ABSTRACT
Background: Gait disorders are common in Parkinson’s disease patients who respond poorly to dopaminergic treatment. Blockade of adenosine A2A receptors is expected to improve gait disorders. Istradefylline is a first-in-class selective adenosine A2A receptor antagonist with benefits for motor complications associated with Parkinson’s disease.
Research design and methods: This multicenter, open-label, single-group, prospective interventional study evaluated changes in total gait-related scores of the Part II/III Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) and Freezing of Gait Questionnaire (FOG-Q) in 31 Parkinson’s disease patients treated with istradefylline. Gait analysis by portable gait rhythmogram was performed.
Results: MDS-UPDRS Part III gait-related total scores significantly decreased at Weeks 4–12 from baseline with significant improvements in gait, freezing of gait, and postural stability. Significant decreases in MDS-UPDRS Part II total scores and individual item scores at Week 12 indicated improved daily living activities. At Week 12, there were significant improvements in FOG-Q, new FOG-Q, and overall movement per 48 h measured by portable gait rhythmogram. Adverse events occurred in 7/31 patients.
Conclusions: Istradefylline improved gait disorders in Parkinson’s disease patients complicated with freezing of gait, improving their quality of life. No unexpected adverse drug reactions were identified.
Trial registration: UMIN-CTR (UMIN000020288).
1. Introduction
Gait disorders are common, troubling symptoms reported in the early stages of Parkinson’s disease (PD). The symptoms of gait disorders include a forward-bent posture, reduced arm swing, short steps, slow walking speed, and toe dragging, as well as a festinating gait and retropulsion [Citation1,Citation2]. Additionally, freezing of gait (FoG) often occurs in patients with a longer duration and advanced stage of disease. The characteristics of FoG include difficulty in initiating walking by stepping forward because the patient is unable to lift their foot from the floor, difficulty in turning while walking, and trembling. This can occur during stressful conditions, such as time pressure to meet a deadline, passing through a narrow gap, or immediately before reaching the target destination [Citation2]. These factors all severely reduce patient quality of life (QOL), and the sudden onset of FoG increases the risk of falls and the potential for limb fractures, which can adversely affect a patient’s mobility even more severely [Citation2–Citation4]. With further progression of the disease, FoG can occur even in ON time, at which point the condition becomes resistant to standard therapy (i.e., dopaminergic treatment), which is commonly administered at the early stage of disease [Citation2]. Therefore, FoG during ON time is particularly problematic in clinical practice.
Unfortunately, these disabling symptoms are often insufficiently controlled by current drug therapies including dopaminergic treatment [Citation5], and because the underlying pathophysiology of FoG is poorly understood, no effective treatment is available. However, nondopaminergic factors are thought to contribute to FoG based on the location of PD pathology in the brain [Citation4–Citation6]. This suggests that drugs acting on adenosine A2A receptors (involved in a nondopaminergic neurotransmitter system present in the basal ganglia, specifically expressed in the striatum and globus pallidus external segment [GPe] [Citation7,Citation8]) might improve gait disorders by restoring imbalanced neuronal signaling in the basal ganglia. Studies of nondopaminergic therapies such as methylphenidate [Citation5] and rivastigmine [Citation9] showed efficacy in reducing FoG, although concerns over potential side effects and cost-effectiveness indicate that the long-term risk-benefit balance is yet to be determined.
