ABSTRACT
Background: Hereditary transthyretin-mediated amyloidosis (hATTR amyloidosis) is a progressive, life-threatening disease. Until recently, tafamidis was the only approved pharmacotherapy. Patisiran significantly improved polyneuropathy and quality of life (QoL) in the phase III APOLLO trial. In the absence of direct comparisons, this analysis aimed to evaluate the comparative efficacy of tafamidis and patisiran in hATTR amyloidosis with polyneuropathy.
Research design and methods: Randomized controlled trial evidence for tafamidis was identified by systematic literature review. Indirect treatment comparisons were performed using the standard pairwise Bucher method for endpoints used in both APOLLO and the tafamidis Fx-005 trial: change from baseline in Neuropathy Impairment Score-lower limbs (NIS-LL), Norfolk QoL-Diabetic Neuropathy questionnaire (QoL-DN), NIS-LL response, and mBMI vs. placebo. Inter-trial population differences were assessed by sensitivity analysis.
Results: The base-case analysis (FAP Stage 1 APOLLO patients vs. intent-to-treat Fx-005 population) suggested patisiran had a greater treatment effect vs. tafamidis for all endpoints, with significant improvements in mean change in NIS-LL (–5.49) and QoL-DN (–13.10) from baseline to Month 18. Similar trends were observed in all sensitivity analyses.
Conclusions: In the absence of direct comparisons, this analysis suggests patisiran has a greater treatment effect than tafamidis in patients with hATTR amyloidosis with polyneuropathy.
1. Introduction
Hereditary transthyretin-mediated amyloidosis (hATTR amyloidosis) is an inherited, heterogeneous, rapidly progressive, life-threatening disease [Citation1–Citation3]. It is caused by a mutation in the transthyretin (TTR) gene that results in misfolded TTR proteins accumulating as amyloid deposits in multiple organs including the nerves, heart, and gastrointestinal tract [Citation1,Citation4,Citation5]. hATTR amyloidosis has an aggressive course with rapid disease progression leading to deteriorating quality of life (QoL), loss of function, and a median survival of 4.7 years following diagnosis [Citation3,Citation6]. It is estimated that there are approximately 10,000 patients worldwide with hATTR amyloidosis with polyneuropathy, although this may be an underestimate as the disease is often underdiagnosed and misdiagnosed resulting from insufficient disease awareness and clinical presentation involving non-specific clinical features that are hallmarks of more common diseases [Citation3,Citation7].
A number of differing measures of disease burden have been used to assess progression of this multisystem disease in clinical trials. Polyneuropathy has been assessed via the Neuropathy Impairment Score (NIS) and related scales, such as NIS-lower limb (NIS-LL; a composite measure of polyneuropathy impairment assessing muscle weakness, sensation loss, and muscle reflexes in the lower limbs [Citation8]), and modified NIS+7 (mNIS+7; a scale developed specifically for hATTR amyloidosis) [Citation9–Citation12]. QoL has been assessed using the Norfolk Quality of Life-Diabetic Neuropathy questionnaire (Norfolk QoL-DN; a validated self-reported measure of QoL for patients with polyneuropathy) [Citation13] and nutritional status has been assessed using modified body mass index (mBMI [BMI × serum albumin]). mBMI is a common assessment of nutritional status in hATTR amyloidosis studies [Citation9,Citation11,Citation12,Citation14,Citation15]. Assessment of ambulation has also been a longstanding measure of neuropathic burden in hATTR amyloidosis and has been captured by the familial amyloidotic polyneuropathy (FAP) staging system and polyneuropathy disability score [Citation14,Citation16].
