Although pharmacotherapeutic intervention is superior to placebo at treatment endpoint in clinical trials, relapse rates after successfully quitting smoking remain high. Contemplation of quitting and quit attempts are common amongst smokers, suggesting that difficulties in quitting and relapse are a greater problem than motivating smokers to attempt to quit. Reduction in tobacco prevalence is attributable mainly to public health strategies and regulatory interventions rather than smoking cessation treatment. At a population level, fewer young people are taking up smoking compared to previous generations and current smokers have a higher mortality rate compared to age-matched non-smokers, resulting in lower population prevalence of current smokers.
It is unclear to what extent tobacco prevalence can be further reduced through public health messages and regulatory interventions and these strategies may be self-limiting, requiring collaboration between public health, regulatory, and smoking cessation researchers to achieve a common goal of reducing tobacco prevalence. This is likely to require targeted interventions. Public health messages and regulatory interventions have been less effective in some socially excluded and socio-economically disadvantaged populations. High rates of smoking persist in populations including; the mentally ill, incarcerated people, and some minority groups. Individualized approaches are like to have a greater role in the foreseeable future. Individualized therapies may include currently approved pharmacotherapies and other pharmacotherapies currently being investigated. Individualized strategies are limited by a paucity of high-quality data investigating the effectiveness and safety of targeted interventions in special populations and further research is long overdue.
Conventional, non-individualized strategies are likely to persist into the future. They are low-cost, easily accessible options, and may be preferred by some individuals.
It is possible that novel agents have a role in future interventions. This may be as monotherapies where they may have safety advantages in special populations, including women during pregnancy and breastfeeding and for people with mental or physical comorbidities or as combination therapies where they may enhance treatment effectiveness. Agents such as N-acetylcysteine, cytisine, nicotine vaccines, and kappa antagonists may have a potential role.
Individualized strategies and novel therapies may result in a greater quit rate, but their impact on the relapse rate remains unclear. Further research is required to improve relapse prevention, which may include novel or conventional pharmacotherapies or combinations of pharmacological and non-pharmacological strategies. Our opinion is that a pluralized approach to smoking cessation, that encompasses individualized settings and personalized approaches, as well as conventional and novel therapies, as well as an emphasis on public health interventions, will all have a role in the future. Individualized settings and personalized approaches receive more attention in countries that have experienced significant falls in rates of tobacco use where smoking rates remain high in special populations only. The future direction of smoking cessation strategies remains largely determined by policymakers whose considerations include the impact of an intervention of the rates of tobacco use at a population level as well as benefits to individuals and special populations.
The role of harm minimization strategies, such as substituting tobacco cigarettes for ENDS, is controversial and its association with the tobacco industry leads to suspicion. However, harm minimization strategies are common for other addictive substances and further research is likely. It is our opinion that a harm minimization option may be beneficial for some smokers who do not intend to quit; however, the tobacco industry should not have a role in the provision of any option.
In coming years the emphasis on individualized strategies is likely to increase, especially as smoking prevalence decreases and remaining smokers are more likely to have complex issues to address. There will also be a greater focus on cessation interventions that include relapse prevention and clinical trials with long follow up period to investigate whether participants remain abstinent. New formulations of existing pharmacotherapies are likely to be developed. Novel therapies will continue to attract interest from researchers and some of these may transit into clinical practice.
No breakthrough pharmacotherapies leading to decisive changes in smoking cessation outcomes are on the horizon; however, some improvements in outcomes should be expected. Public health and regulatory interventions will continue to be the main drivers of further falls in tobacco prevalence.