Istradefylline is a first-in-class selective adenosine A2A receptor antagonist, which is currently only available in Japan for treatment of the wearing-off phenomenon in patients with PD on concomitant treatment with levodopa-containing therapies. Istradefylline lacks significant affinity for receptors/transporters of other neurotransmitters, including dopamine, and does not elicit inhibitory effects on catechol-O-methyltransferase or monoamine oxidase enzymes [Citation10]. Adenosine A2A receptors are highly localized to the striatum and GPe, which is part of the basal ganglia-thalamo-cortical circuit [Citation11]. This circuit has two outputs, a direct and an indirect pathway, which are controlled by dopamine D1 and D2 receptors, respectively. In PD, decreased excitatory activity of the direct pathway and increased inhibitory activity of the indirect pathway causes an imbalance that results in motor dysfunction. It has previously been demonstrated that adenosine A2A receptor activation selectively induces activation of the indirect pathway via dual modulation of receptors in both the striatum and GPe [Citation12]. Blockade of the adenosine A2A receptors in the striatum and GPe antagonizes the hyperexcitability of GABAergic striatopallidal neurons caused by loss of D2 receptor activation in PD, resulting in improvement of motor function via normalization of balance in the basal ganglia [Citation12]. Furthermore, although biochemical receptor interaction between A2A and D2 receptors has been suggested [Citation13], it has also been considered that the effect of A2A receptor function on motor control is at least partially independent of the D2 receptor-mediated mechanism. This is exemplified by preclinical studies demonstrating that an adenosine A2A receptor antagonist or agonist, respectively improve motor dysfunction or further depress motor dysfunction in D2 receptor knockout mice [Citation14,Citation15].
Clinical studies have shown the benefit and tolerability of istradefylline in PD patients with motor complications on levodopa therapy. Patients administered once-daily 20 or 40 mg istradefylline showed a significant reduction in daily OFF time compared with placebo [Citation16–Citation20], with dyskinesia [Citation16,Citation17] or nausea [Citation18] being the most common adverse events (AEs). More recently, a long-term phase 3 study of 308 PD patients with wearing-off symptoms on levodopa therapy reported that 20 mg/day istradefylline (with/without dose adjustment to 40 mg/day) promoted a significantly sustained reduction in OFF time and was well tolerated, although frequent AEs included nasopharyngitis and dyskinesia [Citation19].
These studies suggested that a nondopaminergic agent such as istradefylline might be effective for the treatment of PD. Indeed, caffeine, a nonselective adenosine receptor antagonist, showed clinical benefit regarding FoG in PD patients [Citation21]. Recently, clinical efficacy of istradefylline on gait deficits [Citation22] and postural abnormality [Citation23] were reported. Based on these clinical studies, it was thought that istradefylline can improve the clinical symptoms of gait disorders, including FoG, leading to a better QOL for PD patients.
This was a multicenter, open-label, single-group, prospective interventional study to evaluate the efficacy and safety of istradefylline in improving gait disorders with FoG in PD patients, as measured by the change in the total gait-related score of Part III of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) and Freezing of Gait Questionnaire (FOG-Q). Effects on QOL were assessed using the Parkinson’s Disease Questionnaire-8 (PDQ-8).
2. Patients and methods
2.1. Study design and patients
This study was designed to evaluate the efficacy of istradefylline for gait disorders, including freezing of gait in Parkinson’s disease. The study period was from November 2015 to September 2016 for enrollment and from November 2015 to December 2016 for research at six participating sites throughout Japan. Patients with advanced PD who were treated with levodopa preparations and had wearing-off symptoms and gait disorders complicated with FoG were enrolled.
The main inclusion criteria were as follows: presence of wearing-off phenomena in PD patients currently treated with levodopa-containing products, gait disorders with FoG assessed by physician’s question: ‘Do you sometimes feel that your feet get glued to the floor while walking, making a turn, or when trying to initiate walking?’, stage ≤ IV according to the modified Hoehn and Yahr scale (ON-state), age ≥ 30 years at the time of consent, and written informed consent.
The exclusion criteria were dementia or a score of ≤ 23 on the Mini-Mental State Examination (MMSE); gait disturbance due to other causes; severe dyskinesia as assessed by the investigator/subinvestigator; current treatment with istradefylline; ongoing treatment with deep brain stimulation; lactation, pregnancy, or possible pregnancy; or other conditions judged by the investigator/subinvestigator to be unsuitable for participation in this study (e.g., patients who had undergone orthopedic surgery for gait disorders or had severe psychosis).