Tafamidis, an orally administered TTR protein stabilizer, was approved on the basis of its results in the Fx-005 trial (NCT00409175), a pivotal placebo-controlled trial, which enrolled patients with Stage 1 disease and the V30M (valine to methionine at amino acid 30) mutation in the TTR gene. In this trial, the co-primary endpoints of NIS-LL responder (defined as <2 increase in NIS-LL) and Norfolk QoL-DN were not significantly different from placebo in the intent-to-treat (ITT) population at 18 months. However, a statistically significant treatment benefit was observed in the smaller, efficacy-evaluable population (ITT patients who completed the trial as per protocol) [Citation9]. In more recent open-label studies, neurologic progression has been observed in 40–65% of patients after 12 months of tafamidis treatment [Citation17–Citation20]. In the European Union (EU), tafamidis has been approved for the treatment of transthyretin amyloidosis in adult patients with Stage 1 symptomatic polyneuropathy to delay peripheral neurologic impairment [Citation21–Citation23]. Tafamidis is not currently approved in the US [Citation22].
Patisiran, an RNA interference (RNAi) therapeutic delivered via intravenous infusion, has been approved for the treatment of polyneuropathy associated with hATTR amyloidosis in adults in the US and for the treatment of hATTR amyloidosis in adult patients with Stage 1 or 2 polyneuropathy in the EU. It was evaluated in the randomized, placebo-controlled phase III APOLLO trial (NCT01960348) [Citation16]. Results from APOLLO demonstrated that 18 months of patisiran treatment improved polyneuropathy (as measured by mNIS+7) and QoL (as measured by Norfolk QoL-DN) in the majority of patients with hATTR amyloidosis [Citation16].
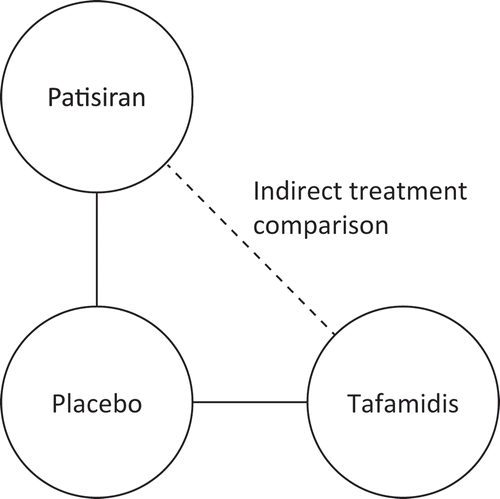
In the absence of direct head-to-head trials, indirect treatment comparison (ITC) assesses the relative effectiveness of two treatments using a common comparator. This methodology is often used to inform healthcare decision-making, as clinical trials rarely compare all treatments of interest [Citation24,Citation25]. A meta-analysis evaluating the results of direct head-to-head comparisons and indirect comparisons using the Bucher methodology reported no evident statistically significant difference between 93% (n = 44) of randomized trials evaluated directly and indirectly [Citation26].
The objective of this analysis was to conduct an indirect, quantitative comparison of the efficacy of patisiran and tafamidis in patients with hATTR amyloidosis with polyneuropathy using currently available data. Data from the APOLLO trial were used for patisiran; tafamidis data were collected from a systematic literature review of all publicly available randomized placebo-controlled trials. Safety was not compared as part of this analysis because of differences in the definitions of safety for data collection between the two studies; however, safety results are summarized for each trial separately.
2. Methods
2.1. Literature search
A systematic literature review was conducted to identify publications of randomized controlled trials that evaluated the efficacy of tafamidis in patients with hATTR amyloidosis with polyneuropathy. This ensured all relevant data from the Fx-005 trial were captured. Searches were conducted in MEDLINE, Embase, the Cochrane Library, and EconLit. Two investigators independently screened all abstracts and assessed their eligibility for inclusion; a third investigator resolved any screening-related disagreements. Articles were then reviewed in full to assess their eligibility for inclusion. As the ITC analyses were conducted based on unpublished data on file from APOLLO, a systematic literature review was not conducted for patisiran.
