ABSTRACT
Background: There is a need for effective, safe and well-tolerated pharmacotherapies for patients with major depressive disorder (MDD) who have inadequate response to antidepressant treatments (ADTs). This analysis aimed to summarize the short-term efficacy and safety of adjunctive brexpiprazole in adults with MDD.
Research design and methods: A pooled analysis of data from the 6-week, randomized, double-blind treatment phases of four studies of adjunctive brexpiprazole 1–3 mg/day versus placebo in outpatients with MDD and inadequate response to ADTs (n = 1,853). Efficacy was measured by Montgomery–Åsberg Depression Rating Scale (MADRS) scores, and safety by treatment-emergent adverse events (TEAEs).
Results: ADT + brexpiprazole 2–3 mg/day showed greater improvement in MADRS Total score from baseline to Week 6 than ADT + placebo (least squares mean difference: −2.15; confidence limits: −2.82, −1.48; p < 0.0001; Cohen’s d effect size: 0.33). TEAEs with incidence ≥5% with ADT + brexpiprazole 1–3 mg/day were akathisia (8.0% versus 2.6% with ADT + placebo), headache (5.8% versus 6.0%), and weight increased (5.8% versus 1.6%).
Conclusions: Adjunctive brexpiprazole is an efficacious and well-tolerated treatment option for adult patients with MDD and inadequate response to ADTs. Study limitations included a lack of active comparator.
1. Introduction
Despite the availability of different classes of antidepressants, many patients with major depressive disorder (MDD) fail to respond to antidepressant treatment (ADT) [Citation1]. Patients with inadequate response to ADT experience a prolonged loss of quality of life and functioning, including work/school, social, and family life functioning [Citation2,Citation3]. Inadequate response to ADT also imposes a significant burden on society, being associated with work productivity loss and unemployment, and increased use of healthcare resources such as emergency departments and hospital admissions [Citation4].
Treatment options for patients with inadequate response to ADT include increasing the dose, switching to another ADT, adding a second ADT, or initiating adjunctive therapy with another medication such as an atypical antipsychotic [Citation5,Citation6]. A review of the evidence for antidepressant augmentation, combination, and switching strategies concluded that current data unequivocally support the use of certain atypical antipsychotics as a first-line option for augmentation in patients who failed first-line ADT [Citation5]. A meta-analysis of 14 studies (N = 3,549) showed that adjunctive treatment with atypical antipsychotics increased the likelihood of antidepressant response and remission, with odds ratios of 1.61 and 1.77, respectively [Citation7]. However, adjunctive antipsychotic treatment was linked to several adverse events, including activating, sedating, and metabolic issues [Citation7]. Thus, for patients with inadequate response to ADT, there is a need for an effective, safe and well-tolerated atypical antipsychotic to help patients gain the most benefit from their ADT.
Brexpiprazole is a serotonin–dopamine activity modulator that acts as a partial agonist at serotonin 5-HT1A and dopamine D2 receptors, and as an antagonist at serotonin 5-HT2A and noradrenaline α1B/α2C receptors, all with subnanomolar affinity [Citation8]. The efficacy and safety of brexpiprazole as adjunctive treatment to ADT over 6 weeks have been demonstrated in four Phase 3 studies in adult patients with MDD and inadequate response to ADTs [Citation9–Citation12]. Brexpiprazole is approved in various countries and regions, including the United States, Canada, Australia, Japan, and the European Union, for the treatment of schizophrenia in adults. Brexpiprazole is also approved in the United States, Canada, and other countries as an adjunctive therapy to antidepressants for the treatment of MDD in adults. The aim of this analysis was to summarize the short-term efficacy and safety of adjunctive brexpiprazole in adults with MDD, based on pooled data from all four short-term Phase 3 studies.
2. Patients and methods
2.1. Study design and patients
This pooled analysis included four short-term, randomized, double-blind, placebo-controlled, Phase 3 studies of adjunctive brexpiprazole in patients with MDD and inadequate response to ADTs. The studies, hereafter referred to as ‘Pyxis’, ‘Polaris’, ‘Sirius’, and ‘Delphinus’, were conducted at multiple sites in North America (Canada, United States) and Europe (France, Germany, Hungary, Poland, Romania, Russia, Serbia, Slovakia, Ukraine). All studies were conducted in accordance with the International Conference on Harmonisation Good Clinical Practice Guideline, and local regulatory requirements. The study protocols were approved by relevant institutional review boards and independent ethics committees (listed in the online supplement to this article). All patients provided written informed consent prior to the start of the studies.