Patients received istradefylline orally for 12 weeks at 20 mg/day for the first 4 weeks, followed by 20 mg/day or an increased dose of 40 mg/day for 8 weeks if they had no tolerability issues (based on the judgement of the investigator/subinvestigator) and still had motor symptoms at Week 4. After Week 4, the dose was reduced to 20 mg/day if the patient exhibited any tolerability issues. The observation period for each patient was 12 weeks after the start of treatment with istradefylline. Details on the use of permitted/restricted concomitant medications or therapies are described in the Supplementary Methods.
This study was approved by the ethics review committees of each study site and conducted in compliance with the Ethical Guidelines for Clinical Studies in Japan, the Declaration of Helsinki, and other applicable regulations/law. All patients provided written informed consent. The study was registered in the UMIN-CTR clinical trials database (https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000023424 [UMIN000020288]).
2.2. Efficacy endpoints
The primary endpoint was change in the total gait-related score of the MDS-UPDRS Part III (items 3.9 arising from chair, 3.10 gait, 3.11 freezing of gait, 3.12 postural stability, and 3.12 posture) before and after the administration of istradefylline. Secondary endpoints included change in each gait-related score of the MDS-UPDRS Part II and Part III, FOG-Q, New Freezing of Gait Questionnaire (NFOG-Q), and PDQ-8 before and after administration of istradefylline. These scores were evaluated at baseline and Weeks 4 and 12.
Free-living gait analysis using a patient diary and a portable gait rhythmogram (PGR) (LSI Medience Co., Tokyo, Japan) was performed at baseline and again at Week 12. The PGR recorded data for 48 h and was worn on the patient’s waist. Acceleration in three dimensions was measured and gait parameters were calculated [Citation24].
2.3. Safety
Safety was assessed by adverse drug reactions (ADRs) (defined as AEs related to the administration of istradefylline). AEs were defined as any unfavorable or unintended signs (including abnormal laboratory findings), symptoms, or diseases that occurred between the start of treatment (Day 1) and the final evaluation at or after Week 12 or early termination, whether or not considered related to istradefylline. Serious AEs (SAEs) were defined as any of the following: death, disability, AEs that were life-threatening or leading to potential disability, AEs requiring hospitalization or prolongation of prior hospitalization, and other medically critical conditions. The frequencies of all AEs and ADRs were summarized by category according to the System Organ Class and Preferred Terms of MedDRA/J v19.1.
2.4. Statistical analysis
The sample size was determined to be 30 based on a calculation to provide 84% power to detect differences in the primary endpoint using a two-sided t-test with a significance level of 5%, assuming that the mean improvement in total gait-related MDS-UPDRS score from baseline is 1.0, with a standard deviation of 2.0, and an autocorrelation of 0.6.
MDS-UPDRS, FOG-Q, and PDQ-8 total and subitem scores were compared using a one-sample t-test. Repeated measures analysis of variance was used for comparisons between baseline and Week 4/Week 12. When a significant difference was detected, a post hoc test was conducted using the Bonferroni method. For the PGR, data were assessed using a one-sample t-test. The Spearman rank test was used to assess correlation. Statistical significance was defined as a two-sided P-level of 5%. All analyses were performed using SAS ver. 9.2 software (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Baseline characteristics
Regarding the disposition of patients in this study, 31 were enrolled, 31 started istradefylline treatment, 28 completed the study course; and three discontinued the study (consent withdrawal, AE, other; n = 1 each; Supplementary Figure 1).
The efficacy analysis set (n = 29) comprised patients not meeting any of the following criteria: not exposed to istradefylline, did not undergo any assessments or observations after the start of treatment with istradefylline, and having significant deviations from the protocol that might have affected efficacy evaluation. The patient who discontinued the study for ‘other’ reasons was included in the efficacy analysis set, received all scheduled treatments, and had data available for analysis. The safety analysis (n = 31) set comprised all patients, excluding those who were not exposed to istradefylline.