2.2. Eligibility criteria
Eligible trials included ≥80% of patients with hATTR amyloidosis with polyneuropathy. Trials had to be randomized controlled trials comparing tafamidis treatment against a common comparator (i.e. placebo in this indirect analysis) and featuring efficacy and QoL outcomes. Single-arm or observational trials were not included in the analysis as indirect comparison methodologies rely on randomized controlled data relative to a common comparator. Full details of the literature review and inclusion/exclusion criteria can be found in the Supplementary Appendix.
2.3. Outcomes
Efficacy outcomes were extracted for analysis if they were measured in both the patisiran APOLLO trial and any qualifying tafamidis trial. The outcomes extracted for analysis from the Fx-005 trial included: mean change in NIS-LL, NIS-LL response, mean change in Norfolk QoL-DN, and mean change in mBMI from baseline to Month 18 compared with placebo. In the APOLLO trial, mean change in Norfolk QoL-DN and mBMI were collected as a priori outcomes. Mean change in NIS-LL was derived from components of the NIS+7, which was also collected a priori as part of the APOLLO trial; NIS-LL response was calculated post hoc from mean change in NIS-LL.
2.4. Statistical analysis
Indirect comparisons between the treatment effects of patisiran and tafamidis were made by comparing least-squares mean differences between drug and placebo from baseline to Month 18 for NIS-LL, Norfolk QoL-DN, and mBMI, and by calculating the odds ratios (ORs) for NIS-LL response. ITCs were performed using the standard pairwise Bucher method [Citation27]. This method compares the magnitude of treatment effect in each trial by evaluating the differences between treatment and placebo arms (). This method preserves the original trial randomization and as a result, the potential intra-trial impact of effect-modifying, non-disease characteristics are removed [Citation27]. Results were considered to be statistically significant when the 95% confidence interval (CI) did not cross 0 (for mean differences) or 1 (for OR). As each trial was powered to detect differences vs. placebo in its full population, rather than vs. non-placebo treatments or smaller subgroups, a lack of statistical significance in this indirect treatment analysis does not imply evidence of no difference between treatments.
3. Results
3.1. Systematic review findings
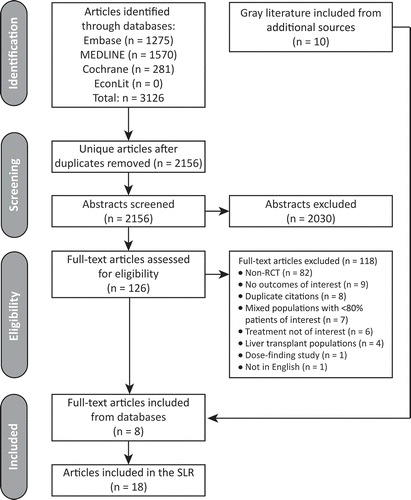
The literature search identified 18 eligible publications (). All articles referred to the same tafamidis phase II/III Fx-005 trial. An overview of the trials included in the analysis (APOLLO and Fx-005) is presented in . The APOLLO trial included 225 patients in the modified ITT population (mITT; all randomized patients who received ≥1 dose of study medication) and the Fx-005 trial included 125 patients in the ITT population (all randomized patients who received ≥1 dose of study medication and who had ≥1 post-baseline assessment of NIS-LL and Norfolk QoL-DN or who discontinued due to liver transplantation). Baseline demographics and disease characteristics of patients in the trials are presented in .
Table 1. Overview of trials included in the analysis.
Table 2. Baseline demographics and disease characteristics of patients in the APOLLO and Fx-005 trials.
3.2. Base-case and sensitivity analysis subgroups
Differences between the APOLLO and Fx-005 populations were observed for age, sex, baseline neuropathy severity (as measured by NIS-LL and FAP stage), mutation type, and prior hATTR amyloidosis pharmacotherapy. In the Fx-005 trial, neither age nor sex was found to modify the effect of outcomes, except a potential effect of sex on mBMI [Citation29]. Subgroup analyses of treatment effects were conducted for the APOLLO trial and found no significant signs of effect modification by age or sex. However, the remaining differences in disease characteristics could not be ruled out as effect modifiers.