An overview of the included studies is presented in . Each of the studies had a similar design. For a full description of the study designs, patients and outcomes, please refer to the individual study publications [Citation9–Citation12].
Table 1. Short-term phase 3 clinical studies of brexpiprazole for the adjunctive treatment of adults with MDD.
In brief, the studies enrolled male or female outpatients aged 18–65 years with a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) [Citation13] diagnosis of single or recurrent, nonpsychotic MDD; a current depressive episode of ≥8 weeks in duration; and an inadequate response to an adequate trial of 1–3 prior ADTs during the current episode (defined as <50% improved according to the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire [Citation14]). At screening and at the start of prospective treatment, patients in Pyxis, Polaris, and Sirius were required to have a 17-item Hamilton Depression Rating Scale (HAM-D17) [Citation15,Citation16] Total score of ≥18, and patients in Delphinus were required to have a Montgomery–Åsberg Depression Rating Scale (MADRS) [Citation17] Total score of ≥26. Key exclusion criteria were treatment with adjunctive antipsychotic medication during the current episode, presenting with suicidal ideation or behavior, substance abuse or dependence, hallucinations or delusions during the current episode, meeting DSM-IV-TR criteria for specified Axis I disorders, or hospitalization within 4 weeks of screening for the current episode.
After a 7–28 day screening phase, patients entered an 8-week (in Delphinus, an 8–10 week) prospective treatment phase in which they received an investigator-determined, open-label ADT, together with single-blind placebo (in Delphinus, double-blind placebo). Patients received the same ADT for the duration of the study. During this phase, patients were assessed for inadequate response to prospective ADT, based on Clinical Global Impressions – Improvement (CGI-I) [Citation18], HAM-D17 and/or MADRS Total score thresholds (see for the definition in each study). In Pyxis and Polaris, the definition of inadequate response was amended during the study so that only patients with consistent inadequate response throughout the prospective treatment phase were randomized. Patients who met the criteria for inadequate response were randomized to 6 weeks of double-blind treatment with adjunctive brexpiprazole or placebo (or, in Delphinus, quetiapine extended-release [XR]; not included in the present analyses). Patients received fixed doses of brexpiprazole in Pyxis (2 mg/day), Polaris (1 mg/day or 3 mg/day), and Sirius (2 mg/day), and flexible doses of brexpiprazole in Delphinus (2–3 mg/day). In the fixed-dose studies, brexpiprazole was titrated as follows: first week, 0.5 mg/day; second week, 1 mg/day; third week onwards, allocated dose. Brexpiprazole was also titrated in Delphinus: first week, 1 mg/day; second week, 2 mg/day; third week onwards, 2–3 mg/day.
Patients meeting any of the following criteria were withdrawn from the trial: an adverse event/concurrent illness/laboratory test abnormality that warranted withdrawal in the investigator’s opinion, use of prohibited concomitant medication, noncompliance with study medication, patient/investigator/sponsor/regulatory authority request, pregnancy, unable to tolerate the minimum therapeutic dose of prospective ADT, unable to tolerate the assigned dose of brexpiprazole/placebo, or lost to follow-up.
2.2. Outcome measures
The MADRS was the primary efficacy measure in each of the four studies. Clinical Global Impressions – Severity of illness (CGI-S) [Citation18] was included as a secondary efficacy measure. During the randomized phase, the MADRS and CGI-S were administered at baseline (prior to dosing at the randomization visit) and at weekly intervals (in Delphinus, at 2-weekly intervals).
Safety was assessed by the incidence of treatment-emergent adverse events (TEAEs), and by measurements of body weight and laboratory parameters (lipids, glucose, and prolactin).