Of the 31 patients enrolled, 15 were male and 16 were female with a mean age of 69.9 years, mean PD duration of 10.71 years, mean number of concomitant anti-Parkinsonian treatments of 3.38, a daily levodopa dose of 469.4 mg, with mean levodopa-equivalent dose of 816.0 ± 463.1 mg/day ().
Table 1. Baseline patient characteristics.
3.2. Efficacy
Regarding the primary efficacy endpoint, the MDS-UPDRS Part III (ON-state) gait-related items total score (with a breakdown of individual scores) was significantly decreased at Week 4 (5.9 ± 4.3; P < 0.001) and maintained until Week 12 (5.7 ± 4.9; P = 0.007) compared with baseline (6.9 ± 4.2), with significant improvement at Week 12 (3.10: gait [1.2 ± 1.1 compared with baseline 1.7 ± 0.9, P = 0.003]; 3.11: FoG [0.7 ± 1.2, compared with baseline 1.0 ± 1.2, P = 0.043]; and 3.12: postural stability [1.4 ± 1.3 compared with baseline 1.7 ± 1.2, P = 0.043]) (, ). Twenty-two patients (75.9%) had improved MDS-UPDRS Part III total score (decreased by 1 point or more) at Week 12.
Table 2. MDS-UPDRS Part II and III (ON-state) gait-related items scores and FOG-Q and NFOG-Q total scores.
Figure 1. Time course of MDS-UPDRS Part III (ON-state) gait-related items total score (a), FOG-Q (b), and PDQ-8 total score (c).
P values calculated vs baseline; one-sample paired t-test with Bonferroni post hoc correction. MDS-UPDRS, Movement Disorder Society-Unified Parkinson’s Disease Rating Scale; FOG-Q, Freezing of Gait Questionnaire; PDQ-8, Parkinson’s Disease Questionnaire-8.
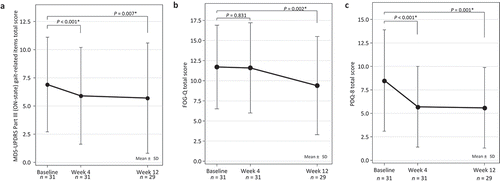
Regarding the secondary efficacy endpoints, the gait-related MDS-UPDRS Part II total score was significantly decreased at Week 12 (5.0 ± 2.8; P = 0.017) compared with baseline (5.9 ± 2.7), as were two individual item scores (2.11: getting out of bed, a car, or a deep chair (1.5 ± 1.0; P = 0.036) and 2.12: walking and balance [1.7 ± 1.1; P = 0.010]) when compared with baseline (1.9 ± 1.0, 2.0 ± 1.0, respectively) (, Supplementary Figure 2). Seventeen patients (58.6%) showed improvements in the MDS-UPDRS Part II total score (decreased by 1 point or more) at Week 12.
Patient-reported FOG-Q total score at Week 4 (11.6 ± 5.6) remained similar to the baseline score (11.7 ± 5.2) but was significantly decreased at Week 12 (9.4 ± 6.1; P = 0.002; ; ). NFOG-Q total score progressively decreased throughout the study period, reaching statistical significance at Week 12 (P = 0.026, Supplementary Figure 3). Twenty (69.0%) and 16 (55.2%) patients had improved FOG-Q and NFOG-Q scores, respectively (decreased by 1 point or more), at Week 12. There was a strong correlation between the FOG-Q and NFOG-Q total scores (r = 0.910, Supplementary Figure 4).
The PGR gait analysis results showed a significant improvement of the mean number of overall movements/48 h at Week 12 (P = 0.015, ), but no significant changes in gait rhythm or acceleration.
Table 3. Gait analysis based on portable gait rhythmogram.