To assess the effect of these characteristics on the indirect treatment analysis, data were collected from four APOLLO subgroups: patients with FAP Stage 1 disease (n = 104), all patients (mITT population), patients with Stage 1 disease and a V30M mutation (n = 44), and patients who were Stage 1 and treatment naïve (n = 48). The Stage 1 disease subgroup was used as the base-case analysis to balance similarity with the Fx-005 population and sample size considerations. Sensitivity analyses were performed with the remaining subgroups. All subgroups were indirectly compared with the Fx-005 trial ITT population.
Baseline neuropathy, QoL, and nutritional status data for the Fx-005 ITT population and APOLLO subgroups are presented in . At baseline, the Fx-005 trial population had milder neuropathy (as measured by NIS-LL) and better QoL (as measured by Norfolk QoL-DN) than the APOLLO base-case subgroup, a trend reflected through the sensitivity analysis subgroups, while nutritional status (as measured by mBMI) was broadly similar.
Table 3. Baseline measures of neuropathy, QoL, and nutritional status for the Fx-005 ITT population and APOLLO base-case and sensitivity analyses populations.
3.3. Analyses
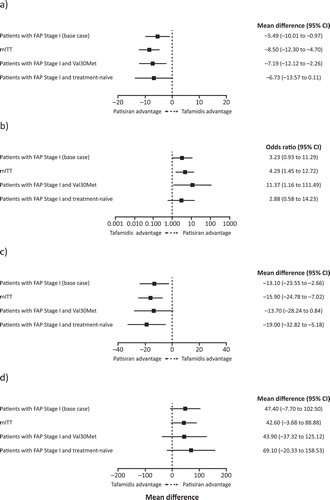
The results of the ITC of patisiran and tafamidis are presented in . Overall, the results of analyses suggest patisiran had a benefit over tafamidis across all outcomes, with every analysis group, and in many cases the difference was statistically significant.
Figure 3. Results of the indirect treatment comparison of Fx-005 ITT population and APOLLO base-case and sensitivity analyses populations for (a) change in mean NIS-LL score; (b) change in mean NIS-LL response; (c) change in mean Norfolk QoL-DN; and (d) change in mean mBMI score. CI: confidence interval; FAP: familial amyloidotic polyneuropathy; mBMI: modified body mass index; mITT: modified intent-to-treat; NIS-LL: Neuropathy Impairment Score-Lower Limb; Norfolk QoL-DN: Norfolk Quality of Life-Diabetic Neuropathy questionnaire.
3.4. NIS-LL
The base-case analysis indicated that patisiran had a significantly greater treatment effect on NIS-LL compared with tafamidis (difference in mean change from baseline to Month 18 of –5.49; 95% CI: –10.01 to –0.97). This greater benefit of patisiran vs. tafamidis on NIS-LL was supported by the sensitivity analysis, with significant differences observed in the mITT subgroup (–8.50; 95% CI: –12.30 to –4.70), and the FAP Stage 1 and V30M subgroup (–7.19; 95% CI: –12.12 to –2.26), despite the small sample size. There was also a trend toward a greater treatment effect with patisiran vs. tafamidis in the Stage 1 and treatment-naïve subgroup ()).
3.5. NIS-LL response
For NIS-LL response, the base-case analysis trended toward a greater treatment effect for patisiran compared with tafamidis (OR 3.23; 95% CI: 0.93–11.29). This trend was supported by a significantly greater treatment effect for patisiran vs. tafamidis in the mITT subgroup (OR 4.29; 95% CI: 1.45–12.72) and FAP Stage 1 and V30M subgroup (OR 11.37; 95% CI: 1.16–111.49). A trend toward a greater treatment effect was observed in the Stage 1 and treatment-naïve subgroup ()).