2.3. Data analysis
Efficacy analyses comprised the mean changes from baseline in MADRS Total score, 6-item MADRS core symptom subscale (MADRS6) score, MADRS item scores, and CGI-S score; and the MADRS response and remission rates. The baseline value was defined as the last value obtained prior to randomization. The MADRS6 was derived from the MADRS using the following six items: apparent sadness, reported sadness, inner tension, lassitude, inability to feel, and pessimistic thoughts [Citation19]. MADRS response was defined as a ≥50% reduction from baseline in MADRS Total score, and MADRS remission was defined as a MADRS Total score of ≤10 points together with a ≥50% reduction from baseline. Efficacy analyses were performed in the efficacy sample, defined as all randomized patients (based on inadequate response criteria per final protocols) who received at least one dose of double-blind medication and who had a baseline and at least one post-baseline MADRS Total evaluation in the randomized treatment phase. Two brexpiprazole doses were investigated in pooled efficacy analyses: 2–3 mg/day (the recommended dose range for MDD in the United States [Citation20]), and 2 mg/day (the recommended dose for MDD in some other countries). The pooled 2–3 mg/day analyses included data for 2 mg/day from Pyxis, 3 mg/day from Polaris, 2 mg/day from Sirius, and 2–3 mg/day from Delphinus, versus placebo data from all four studies. The pooled 2 mg/day analyses included data for 2 mg/day from Pyxis, 2 mg/day from Sirius, and those patients from Delphinus who received only 2 mg/day during the flexible-dose phase, versus placebo data from Pyxis, Sirius, and Delphinus. Least squares (LS) mean changes from baseline, differences from placebo (with 95% confidence limits [CLs]), and p-values were calculated using mixed model for repeated measures, with terms of treatment, site nested within study, visit, treatment by visit and baseline by visit interaction. Cohen’s d effect sizes were also calculated. MADRS response and remission analyses used the Cochran–Mantel–Haenszel general association test, controlling for study site, with last observations carried forward. All analyses were performed at the 5% level using two-sided tests of significance with no correction for multiple comparisons.
Safety analyses were performed in the safety sample, defined as all randomized patients (based on inadequate response criteria from before the protocol amendments in Pyxis and Polaris, to capture all patients who took the drug) who received at least one dose of double-blind medication in the randomized treatment phase. Brexpiprazole doses of 1–3 mg/day (i.e. all patients receiving brexpiprazole) were pooled for the safety analyses, as were placebo data. In addition, to evaluate a potential dose effect on the incidence of TEAEs, pooled brexpiprazole 1 mg/day, 2 mg/day, and 3 mg/day groups were created using data from the fixed-dose studies. Descriptive statistics were used to present the safety data. In addition, numbers needed to treat for one additional patient to be harmed (NNHs) were calculated based on the risk difference between ADT + brexpiprazole and ADT + placebo for the incidence of certain TEAEs of interest. The 95% CLs for NNHs were calculated as the inverse of the 95% CLs for the risk difference. In Delphinus, to ensure comprehensive blinding, laboratory parameters were not measured at randomization or Week 6, and thus data from Delphinus were not included in the laboratory parameter analyses.
Analyses were performed using SAS® 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Patients
Across the four studies, a total of 1,853 patients were randomized to ADT + brexpiprazole 1–3 mg (n = 1,033) or ADT + placebo (n = 820). An additional 100 patients were randomized to ADT + quetiapine XR; these patients are not included in the present analyses. The proportion of patients who completed treatment was similar in the ADT + brexpiprazole (n = 948; 91.8%) and ADT + placebo (n = 768; 93.7%) groups. The most frequently reported reasons for discontinuation were that the patient withdrew consent (ADT + brexpiprazole: n = 29, 2.8%; ADT + placebo: n = 25, 3.0%), adverse events (n = 24, 2.3%; n = 6, 0.7%), and that the patient met withdrawal criteria (n = 14, 1.4%; n = 7, 0.9%). For the latter category, the specific withdrawal criteria were a positive urine drug screen (ADT + brexpiprazole: n = 10; ADT + placebo: n = 5), pregnancy (n = 2; n = 1), use of restricted medication (n = 2; n = 0), and noncompliance with study medication (n = 0; n = 1).
Two randomized patients did not receive study medication. Thus, for the present analyses, the pooled safety sample comprised 1,032 patients in the ADT + brexpiprazole 1–3 mg group and 819 patients in the ADT + placebo group. At the dose ranges of interest, and including only those patients who met inadequate response criteria per final protocols and who had a post-baseline MADRS Total evaluation, the pooled efficacy sample comprised A) 770 patients in the ADT + brexpiprazole 2–3 mg group and 788 patients in the ADT + placebo group, and B) 486 patients in the ADT + brexpiprazole 2 mg group and 585 patients in the ADT + placebo group.
Baseline demographic and clinical characteristics were similar between treatment groups (). The mean age was 44 years, and 69.9% of patients were female. On average, patients were moderately ill at baseline, with a mean CGI-S score of 4.2.