PDQ-8 total scores were significantly decreased at Week 4 (5.7 ± 4.3; P < 0.001) and were maintained at the same level at Week 12 (5.6 ± 4.3; P = 0.001, , Supplementary Table 1) compared with the baseline score (8.5 ± 5.4). The following sub-item scores showed significant changes at Week 12 compared with baseline: 5. Problems with your concentration, e.g. when reading or watching TV? (−0.6 ± 1.1; P = 0.006); 7. Painful muscle cramps or spasms? (−0.7 ± 1.1, P = 0.005); and 8. Embarrassed in public due to having Parkinson’s disease? (−0.7 ± 1.0, P = 0.001) (Supplementary Table 1).
3.3. Safety
Overall, AEs were reported in 7/31 patients (22.6%) and included dyskinesia in two patients (6.5%), and cellulitis, pneumonia, insomnia, diarrhea, and headache in one patient each (3.2%). One SAE (pneumonia) was observed in a 76-year-old male patient who was hospitalized. His pneumonia was treated successfully and he was discharged. ADRs were reported in 4/31 patients (12.9%); these included dyskinesia in two patients (6.5%), and insomnia and chest pain in one patient each (3.2%). All ADRs were mild in severity, except the case of chest pain (moderate), and entered remission or resolved. No patient discontinued the study drug because of an ADR, and no serious ADRs or previously unreported ADRs were observed.
4. Discussion
This study evaluated the efficacy and safety of istradefylline for gait disorders, including FoG during ON time, which severely reduces the QOL of PD patients. Our results demonstrated improvements in gait disorders as measured by MDS-UPDRS Part II and Part III (ON-state) gait-related scores, FOG-Q, NFOG-Q, PDQ-8, and gait analysis.
Gait disorders, especially ON-state, in patients with advanced PD do not respond well to treatment with levodopa or electrical subthalamic nucleus stimulation. Such patients often have cognitive decline and treatment with nondopaminergic medication is considered a new therapeutic strategy [Citation5,Citation6]. Nondopaminergic pathways involving the adenosine A2A receptor might contribute to gait disorders observed in PD patients. Adenosine A2A receptors are highly localized to the striatum and GPe, which is part of the basal ganglia thalamocortical circuit [Citation11], and more specifically, are predominantly expressed postsynaptically on medium spiny neurons of the indirect GABAergic striatopallidal output pathway. Adenosine acting on these A2A receptors causes excessive activation of the indirect pathway in both the striatum and GPe; so-called ‘dual excitation’ of the indirect pathway [Citation25]. In contrast, D2 receptors, also expressed on medium spiny neurons, exert their effect on motor function by suppressing indirect output activity. As a consequence, A2A receptors and D2 receptors in the basal ganglia act to control the activity of the indirect output to the GPe through their opposing influences on the same pathway. Therefore, A2A antagonists support D2 receptor-mediated function specifically on this pathway [Citation26].
Efficacy of caffeine, a nonselective adenosine receptor antagonist, for FoG was experienced by some PD patients [Citation21]. However, a recent prospective study (Café-PD) reported no clinically important improvements of PD-related motor manifestations [Citation27]. The outcomes of caffeine may be attributed to less selectivity and weak affinity for the adenosine A2A receptor [Citation10], along with multiple other biochemical activities of caffeine [Citation28]. In a recent study, a significant decrease in the FOG-Q score in PD patients was reported after 1-month treatment with istradefylline [Citation22]. Another 3-month study that evaluated the efficacy of istradefylline showed that 50% of patients had an improvement in postural abnormalities, although an effect on FoG was not observed. However, in that study, only 5 of 21 patients were administered a 40-mg dose of istradefylline [Citation23], whereas in the present study, 28 of 29 patients received a 40-mg dose. Istradefylline is a selective A2A receptor antagonist with approximately 1000-fold higher affinity than caffeine [Citation10], and it reduces activity of the indirect pathway [Citation29], which is the mode of action of the anti-parkinsonian effect of adenosine A2A receptor antagonists [Citation30]. Furthermore, an adenosine A2A antagonist has been shown to modify increased suppression outputs from the basal ganglia projecting to the thalamocortical loop in PD patients [Citation31]. Moreover, in rat studies, A2A receptor antagonists improved initiation of movement and sensory motor integration in the 6-hydroxydopamine PD rat model [Citation32], and istradefylline promoted release of dopamine and improved cognitive performance in prefrontal cortex-lesioned rats [Citation33].