3.6. Norfolk QoL-DN
Similar to the NIS-LL results, the base-case analysis indicated a significantly greater treatment effect for patisiran compared with tafamidis for the difference in mean change from baseline to Month 18 in Norfolk QoL-DN (–13.10; 95% CI: –23.55 to –2.66). In the sensitivity analysis, significantly greater treatment effects for patisiran vs. tafamidis were also observed in the mITT subgroup (–15.90; 95% CI: –24.78 to –7.02), and Stage 1 and treatment-naïve subgroup (–19.00; 95% CI: –32.82 to –5.18). A trend toward a greater treatment effect was observed in the Stage 1 and V30M subgroup ()).
3.7. mBMI
For mBMI, the base-case analysis trended toward a greater treatment effect for patisiran compared with tafamidis for the difference in mean change from baseline to Month 18 (+47.40; 95% CI: –7.70 to 102.50). This was supported by the sensitivity analyses which saw similar trends in all subgroups despite small sample sizes ()).
3.8. Safety
Safety outcomes were not compared as part of this ITC because of differences in definitions of adverse events (AEs) and serious AEs (SAEs) between trials. In the APOLLO and Fx-005 clinical trials, each active treatment was considered to be generally well tolerated, with low rates of discontinuation due to AEs (). In each trial, the frequency of AEs and SAEs was similar between treatment and placebo groups. Treatment discontinuations due to AEs were more frequent with placebo (14.3%) than with patisiran (4.7%) in the APOLLO trial but were of similar frequency in placebo (4.8%) and tafamidis (6.2%) groups in the Fx-005 trial [Citation9,Citation16]. Deaths occurred in seven (4.7%) and six patients (7.8%) in the patisiran and placebo groups, respectively, in the APOLLO trial. In the Fx-005 trial, deaths were reported in two (3.1%) and three patients (4.8%) from the tafamidis and placebo groups, respectively. In both studies, no deaths were considered treatment related. In addition, there were no clinically relevant changes in laboratory values related to patisiran (including platelet counts and indicators of liver or kidney function) or tafamidis (including thyroid function) in either trial [Citation9,Citation16].
Table 4. Key safety outcomes in the APOLLO and Fx-005 trials.
4. Discussion
hATTR amyloidosis is a rare, genetic, rapidly progressive, and life-threatening disease. Tafamidis was approved in the EU and select other countries based on its results in the Fx-005 trial, which enrolled patients with Stage 1 disease and the V30M mutation. Patisiran has been approved in the US and EU based on results of the pivotal APOLLO trial, which enrolled hATTR amyloidosis patients across a broad range of disease stages and mutations. Due to the multi-systemic nature of this disease and its rapid progression, careful consideration is required in deciding the most appropriate course of treatment for patients. The current analysis provides an indirect, in-depth, quantitative comparison of patisiran and tafamidis in patients with hATTR amyloidosis with polyneuropathy, based on currently available data. ITCs are commonly used in the absence of comparative efficacy data from head-to-head trials, and have been often utilized in healthcare decision-making [Citation26]. While both trials enrolled patients with hATTR amyloidosis with polyneuropathy, there were differences in baseline characteristics, notably for age, sex, mutation type, severity of polyneuropathy, and prior hATTR amyloidosis pharmacotherapy use. These differences may be partly explained by the Fx-005 trial being conducted only in patients with the V30M mutation vs. the APOLLO trial which included patients with any TTR mutation. It has been observed that patients with V30M mutations in some endemic areas, such as Portugal, often have a disease onset before 40 years of age and develop progressive sensory-motor and autonomic neuropathy, leading to life-threatening cachexia if untreated. Conversely, patients with non-V30M mutation or V30M mutation but with a later age onset (>50 years) are more likely to be male and demonstrate a more varied disease phenotype [Citation30,Citation31]. To control for these differences a number of sensitivity analyses were performed, including for mutation type.