Table 2. Baseline demographic and clinical characteristics (randomized sample).
3.2. Efficacy
3.2.1. Individual studies
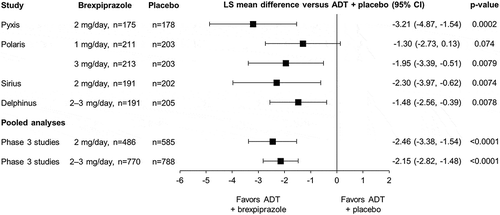
Across the four individual studies, ADT + brexpiprazole 2 mg (Pyxis, Sirius), 3 mg (Polaris), and 2–3 mg (Delphinus) showed greater improvement in depressive symptoms from baseline to Week 6 than ADT + placebo, as measured by change in MADRS Total score (all p < 0.01) (). The Cohen’s d effect sizes were 0.42 in Pyxis, 0.27 in Polaris, 0.28 in Sirius, and 0.28 in Delphinus. The ADT + brexpiprazole 1 mg group (Polaris) showed a numerical benefit only (p = 0.074; Cohen’s d: 0.18), and is not discussed further in this section.
Figure 1. Estimated treatment effect for ADT + brexpiprazole: mean change in MADRS Total score from baseline to Week 6 (efficacy sample).
Mixed model for repeated measures. ADT: antidepressant treatment; CI: confidence interval; LS: least squares; MADRS: Montgomery–Åsberg Depression Rating Scale.
ADT + brexpiprazole showed benefits over placebo on MADRS6 score change from baseline to Week 6 in each study, with Cohen’s d effect sizes of 0.44 in Pyxis (p < 0.0001), 0.25 in Polaris (p = 0.015), 0.26 in Sirius (p = 0.012), and 0.30 in Delphinus (p = 0.0043). The Cohen’s d effect sizes for CGI-S score change versus placebo at Week 6 were 0.38 in Pyxis (p = 0.0006), 0.23 in Polaris (p = 0.021), 0.19 in Sirius (p = 0.071), and 0.22 in Delphinus (p = 0.035).
3.2.2. Pooled 2–3 mg/day analysis
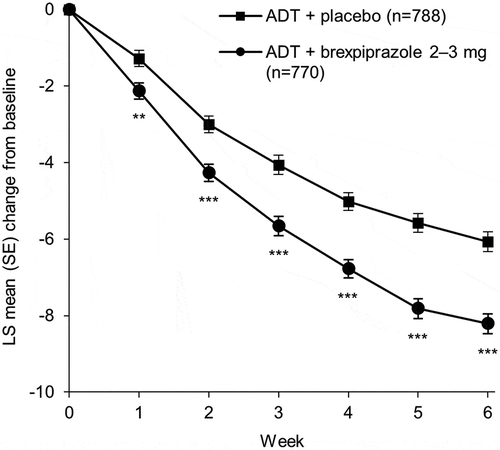
In the first pooled analysis, ADT + brexpiprazole 2–3 mg showed greater improvement in MADRS Total score from baseline to Week 6 than ADT + placebo, with a Cohen’s d effect size of 0.33 (p < 0.0001) (; ). Benefits over placebo (p < 0.01) were observed at all visits ().
Table 3. Effects of brexpiprazole and placebo as adjunct to antidepressant treatment on efficacy endpoints at Week 6 (efficacy sample).
Figure 2. Mean change in MADRS Total score from baseline to Week 6, pooled analysis (efficacy sample).
**p < 0.01, ***p < 0.001 versus ADT + placebo; mixed model for repeated measures. Mean (SD) MADRS Total score at baseline: ADT + placebo, 26.3 (5.6); ADT + brexpiprazole 2–3 mg, 26.4 (5.5). ADT: antidepressant treatment; LS: least squares; MADRS: Montgomery–Åsberg Depression Rating Scale; SD: standard deviation; SE: standard error.
Benefits were also seen for ADT + brexpiprazole 2–3 mg versus ADT + placebo at Week 6 on core depressive symptoms (MADRS6; Cohen’s d: 0.30; p < 0.0001) and severity of illness (CGI-S; Cohen’s d: 0.24; p < 0.0001) ()). Benefits over ADT + placebo (p < 0.05) were observed for eight out of ten MADRS items, including all six core items ()). MADRS response was more common with ADT + brexpiprazole 2–3 mg than ADT + placebo (p = 0.0007; )). MADRS remission was also more likely with ADT + brexpiprazole 2–3 mg (p = 0.023; )).
