ABSTRACT
Background: Vildagliptin is a dipeptidyl peptidase-4 inhibitor that reduces glycemia in patients with type 2 diabetes mellitus (T2DM). When approved in 2013, data on vildagliptin combined with >750 mg/day metformin in Japanese patients were limited. There is a need to confirm the safety and efficacy of vildagliptin in combination with oral antidiabetic drugs (OADs).
Research design and methods: This 52-week post-marketing surveillance (PMS) observational study in Japanese T2DM patients evaluated the safety and efficacy of vildagliptin in combination with OADs including high-dose metformin or insulin but excluding combination with sulfonylureas alone.
Results: During this survey of 3006 Japanese T2DM patients, 13.61% of patients experienced adverse events (AEs) and 2.20% reported a serious AE (SAE). The frequency of AEs/SAEs was similar when in combination with biguanides (12.93%/1.46%), metformin ≥1000 mg/day (12.92%/1.22%), metformin <1000 mg/day (12.62%/1.54%), thiazolidine derivatives (16.71%/2.86%), α-glucosidase inhibitors (13.18%/1.90%), rapid-acting insulin secretagogues (glinides) (20.41%/5.71%), or insulin (15.87%/2.47%). The mean ± SD changes from baseline at endpoint in glycated hemoglobin and fasting blood glucose were −0.76 ± 1.27% and −23.3 ± 57.3 mg/dL, respectively, and these changes were consistent, regardless of concomitant OAD.
Conclusions: Long-term vildagliptin combination therapy is safe and effective in Japanese T2DM patients in real-world settings.
1. Introduction
Vildagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor that enhances incretin hormone activity, sustains insulin levels, and reduces glycemia in type 2 diabetes mellitus (T2DM) [Citation1,Citation2]. Several previous clinical trials have shown that vildagliptin monotherapy and add-on therapy are tolerable and effective in patients with poor glycemic control [Citation3–Citation7]. Vildagliptin (Equa® [Novartis] tablets, 50 mg) was approved for the treatment of T2DM in February 2013, after the initial indication of combination therapy with sulfonylureas (SUs) was approved in January 2010 in Japan. However, at the time of the 2013 approval, insufficient Japanese data regarding vildagliptin combination therapy with high-dose metformin (Met; >750 mg/day) were available to inform treatment decisions. In addition, there was a need for additional safety and efficacy data related to the combination of vildagliptin with other oral antidiabetic drugs (OADs) such as biguanides (BGs), thiazolidine derivatives (TZDs), α-glucosidase inhibitors (αGIs), and rapid-acting insulin secretagogues (glinides), or insulin.
Before the indication of vildagliptin for T2DM in Japan, the only published vildagliptin combination study was a 52-week Japanese clinical study that investigated the safety of combination therapy with Met, TZDs, αGIs, or glinides [Citation8]. In that study, 58 patients were treated with Met, of which 27 patients received Met at doses of 500 mg/day, 29 patients received 750 mg/day, and only 2 patients received ≥1000 mg/day. Notably, despite the wide range of approved dosages for Met, few patients in the above study received ≥1000 mg/day [Citation8]. The global EDGE study previously demonstrated the efficacy and tolerability of second-line therapy with vildagliptin in T2DM; however, this global study did not focus on Japanese patients [Citation9].
Therefore, this 52-week post-marketing surveillance (PMS) aimed to confirm the safety and efficacy of vildagliptin in Japanese patients by evaluating the long-term safety and efficacy profiles of vildagliptin used in combination with OADs including high-dose Met or insulin but excluding SU monotherapy, who were first prescribed vildagliptin in the real-world setting.
2. Patients and methods
2.1. Patients
Patients were registered from April 2013 to March 2015 across 479 sites in Japan. Patients were included if they had never been prescribed vildagliptin for T2DM. Additionally, to be included in the present study, patients had to be receiving vildagliptin in combination with non-SU OADs or insulin. Vildagliptin was prescribed and administered in accordance with the package insert [Citation10]. Patients were orally administered 50 mg of vildagliptin in the morning and 50 mg in the evening before or after a meal. Dosing could be reduced to a single 50-mg dose in the morning if the patient’s condition required it.
This study was conducted in accordance with the Japanese Good Post-marketing Study Practice (GPSP) regulations.
2.2. Study design
In this non-interventional, single-cohort, observational study, patients were centrally registered within 2 weeks of beginning vildagliptin therapy, and data were collected via an electronic data capture system. The planned sample size was 3000 patients and the observation period was 1 year (52 weeks) from the start of vildagliptin administration. The investigator filled out the case report form (CRF) on the electronic data capture system for each registered patient and submitted it at the end of the observation period (12 months) or within 3 months if the patient discontinued the study. The clinical course and laboratory parameters were measured during routine consultations, wherever possible, with no discrimination between outpatient visits or hospital inpatient visits. The observed values and the presence/absence of changes constituting adverse events (AEs) were recorded at or within 4 weeks before the date of treatment initiation, and at Week 12 (± 6 weeks), Week 24 (± 6 weeks), Week 36 (−6/+8 weeks), Week 52 (−8/+6 weeks), or at the time of discontinuation.
2.3. Study items
2.3.1. Demographic and treatment details
The investigator recorded background variables and data, such as sex, age, duration of illness, medical complications, disease history, and body mass index (BMI). Additionally, patients with cardiac, hepatic, or renal impairments had their severity of dysfunction evaluated. Daily dose, glycemic control status, and concomitant antidiabetic agents (including insulin) were also recorded.