The current analyses suggest a greater benefit of patisiran vs. tafamidis for patients with Stage 1 hATTR amyloidosis. This benefit was observed across all measures of neuropathic function, QoL, and nutritional status in patients with Stage 1 disease; this benefit was demonstrated regardless of V30M mutation status or previous stabilizer use and, in many cases, the difference was statistically significant despite small sample sizes. This is consistent with the findings of the APOLLO trial, which found significant treatment effects for patisiran- vs. placebo-treated patients across all patient subtypes, regardless of patients’ disease stage or mutation [Citation16].
Analysis of the data suggests that patisiran had a greater effect than tafamidis on measures of polyneuropathy, a cardinal feature of this disease, as assessed by NIS-LL and NIS-LL response. In the Fx-005 trial, tafamidis demonstrated an approximate 3-point difference in mean NIS-LL scores compared with placebo, equivalent to 52% less neurologic deterioration in patients with Stage 1 hATTR amyloidosis with polyneuropathy [Citation9]. In the subgroup of patients from the APOLLO trial with Stage 1 disease, there was an approximate 9-point difference in mean NIS-LL scores for patisiran vs. placebo, equivalent to 92% less neurologic deterioration on average among patisiran-treated patients.
Patisiran was also associated with an improvement in patients’ QoL relative to tafamidis, as measured by the difference in mean change for Norfolk QoL-DN scores. Finally, the results of these analyses suggest a greater effect on nutritional status, as measured by mBMI, for patisiran- over tafamidis-treated patients.
In the APOLLO and Fx-005 trials, each treatment was generally well tolerated, with low rates of discontinuations due to AEs in the active treatment arms [Citation9,Citation16]. In each trial the frequency of AEs, SAEs, and deaths was similar between treatment and placebo arms, with no deaths considered treatment related [Citation9,Citation16]. The long-term safety of tafamidis (up to 6 years) has been assessed in an open-label extension study in which this agent was generally well tolerated [Citation32]. The long-term safety of patisiran is currently being assessed via a global open-label extension study, with patients having received up to 4 years of treatment at the time of publication (NCT02510261).
4.1. Limitations
As discussed above, while both trials enrolled patients with hATTR amyloidosis with polyneuropathy, there were differences in baseline characteristics, notably for mutation type, severity of polyneuropathy, and prior hATTR amyloidosis pharmacotherapy use. To help mitigate this in the current analysis, sensitivity analyses were conducted comparing a number of APOLLO subgroups against the tafamidis data. In the current comparison, the baseline values for neuropathy severity, mutation type, and prior hATTR amyloidosis pharmacotherapy were considered potential treatment effect modifiers. Sensitivity analyses were therefore conducted on three subgroups from the APOLLO trial, with results trending similarly across all subgroups and outcomes. This suggests that the findings from the ITC are likely to be valid with respect to population differences between the two trials. Age and sex were not accounted for in the sensitivity analyses as they have not been demonstrated to modify treatment effects in hATTR amyloidosis and so are unlikely to contribute to bias in the analyses.
Another limitation of this ITC is the relatively small number of patients involved in the two pivotal trials. While larger sample sizes would contribute to the strength of the findings, the rarity of hATTR amyloidosis and challenges in diagnosis result in a limited number of patients being available for clinical studies. If additional appropriate clinical data become available in the future, this ITC could be reperformed. However, trials directly comparing the treatments in this patient population would be most informative. Finally, indirect comparisons between these two therapies could not be conducted among patients with non-V30M mutations or more advanced neuropathy stages, as these patients were not enrolled or evaluated as part of the tafamidis Fx-005 trial.
5. Conclusions
Overall, the results of this ITC suggest patisiran has a greater benefit compared with tafamidis in patients with FAP Stage 1 hATTR amyloidosis with polyneuropathy. This benefit was seen across all endpoints measured, with statistically significant differences observed for differences in mean change from baseline in NIS-LL and Norfolk QoL-DN. Similar results were also observed across all sensitivity analyses. Although these findings have important limitations, the consistency of the results across subgroups, which were analyzed to address these limitations, supports the validity of the ITC. This analysis provides an in-depth and quantitative comparison of the efficacy data currently available.