3.2.3. Pooled 2 mg/day analysis
In the second pooled analysis, ADT + brexpiprazole 2 mg showed greater improvement in MADRS Total score from baseline to Week 6 than ADT + placebo (Cohen’s d: 0.33; p < 0.0001) (; )). Benefits were also seen for core depressive symptoms, severity of illness, MADRS response, and MADRS remission ()).
3.3. Safety
The proportion of patients who experienced at least one TEAE during treatment with ADT + brexpiprazole 1–3 mg was 57.7%, compared with 48.7% for those receiving ADT + placebo. TEAEs with an incidence of ≥5% in the ADT + brexpiprazole 1–3 mg group were akathisia (NNH: 19), headache (NNH: −591), and weight increased (NNH: 24) (). The incidence of akathisia increased with brexpiprazole dose, from 4.4% with 1 mg to 7.9% with 2 mg and 13.5% with 3 mg. EPS-related TEAEs other than akathisia were reported by 61 patients (5.9%) in the ADT + brexpiprazole 1–3 mg group, with no dose effect, and by 20 patients (2.4%) in the ADT + placebo group. The discontinuation rate due to TEAEs was 2.2% with ADT + brexpiprazole 1–3 mg and 0.6% with ADT + placebo. TEAEs leading to discontinuation in more than one patient receiving ADT + brexpiprazole 1–3 mg were akathisia, 7 patients (0.7%); anxiety, 2 patients (0.2%); and restlessness, 2 patients (0.2%).
Table 4. Treatment-emergent adverse events (safety sample).
The mean (standard deviation) increase in body weight from baseline to Week 6 was 1.4 (2.1) kg in the ADT + brexpiprazole 1–3 mg group (n = 947), and 0.3 (1.7) kg in the ADT + placebo group (n = 767). The proportion of patients with ≥7% weight increase at any time post-baseline was 4.3% (44/1,025) in the ADT + brexpiprazole 1–3 mg group and 1.8% (15/814) in the ADT + placebo group. The proportions with ≥7% weight decrease at any time post-baseline were 0.2% (2/1,025) for ADT + brexpiprazole 1–3 mg and 0.4% (3/814) for ADT + placebo. Effects on lipids, glucose, and prolactin were small and not considered to be clinically meaningful ().
Table 5. Mean change in laboratory parameters from baseline to Week 6 (safety sample).
4. Discussion
Supporting the findings of four individual short-term studies [Citation9–Citation12], the present analysis of pooled data shows that adjunctive brexpiprazole 2–3 mg/day improves symptoms of depression compared with adjunctive placebo in patients with MDD and an inadequate response to ADTs. The magnitude of the benefit for adjunctive brexpiprazole over adjunctive placebo was 2.15 points, which is above the minimum clinically important difference for MADRS Total score (estimated at around 2 points) [Citation21,Citation22]. This benefit corresponds to a standardized effect size of 0.33 based on Cohen’s d, which can be interpreted as a small-to-medium benefit [Citation23]. In a prior meta-analysis of adjunctive atypical antipsychotics in MDD, aripiprazole, olanzapine–fluoxetine combination (OFC), quetiapine, and risperidone also showed small-to-medium standardized effect sizes on depressive symptoms (0.31 on average), with no significant differences between agents [Citation7].
Adjunctive brexpiprazole 2 mg/day also improved symptoms of depression compared with adjunctive placebo in patients with MDD and an inadequate response to ADTs. On average, there was little difference in efficacy between the 2–3 mg and 2 mg doses. Individual patients may benefit from the 3 mg dose, however, and the option of increasing the dose to 3 mg gives clinicians more flexibility in the treatment of their patients, in regions where this dose is permitted.
Cohen’s d effect sizes for improvement in MADRS Total score were relatively consistent across pooled analyses (both 0.33) and three of the individual studies (0.27–0.28), although the effect size was higher in Pyxis (0.42).
Benefits were also observed on the CGI-S and for response and remission rates, providing clinically meaningful endpoints in support of adjunctive brexpiprazole in MDD. Of note, adjunctive brexpiprazole showed a benefit on the core depressive symptoms scale (MADRS6), and was generally associated with greater improvement on core symptoms (apparent sadness, reported sadness, inner tension, lassitude, inability to feel, and pessimistic thoughts) than on non-core symptoms. This suggests that brexpiprazole exerts its effects in MDD by treating the core symptoms of the disease [Citation24]. Adjunctive aripiprazole and OFC have also shown benefits on HAM-D and MADRS core depressive symptom subscales, respectively, in post hoc analyses of randomized controlled trials [Citation25,Citation26].