The core elements of gait disturbance in PD may include the following: (1) decreased activity of the corticospinal tract, (2) degradation of function of motion program generation and attitude control mechanism in cortical association areas, and (3) dysfunction of control mechanism of walking and muscle tone in the spinal cord from the brainstem [Citation34]. In patients with FoG, blood flow in the frontal lobe areas is known to be reduced [Citation4]. Based on these findings, together with the outcome of the present study, istradefylline might mediate its effects on gait disorders, including FoG, by improving function in networks related to locomotor control, such as the pedunculopontine nucleus and thalamocortical loop network [Citation34].
The results from the present gait analysis by PGR suggest possible mechanisms underlying the improvement in gait clinical scores. For smooth execution of gait movements, elaborate neural controls are necessary in the following elements: (1) postural stability, (2) manifestation (release) of gait movements, and (3) effective propelling forces and rhythms in gait cycles. Notably, the improvements in gait scores were not associated with improvements in force or rhythm. However, improvements were observed in walking balance and execution of movements (e.g., a decrease in freezing and an increase in amount of overall movements per 48 h). These results suggest that the istradefylline-induced improvement might be attributed to mechanisms (1) and (2) listed above.
FoG severely affects the QOL of PD patients [Citation35]. In the current study, PDQ-8 scores were significantly decreased at Week 4 and were maintained at the same level until Week 12. Although there are several factors that affect the QOL of PD patients, gait disorders play an important role. Therefore, istradefylline might improve the QOL of PD patients by reducing the clinical symptoms of gait disorders related with PD, including FoG.
This study also demonstrated the safety profile of istradefylline, with neither serious ADRs nor unexpected ADRs observed. Our safety results were consistent with those of previous clinical studies and show that istradefylline is well tolerated in combination with dopaminergic anti-Parkinsonian drugs, indicating the benefit of the drug in PD patients with wearing-off phenomena.
Our study had some limitations, including its small sample size, which might lead to bias (particularly when subjecting subscores to statistical tests) or the increased likelihood of type II error. Furthermore, this study was not controlled, blinded, or randomized; thus, the placebo effect should be considered when interpreting the results.
5. Conclusions
Regarding the clinical implications of this study, istradefylline improved QOL with associated reductions in gait disturbance. Gait disturbances represent a large therapeutic need, as these cannot be adequately controlled by other existing therapies. Treatment with istradefylline might be beneficial to patients who are not responsive to dopaminergic therapies. This was the first multicenter collaborative study that evaluated the efficacy of istradefylline for gait disorder and FoG. The promising results from our study suggest that further studies involving larger patient numbers and a randomized, controlled design are warranted.
Role of the sponsor
Kyowa Hakko Kirin Co., Ltd. was involved in the process of study design and protocol development.
Declaration of interest
H Shimura has received lecture fees from Kyowa Hakko Kirin Co., Ltd. K Kitagawa has received consulting fees from Kyowa Hakko Kirin Co., Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
M Iijima participated in the conception, organization, and execution of the research project; review and critique of the statistical analysis; and writing of the manuscript.
S Orimo participated in the conception, organization, and execution of the research project; execution of the statistical analysis; and review and critique of the manuscript.
H Terashi participated in the conception, organization, and execution of the research project; review and critique of the statistical analysis; and review and critique of the manuscript.
M Suzuki participated in the organization and execution of the research project; and review and critique of the manuscript.