Author contributions
V Planté-Bordeneuve and M Polydefkis were involved in the design, interpretation of the data, the drafting of the paper or revising it critically for intellectual content, and the final approval of the version to be published.
H Lin, J Gollob, and S Agarwal were involved in the conception and design, analysis and interpretation of the data; the drafting of the paper or revising it critically for intellectual content; and the final approval of the version to be published
M Betts, K Fahrbach, and M Chitnis were involved in the analysis and interpretation of the data; the drafting of the paper or revising it critically for intellectual content; and the final approval of the version to be published
All authors agree to be accountable for all aspects of the work
Declaration of interest
V Planté-Bordeneuve reports grant and advisory board fees from Alnylam Pharmaceuticals, non-financial support from Pfizer, non-financial support and advisory board fees from Ionis Pharmaceuticals, and advisory board fees from Eidos Therapeutics, outside the submitted work. M Polydefkis declares personal fees from Alnylam Pharmaceuticals, grants and consultancy fees from Pfizer, expenses from Ionis Pharmaceuticals, and consultancy fees from Vertex Pharmaceuticals, Biogen, and Chromocell, outside the submitted work. H Lin and S Agarwal are employees of Alnylam Pharmaceuticals. J Gollob is a former employee of Alnylam Pharmaceuticals and current Chief Medical Officer with Kymera Therapeutics. K Fahrbach, M Chitnis and M Betts are employees of Evidera, who received funding from Alnylam Pharmaceuticals to conduct this research. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Data availability statement
Due to the sensitive nature of the data used in this study, the dataset will not be made available to other researchers. However, requests from qualified researchers for additional analyses may be sent to Alnylam Pharmaceuticals ([email protected]).
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (23.4 KB)Acknowledgments
This study uses data from trials NCT00409175 and NCT01960348.
Supplementary material
Supplementary material for this article can be accessed here
Additional information
Funding
References
- Hanna M. Novel drugs targeting transthyretin amyloidosis. Curr Heart Fail Rep. 2014;11(1):50–57.
- Damy T, Judge DP, Kristen AV, et al. Cardiac findings and events observed in an open-label clinical trial of tafamidis in patients with non-val30met and non-val122Ile hereditary transthyretin amyloidosis. J Cardiovasc Transl Res. 2015;8(2):117–127.
- Hawkins PN, Ando Y, Dispenzeri A, et al. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47(8):625–638.
- Mohty D, Damy T, Cosnay P, et al. Cardiac amyloidosis: updates in diagnosis and management. Arch Cardiovasc Dis. 2013;106(10):528–540.
- Adams D, Coelho T, Obici L, et al. Rapid progression of familial amyloidotic polyneuropathy: a multinational natural history study. Neurology. 2015;85(8):675–682.
- Swiecicki PL, Zhen DB, Mauermann ML, et al. Hereditary ATTR amyloidosis: a single-institution experience with 266 patients. Amyloid. 2015;22(2):123–131.
- Schmidt HH, Waddington-Cruz M, Botteman MF, et al. Estimating the global prevalence of transthyretin familial amyloid polyneuropathy. Muscle Nerve. 2018;57(5):829–837.
- Bril V. NIS-LL: the primary measurement scale for clinical trial endpoints in diabetic peripheral neuropathy. Eur Neurol. 1999;41(Suppl. 1):8–13.
- Coelho T, Maia LF, Da Silva AM, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79(8):785–792.
- Berk JL, Suhr OB, Obici L, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310(24):2658–2667.
- Adams D, Suhr OB, Dyck PJ, et al. Trial design and rationale for APOLLO, a phase 3, placebo-controlled study of patisiran in patients with hereditary ATTR amyloidosis with polyneuropathy. BMC Neurol. 2017;17(1):181.
- Dyck PJ, Kincaid JC, Dyck PJB, et al. Assessing mNIS+7IONIS and international neurologists’ proficiency in a familial amyloidotic polyneuropathy trial. Muscle Nerve. 2017;56(5):901–911.