In prior pooled analyses of randomized, controlled trials in MDD, adjunctive brexpiprazole was efficacious in subgroups of patients with anxious distress, anxious depression, and irritability at baseline [Citation27,Citation28]. These pooled analyses were supported by the results of exploratory open-label studies [Citation29,Citation30]. Furthermore, an exploratory open-label study found that physiologic measures of sleep and daytime alertness were improved in patients with sleep disturbances treated with adjunctive brexpiprazole [Citation31]. Elderly patients (≥65 years) with MDD have shown improvements in depressive symptoms, social functioning, and quality of life during open-label treatment with adjunctive brexpiprazole [Citation32]. The benefits of brexpiprazole in anxious, irritated, and sleep-disturbed patients is consistent with its partial agonism at 5-HT1A and D2/D3 receptors, and its antagonism at 5-HT2A and α1 receptors [Citation8,Citation30,Citation31]. In the future, factor analysis of symptom clusters might be warranted to better understand the therapeutic specificity benefits of brexpiprazole, thereby assisting physicians in choosing the best treatment for different subsets of patients.
Although patient functioning was not investigated in the present analysis, a prior pooled analysis of randomized, controlled trials in MDD showed that adjunctive brexpiprazole improved functioning over 6 weeks versus placebo, with specific benefits in the domains of social and family life [Citation33]. Furthermore, an open-label study of adjunctive brexpiprazole in young patients (18–35 years) with MDD who were in a work or school environment showed improvements from baseline in multiple domains of functioning, including work/school functioning, suggesting that brexpiprazole has the potential to reduce the socioeconomic burden of depression [Citation34].
The long-term efficacy and safety of adjunctive brexpiprazole in MDD have been investigated in an open-label study [Citation35] and a randomized, double-blind, placebo-controlled study [Citation36]. The open-label study, which enrolled almost 3,000 patients, showed that treatment with adjunctive brexpiprazole 0.5–3 mg/day in MDD was generally well tolerated for up to 52 weeks (weight increase was the only TEAE with incidence ≥10%), and was associated with continued improvement in efficacy measures and functional outcomes. In the 24-week, randomized study, which used an untried study design, adjunctive brexpiprazole 1–3 mg/day did not differentiate from adjunctive placebo on the primary endpoint of full remission. As this study had a novel design, a number of design elements may have contributed to the study results.
Atypical antipsychotics as a group, while being effective augmentation strategies in MDD, are associated with tolerability concerns. A meta-analysis of randomized controlled trials of adjunctive antipsychotics showed that aripiprazole was associated with the greatest risk of akathisia, quetiapine was associated with the greatest risk of sedation, and OFC was associated with the greatest risk of abnormal metabolic laboratory results and weight gain [Citation7]. The present pooled analysis showed that adjunctive brexpiprazole 1–3 mg was associated with a low discontinuation rate due to TEAEs (2.2%). Akathisia and sedating adverse events had a relatively low incidence (8.0% for akathisia and <5% for each sedating TEAE), although akathisia adverse events appeared to be dose-related. NNH can be used to gain an understanding of the frequency of seeing an adverse event; an NNH below 10 is considered likely to be more clinically relevant [Citation37]. The NNH for akathisia as a TEAE was 19 with brexpiprazole; a review of other adjunctive antipsychotics found corresponding NNHs of 5 for aripiprazole and 91 for quetiapine XR [Citation38]. NNHs for somnolence and sedation with brexpiprazole were 28 and −339, respectively; the review found corresponding values of 5 and 10 for quetiapine XR, 10 and 18 for OFC, and 43 and 42 for aripiprazole [Citation38]. Thus, brexpiprazole appears to be less activating than aripiprazole and less sedating than quetiapine XR and OFC. The mean weight gain with brexpiprazole was 1.4 kg over 6 weeks, and there was no indication of an adverse effect on metabolic parameters or prolactin. Overall, brexpiprazole appears to be well tolerated by patients with MDD and inadequate response to ADTs.