A Hayashi participated in the conception, organization, and execution of the research project; and review and critique of the manuscript.
H Shimura participated in the organization and execution of the research project; and review and critique of the manuscript.
H Mitoma participated in the conception and organization of the research project; design and review/critique of the statistical analysis; and review and critique of the manuscript.
K Kitagawa participated in the organization and execution of the research project; and review and critique of the manuscript.
Y Okuma participated in the conception, organization, and execution of the research project; review and critique of the statistical analysis; and review and critique of the manuscript.
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Supplemental Material
Download Zip (107.6 KB)Acknowledgments
The authors would like to thank the patients and investigators at the following participating sites: Tokyo Women’s Medical University, Kanto Central Hospital, Juntendo University Shizuoka Hospital, Tokyo Medical University, Katsushika Medical Center, The Jikei University School of Medicine, and Juntendo University Urayasu Hospital. Writing and editorial assistance was provided by JL Croxford, PhD, and Mary Richardson, MSc, of Edanz Medical Writing, and funded by Kyowa Hakko Kirin Co., Ltd. Results from this manuscript were previously presented as a poster presentation at the XXIII World Congress of Neurology (WCN 2017), in Kyoto Japan on September 16–20, 2017.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Morris ME, Iansek R, Matyas TA, et al. The pathogenesis of gait hypokinesia in Parkinson’s disease. Brain. 1994;117(Pt 5):1169–1181.
- Okuma Y. Practical approach to freezing of gait in Parkinson’s disease. Pract Neurol. 2014;14(4):222–230.
- Kerr GK, Worringham CJ, Cole CH, et al. Predictors of future falls in Parkinson disease. Neurology. 2010;75(2):116–124.
- Okuma Y, Yanagisawa N. The clinical spectrum of freezing of gait in Parkinson’s disease. Mov Disord. 2008;23(Suppl 2):S426–S430.
- Nonnekes J, Snijders AH, Nutt JG, et al. Freezing of gait: a practical approach to management. Lancet Neurol. 2015;14(7):768–778.
- Devos D, Defebvre L, Boedet R. Dopaminergic and non-dopaminergic pharmacological hypotheses for gait disorders in Parkinson’s disease. Fundam Clin Pharmacol. 2010;24(4):407–421.
- Schiffmann SN, Vanderhaeghen JJ. Adenosine A2 receptors regulate the gene expression of striatopallidal and striatonigral neurons. J Neurosci. 1993;13(3):1080–1087.
- Kalia LV, Brotchie JM, Fox SH. Novel nondopaminergic targets for motor features of Parkinson’s disease: review of recent trials. Mov Disord. 2013;28(2):131–144.
- Henderson EJ, Lord SR, Brodie MA, et al. Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(3):249–258.
- Saki M, Yamada K, Koshimura E, et al. In vitro pharmacological profile of the A2A receptor antagonist istradefylline. Naunyn Schmiedebergs Arch Pharmacol. 2013;386(11):963–972.
- Rosin DL, Robeva A, Woodard RL, et al. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol. 1998;401(2):163–186.
- Mori A, LeWitt P, Jenner P. The story of Istradefylline – the first approved A2A antagonist for the treatment of Parkinson’s Disease. In: Morelli M, Simola N, Wardas J, editors. The adenosinergic system: a non-dopaminergic target in Parkinson’s disease. Switzerland: Springer International Publishing; 2015. p. 273–289.
- Fuxe K, Ferré S, Genedani S, et al. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92(1–2):210–217.
- Aoyama S, Kase H, Borrelli E. Rescue of locomotor impairment in dopamine D2 receptor-deficient mice by an adenosine A2A receptor antagonist. J Neurosci. 2000;20(15):5848–5852.
- Chen JF, Moratalla R, Impagnatiello F, et al. The role of the D2 dopamine receptor (D2R) in A2A adenosine receptor (A2AR)-mediated behavioral and cellular responses as revealed by A2A and D2 receptor knockout mice. Proc Natl Acad Sci USA. 2001;98(4):1970–1975.