- Vinik EJ, Hayes RP, Oglesby A, et al. The development and validation of the Norfolk QOL-DN, a new measure of patients’ perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther. 2005;7(3):497–508.
- Suhr O, Danielsson A, Holmgren G, et al. Malnutrition and gastrointestinal dysfunction as prognostic factors for survival in familial amyloidotic polyneuropathy. J Intern Med. 1994;235(5):479–485.
- Coelho T, Vinik A, Vinik EJ, et al. Clinical measures in transthyretin familial amyloid polyneuropathy. Muscle Nerve. 2017;55(3):323–332.
- Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21.
- Cortese A, Vita G, Luigetti M, et al. Monitoring effectiveness and safety of tafamidis in transthyretin amyloidosis in Italy: a longitudinal multicenter study in a non-endemic area. J Neurol. 2016;263(5):916–924.
- Ando Y, Sekijima Y, Obayashi K, et al. Effects of tafamidis treatment on transthyretin (TTR) stabilization, efficacy, and safety in Japanese patients with familial amyloid polyneuropathy (TTR-FAP) with Val30Met and non-Val30Met: a phase III, open-label study. J Neurol Sci. 2016;362:266–271.
- Plante-Bordeneuve V, Gorram F, Salhi H, et al. Long-term treatment of transthyretin familial amyloid polyneuropathy with tafamidis: a clinical and neurophysiological study. J Neurol. 2017;264(2):268–276.
- Lozeron P, Theaudin M, Mincheva Z, et al. Effect on disability and safety of tafamidis in late onset of Met30 transthyretin familial amyloid polyneuropathy. Eur J Neurol. 2013;20(12):1539–1545.
- Scott LJ. Tafamidis: a review of its use in familial amyloid polyneuropathy. Drugs. 2014;74(12):1371–1378.
- Adams D, Cauquil C, Labeyrie C, et al. TTR kinetic stabilizers and TTR gene silencing: a new era in therapy for familial amyloidotic polyneuropathies. Expert Opin Pharmacother. 2016;17(6):791–802.
- Pfizer Limited, summary of product characteristics: Vyndaqel 20 mg soft capsules. 2011. [cited 2018 Nov 03]. Available from: https://www.ema.europa.eu/documents/product-information/vyndaqel-epar-product-information_en.pdf
- Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14(4):417–428.
- Jansen JP, Trikalinos T, Cappelleri JC, et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC good practice task force report. Value Health. 2014;17(2):157–173.
- Song F, Altman DG, Glenny AM, et al. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326(7387):472.
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691.
- Keohane D, Schwartz J, Gundapaneni B, et al. Tafamidis delays disease progression in patients with early stage transthyretin familial amyloid polyneuropathy: additional supportive analyses from the pivotal trial. Amyloid. 2017;24(1):30–36.
- Food and drug administration, US food and drug administration center for drug evaluation and research doNP. Vyndaqel (tafamidis meglumine) Background Package. 2012. [cited 2017 May 01]. Available from: https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/peripheralandcentralnervoussystemdrugsadvisorycommittee/ucm304830.pdf
- Parman Y, Adams D, Obici L, et al. Sixty years of transthyretin familial amyloid polyneuropathy (TTR-FAP) in Europe: where are we now? A European network approach to defining the epidemiology and management patterns for TTR-FAP. Curr Opin Neurol. 2016;29(Suppl. 1):S3–S13.
- Suhr OB, Lundgren E, Westermark P. One mutation, two distinct disease variants: unravelling the impact of transthyretin amyloid fibril composition. J Intern Med. 2017;281(4):337–347.
- Barroso FA, Judge DP, Ebede B, et al. Long-term safety and efficacy of tafamidis for the treatment of hereditary transthyretin amyloid polyneuropathy: results up to 6 years. Amyloid. 2017;24(3):194–204.