Although limited by its post hoc nature, this analysis confirmed the results of the individual brexpiprazole studies in a large sample of almost 2,000 patients. By increasing the sample size compared with the individual studies, these pooled analyses increase precision and thereby increase confidence in the efficacy and safety results. The analysis is limited by the lack of an adjunctive active comparator, such as another approved adjunctive antipsychotic. No correction was made for multiple comparisons. Each of the included studies was funded by the pharmaceutical industry, and independent studies are lacking. Finally, as with all clinical trials, the patient selection criteria (outpatients with no psychotic symptoms and low suicide risk) and restrictions regarding concomitant medications and comorbidities mean that the results are of limited generalizability to a broader patient population.
5. Conclusion
Adjunctive brexpiprazole is an efficacious and well-tolerated treatment option for adult patients with MDD and inadequate response to ADTs.
Declaration of interest
ME Thase reports the following relationships over the past 3 years: He has served as an advisor/consultant for Acadia, Alkermes, Allergan (Forest, Naurex), AstraZeneca, Cerecor, Eli Lilly and Company, Fabre-Kramer, Gerson Lehrman Group, Guidepoint Global, Johnson & Johnson (Janssen, Ortho-McNeil), Lundbeck A/S, MedAvante, Merck & Co, Moksha8, Nestlé (Pamlab), Novartis, Otsuka, Pfizer, Shire, Sunovion and Takeda. He has received grant support from Acadia, the Agency for Healthcare Research and Quality, Alkermes, Assurex, Avanir, Forest Laboratories, Intra-Cellular, Janssen Pharmaceuticals, the National Institute of Mental Health, Otsuka, PCORI and Takeda. He also has equity holdings wth MedAvante Inc and receives royalties from American Psychiatric Press, Guilford Publications, Herald House and W.W. Norton & Company Inc. Finally, he declares that his spouse is employed by Peloton Advantage, which does business with AstraZeneca, GlaxoSmithKline, and Pfizer. P Zhang, C Weiss and M Hobart are full-time employees of Otsuka Pharmaceutical Development & Commercialization Inc while S Rasmussen Meehan is a full-time employee of H. Lundbeck A/S. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved with the concept and design, the analysis and interpretation, the drafting of the paper and revising it critically for intellectual content. The authors also approved the final manuscript and agree to be accountable for all aspects of the work.
Acknowledgements
Writing support was provided by Chris Watling, PhD, assisted by his colleagues at Cambridge Medical Communication Ltd (Cambridge, UK), and funded by Otsuka Pharmaceutical Development & Commercialization Inc. and H. Lundbeck A/S.
Additional information
Funding
References
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917.
- Mauskopf JA, Simon GE, Kalsekar A, et al. Nonresponse, partial response, and failure to achieve remission: humanistic and cost burden in major depressive disorder. Depress Anxiety. 2009;26(1):83–97.
- Trivedi MH, Corey-Lisle PK, Guo Z, et al. Remission, response without remission, and nonresponse in major depressive disorder: impact on functioning. Int Clin Psychopharmacol. 2009;24(3):133–138.
- Knoth RL, Bolge SC, Kim E, et al. Effect of inadequate response to treatment in patients with depression. Am J Manag Care. 2010;16(8):e188–e196.
- Connolly KR, Thase ME. If at first you don’t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs. 2011;71(1):43–64.
- Patkar AA, Pae CU. Atypical antipsychotic augmentation strategies in the context of guideline-based care for the treatment of major depressive disorder. CNS Drugs. 2013;27(Suppl 1):S29–S37.
- Spielmans GI, Berman MI, Linardatos E, et al. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10(3):e1001403.
- Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin–dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589–604.
- Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a Phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76(9):1224–1231.
- Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76(9):1232–1240.
- Hobart M, Skuban A, Zhang P, et al. A randomized, placebo-controlled study of the efficacy and safety of fixed-dose brexpiprazole 2 mg/d as adjunctive treatment of adults with major depressive disorder. J Clin Psychiatry. 2018;79(4):17m12058.
- Hobart M, Skuban A, Zhang P, et al. Efficacy and safety of flexibly dosed brexpiprazole for the adjunctive treatment of major depressive disorder: a randomized, active-referenced, placebo-controlled study. Curr Med Res Opin. 2018;34(4):633–642.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed text revision. Washington (DC): American Psychiatric Association; 2000.
- Chandler GM, Iosifescu DV, Pollack MH, et al. Validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322–325.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296.
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389.
- Guy W. ECDEU assessment manual for psychopharmacology, revised. Rockville (MD): National Institute of Mental Health; 1976.