Declaration of interest
S Dodd has received grant support from Stanley Medical Research Institute, the NHMRC, Beyond Blue, Australian Rotary Health (ARHRF), the Simons Foundation, the Geelong Medical Research Foundation, the Fondation FondaMental, the Harry Windsor Foundation, Eli Lilly and Company, GlaxoSmithKline, Organon, Mayne Pharma and Servier. He has also received speaker’s fees from Eli Lilly and Company and advisory board fees from Eli Lilly and Company and Novartis as well as conference travel support from Servier. M Berk has received grant support from the National Institutes of Health, the Simons Autism Foundation, the Cancer Council of Victoria, the CRC for Mental Health, the Stanley Medical Research Foundation, MBF, the NHMRC, Beyond Blue, the Geelong Medical Research Foundation, Bristol Myers Squibb, Eli Lilly, and Company GlaxoSmithKline, Organon, Novartis, Mayne Pharma and Servier. He has also been a speaker for AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Janssen Cilag, Lundbeck, Merck & Co, Pfizer, Sanofi, Servier, Solvay and Wyeth, and has served as a consultant to AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Janssen Cilag, Lundbeck and Servier and is a co-inventor on two provisional patents regarding the use of NAC and related compounds for psychiatric indications, assigned to the Mental Health Research Institute. OM Dean has received grant support from the Brain and Behavior Foundation, Simons Autism Foundation, Stanley Medical Research Institute, Deakin University, Eli Lilly and Company, the NHMRC and the Australasian Society for Bipolar and Depressive Disorders (ASBDD)/Servier. She has also received, in kind, support from BioMedica Nutracuticals, NutritionCare and Bioceuticals. N Gómez-Coronado has received grant support from the Fundación Española de Psiquiatría y Salud Mental. She has also received speaker´s fees from Janssen Cilag, Otsuka, Lundbeck, Pfizer Inc and research fees from Janssen Cilag and Adamed. She has also received travel grants from Janssen Cilag and Lundbeck. DI Lubman has received support from the Colonial Foundation, and has received travel support and speaker honoraria from AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, Janssen Pharmaceuticals, Organon and Pfizer Inc. He has also received investigator‐initiated research grants from Bristol-Myers Squibb and Pfizer Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Gomez-Coronado N, Walker AJ, Berk M, et al. Current and emerging pharmacotherapies for cessation of tobacco smoking. Pharmacotherapy. 2018 Feb;38(2):235–258.
- Cook BL, Wayne GF, Kafali EN, et al. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA. 2014 Jan 8;311(2):172–182.
- Stead LF, Buitrago D, Preciado N, et al. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013 May;31(5):CD000165.
- Prochaska JJ, Gill P, Hall SM. Treatment of tobacco use in an inpatient psychiatric setting. Psychiatr Serv. 2004 Nov;55(11):1265–1270.
- Rigotti NA, Regan S, Levy DE, et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014 Aug 20;312(7):719–728.
- Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012 Nov 14;11:CD000146.
- Hartmann-Boyce J, Stead LF, Cahill K, et al. Efficacy of interventions to combat tobacco addiction: cochrane update of 2013 reviews. Addiction. 2014 Sep;109(9):1414–1425.
- Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015 Feb 17;313(7):687–694.
- Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. Am J Prev Med. 2013 Mar;44(3):207–215.
- Hartmann-Boyce J, McRobbie H, Bullen C, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2016 Sep 14;9:CD010216.
- Pepper JK, Brewer NT. Electronic nicotine delivery system (electronic cigarette) awareness, use, reactions and beliefs: a systematic review. Tob Control. 2014 Sep;23(5):375–384.
- Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014 Jan;8(1):CD000031.
- Prado E, Maes M, Piccoli LG, et al. N-acetylcysteine for therapy-resistant tobacco use disorder: a pilot study. Redox Rep. 2015 Sep;20(5):215–222.
- Grella SL, Funk D, Coen K, et al. Role of the kappa-opioid receptor system in stress-induced reinstatement of nicotine seeking in rats. Behav Brain Res. 2014 May 15;265:188–197.
- Halpern SD, Harhay MO, Saulsgiver K, et al. A pragmatic trial of E-cigarettes, incentives, and drugs for smoking cessation. N Engl J Med. 2018 Jun 14;378(24):2302–2310.
- Hartmann-Boyce J, Cahill K, Hatsukami D, et al. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2012 Aug;15(8):CD007072.
- Schoberberger R, Bohm G, Schroeder Y. Heavy dependent nicotine smokers–newfound lifestyle appreciation after quitting successfully. Experiences from inpatient smoking cessation therapy. Public Health. 2015 May;129(5):539–544.
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008 Apr;83(4):531–541.
- Sansores RH, Ramirez-Venegas A, Arellano-Rocha R, et al. Use of varenicline for more than 12 months for smoking cessation in heavy chronic obstructive pulmonary disease smokers unmotivated to quit: a pilot study. Ther Adv Respir Dis. 2016 Oct;10(5):383–390.
- Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017 Mar 20;38:165–185.
- Taylor G, McNeill A, Girling A, et al. Change in mental health after smoking cessation: systematic review and meta-analysis. Bmj. 2014 Feb 13;348:g1151.
- Thomson D, Berk M, Dodd S, et al. Tobacco use in bipolar disorder. Clin Psychopharmacol Neurosci. 2015 Apr 30;13(1):1–11.
- Balmford J, Borland R, Hammond D, et al. Adherence to and reasons for premature discontinuation from stop-smoking medications: data from the ITC Four-Country Survey. Nicotine Tob Res. 2011 Feb;13(2):94–102.
- Borland R, Balmford J, Swift E. Effects of timing of initiation and planning on smoking cessation outcomes: study protocol for a randomised controlled trial. BMC Public Health. 2013 Mar 18;13:235.