- Mizuno Y, Hasegawa K, Kondo T, et al. Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease: a randomized, controlled study. Mov Disord. 2010;25(10):1437–1443.
- Mizuno Y, Kondo T., Japanese Istradefylline Study Group. Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Mov Disord. 2013;28(8):1138–1141.
- Hauser RA, Hubble JP, Truong DD, et al. Randomized trial of the adenosine A(2A) receptor antagonist istradefylline in advanced PD. Neurology. 2003;61(3):297–303.
- Kondo T, Mizuno Y, Japanese Istradefylline Study Group. A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin Neuropharmacol. 2015;38(2):41–46.
- LeWitt PA, Guttman M, Tetrud JW, et al. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005). Ann Neurol. 2008;63(3):295–302.
- Kitagawa M, Houzen H, Tashiro K. Effects of caffeine on the freezing of gait in Parkinson’s disease. Mov Disord. 2007;22(5):710–712.
- Matsuura K, Kajikawa H, Tabei KI, et al. The effectiveness of istradefylline for the treatment of gait deficits and sleepiness in patients with Parkinson’s disease. Neurosci Lett. 2018;662:158–161.
- Suzuki K, Miyamoto T, Miyamoto M, et al. Could istradefylline be a treatment option for postural abnormalities in mid-stage Parkinson’s disease? J Neurol Sci. 2018;385:131–133.
- Mitoma H, Yoneyama M, Orimo S. 24-hour recording of parkinsonian gait using a portable gait rhythmogram. Intern Med. 2010;49(22):2401–2408.
- Mori A, Shindou T. Modulation of GABAergic transmission in the striatopallidal system by adenosine A2A receptors: a potential mechanism for the antiparkinsonian effects of A2A antagonists. Neurology. 2003;61(11Suppl 6): S44–S48. Erratum in: Neurology. 2004;62(2):348.
- Hickey P, Stacy M. Adenosine A2A antagonists in Parkinson’s disease: what’s next? Curr Neurol Neurosci Rep. 2012;12(4):376–385.
- Postuma RB, Anang J, Pelletier A, et al. Caffeine as symptomatic treatment for Parkinson disease (Café-PD): a randomized trial. Neurology. 2017;89(17):1795–1803.
- Fredholm BB, Bättig K, Holmén J, et al. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51(1):83–133.
- Ochi M, Koga K, Kurokawa M, et al. Systemic administration of adenosine A(2A) receptor antagonist reverses increased GABA release in the globus pallidus of unilateral 6-hydroxydopamine-lesioned rats: a microdialysis study. Neuroscience. 2000;100(1):53–62.
- Mori A. Mode of action of adenosine A2A receptor antagonists as symptomatic treatment for Parkinson’s disease. In: Mori A, editor. International review of neurobiology. Adenosine receptors in neurology and psychiatry. Amsterdam, The Netherlands: Elsevier B.V.; 2014. p. 87–116.
- Black KJ, Koller JM, Campbell MC, et al. Quantification of indirect pathway inhibition by the adenosine A2a antagonist SYN115 in Parkinson disease. J Neurosci. 2010;30(48):16284–16292.
- Pinna A, Pontis S, Borsini F, et al. Adenosine A2A receptor antagonists improve deficits in initiation of movement and sensory motor integration in the unilateral 6-hydroxydopamine rat model of Parkinson’s disease. Synapse. 2007;61(8):606–614.
- Kadowaki HT, Kobayashi M, Mori A, et al. Effects of the adenosine A2A antagonist istradefylline on cognitive performance in rats with a 6-OHDA lesion in prefrontal cortex. Psychopharmacology (Berl). 2013;230(3):345–352.
- Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord. 2013;28(11):1483–1491.
- Moore O, Peretz C, Giladi N. Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait. Mov Disord. 2007;22(15):2192–2195.