- Bech P, Tanghøj P, Andersen HF, et al. Citalopram dose-response revisited using an alternative psychometric approach to evaluate clinical effects of four fixed citalopram doses compared to placebo in patients with major depression. Psychopharmacology (Berl). 2002;163(1):20–25.
- Rexulti® (brexpiprazole) tablets, for oral use. Prescribing information; 2018 [ cited 2019 Apr 26]. Available from: https://www.otsuka-us.com/media/static/Rexulti-PI.pdf
- Duru G, Fantino B. The clinical relevance of changes in the Montgomery–Åsberg Depression Rating Scale using the minimum clinically important difference approach. Curr Med Res Opin. 2008;24(5):1329–1335.
- Montgomery SA, Möller HJ. Is the significant superiority of escitalopram compared with other antidepressants clinically relevant? Int Clin Psychopharmacol. 2009;24(3):111–118.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale (NJ): Lawrence Erlbaum Associates; 1988.
- Nelson JC, Weiller E, Zhang P, et al. Efficacy of adjunctive brexpiprazole on the core symptoms of major depressive disorder: a post hoc analysis of two pooled clinical studies. J Affect Disord. 2018;227:103–108.
- Nelson JC, Mankoski R, Baker RA, et al. Effects of aripiprazole adjunctive to standard antidepressant treatment on the core symptoms of depression: a post-hoc, pooled analysis of two large, placebo-controlled studies. J Affect Disord. 2010;120(1–3):133–140.
- Tohen M, Case M, Trivedi MH, et al. Olanzapine/fluoxetine combination in patients with treatment-resistant depression: rapid onset of therapeutic response and its predictive value for subsequent overall response in a pooled analysis of 5 studies. J Clin Psychiatry. 2010;71(4):451–462.
- Thase ME, Weiller E, Zhang P, et al. Adjunctive brexpiprazole in patients with major depressive disorder and anxiety symptoms: post hoc analyses of three placebo-controlled studies. Neuropsychiatr Dis Treat. 2018;15:37–45.
- Fava M, Weiller E, Zhang P, et al. Efficacy of brexpiprazole as adjunctive treatment in major depressive disorder with irritability: post hoc analysis of 2 pivotal clinical studies. J Clin Psychopharmacol. 2017;37(2):276–278.
- Davis LL, Ota A, Perry P, et al. Adjunctive brexpiprazole in patients with major depressive disorder and anxiety symptoms: an exploratory study. Brain Behav. 2016;6(10):e00520.
- Fava M, Ménard F, Davidsen CK, et al. Adjunctive brexpiprazole in patients with major depressive disorder and irritability: an exploratory study. J Clin Psychiatry. 2016;77(12):1695–1701.
- Krystal AD, Mittoux A, Meisels P, et al. Effects of adjunctive brexpiprazole on sleep disturbances in patients with major depressive disorder: an open-label, flexible-dose, exploratory study. Prim Care Companion CNS Disord. 2016;18(5). DOI:10.4088/PCC.15m01914
- Lepola U, Hefting N, Zhang D, et al. Adjunctive brexpiprazole for elderly patients with major depressive disorder: an open-label, long-term safety and tolerability study. Int J Geriatr Psychiatry. 2018;33(10):1403–1410.
- Hobart M, Zhang P, Weiss C, et al. Adjunctive brexpiprazole and functioning in major depressive disorder: a pooled analysis of six randomized studies using the Sheehan Disability Scale. Int J Neuropsychopharmacol. 2019;22(3):173–179.
- Weisler RH, Ota A, Tsuneyoshi K, et al. Brexpiprazole as an adjunctive treatment in young adults with major depressive disorder who are in a school or work environment. J Affect Disord. 2016;204:40–47.
- Hobart M, Zhang P, Skuban A, et al. A long-term, open-label study to evaluate the safety and tolerability of brexpiprazole as adjunctive therapy in adults with major depressive disorder. J Clin Psychopharmacol. 2019;39(3):203–209.
- Bauer M, Hefting N, Lindsten A, et al. A randomised, placebo-controlled 24-week study evaluating adjunctive brexpiprazole in patients with major depressive disorder. Acta Neuropsychiatr. 2019;31(1):27–35.
- Citrome L, Ketter TA. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract. 2013;67(5):407–411.
- Citrome L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol. 2017;37(2):138–147.