2.3.2. Safety outcomes
AEs and adverse drug reactions (ADRs) that occurred during the surveillance and up to 30 days after treatment completion or discontinuation were recorded and coded using the Japanese Medical Dictionary for Regulatory Activities (MedDRA-J) version 20.0. The AEs listed in the risk management plan (RMP) included increased transaminase levels and drug-induced hepatitis, angioedema, acute pancreatitis, skin lesion, hypoglycemia, serious infection, cardiovascular risk, muscular events, neuropsychiatric events, breast cancer, and pancreatic carcinoma.
2.3.3. Efficacy outcomes
Efficacy was evaluated by analyzing the levels of HbA1c (National Glycohemoglobin Standardization Program [NGSP] value) and fasting blood glucose (FBG) at baseline and then at Week 12, Week 24, Week 36, and Week 52, or at discontinuation. The changes in HbA1c and FBG levels from baseline to endpoint were also assessed.
Blood glucose control was evaluated by the study investigators and involved a comprehensive evaluation including HbA1c, blood glucose results, clinical features of the patient and clinical findings. ‘Excellent’ or ‘good’ were defined as a response whereas ‘inadequate/poor’, ‘bad’, and ‘not assessable’ assessments were regarded as a non-response. The number and proportions of patients with a response or a non-response were calculated.
These efficacy outcomes were also stratified by the different types of concomitant antidiabetic agents (including OADs and insulin) used and by baseline characteristics including sex, age, BMI, and prior treatment with DPP-4 inhibitors.
2.3.4. Laboratory parameters
Liver enzymes (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) were measured and any changes that constituted an AE were evaluated at baseline, Week 12, Week 24, Week 36, Week 52, or at discontinuation.
2.4. Statistical methods
The planned sample size was 3000 patients based on the probability to detect ADRs at an incidence of 0.1% and a confidence level of 95%. The safety analysis population consisted of CRF-locked patients who were eligible for treatment, whereas the efficacy analysis population consisted of all patients in the safety analysis set who also had their glycemic control status assessed.
Summary statistics (mean ± SD) were used for quantitative parameters such as HbA1c, FBG, safety outcomes (frequency of AEs and ADRs), and laboratory parameters. Categorical variables are presented as number (%). Inter-group comparisons were tested using the Fisher’s exact test for unpaired, nominal 2-group data, or the Mann–Whitney test for continuous data (3 groups or more; Fisher’s exact test was used for 2 × 2). Paired t-tests were used for paired continuous data. The 95% confidence intervals (CIs) of the mean were calculated and a p-value of 0.05 was considered to be statistically significant. For the statistical analyses, SAS® System version 9.13 software (SAS Institute Inc., Cary, NC, USA) was used.
3. Results
3.1. Patients
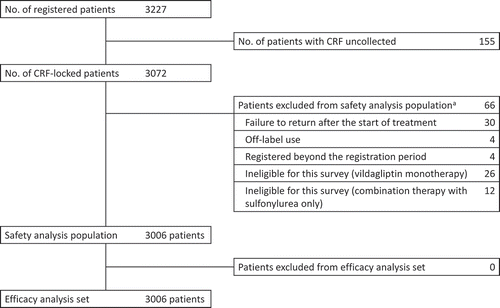
This study registered 3227 patients by the cutoff date, with CRFs available for 3072 patients (). The safety analysis population consisted of 3006 patients with 66 patients excluded because of failure to visit the hospital once the study started (n = 30), ineligibility because of vildagliptin monotherapy (n = 26), ineligibility because of vildagliptin combined with SU therapy only (n = 12), and other reasons. Of the 3006 patients included in the safety analysis population, no further patients were excluded from the efficacy analysis population.
Figure 1. Patient disposition
The baseline patient characteristics are shown in . In brief, 59.7% of patients were male, the mean ± SD age was 63.8 ± 12.8 years and mean ± SD BMI was 25.8 ± 4.6 kg/m2. Prior treatment with a DPP-4 inhibitor was reported in 7.9% of patients and the disease durations were <5 years (16.5%), 5 to <10 years (17.1%), ≥10 years (28.2%), and unknown (38.1%). The baseline patient characteristics by concomitant antidiabetic agents are shown in Table S1.
Table 1. Patient baseline characteristics
3.2. Safety
In the safety analysis population, 609 AEs occurred in 13.61% (n = 409) of patients, of which 2.20% (n = 66) experienced 89 serious AEs (). When stratified by concomitant antidiabetic agents, the percentage (number) of patients with AEs was 20.41% (n = 50) in the glinide subgroup, 15.87% (n = 109) in the insulin subgroup, 16.71% (n = 70) in the TZD subgroup, 13.18% (n = 104) in the αGI subgroup, 12.93% (n = 212) in the BGs subgroup, 12.92% (n = 85) in the Met ≥1000 mg/day subgroup, 12.62% (n = 115) in the Met <1000 mg subgroup, and 0.0% (n = 0) in the sodium glucose co-transporter 2 inhibitor (SGLT2) subgroup (). The most common AEs in the safety analysis population were increased glycosylated hemoglobin (1.00%, n = 30); diabetes mellitus (0.83%, n = 25); increased blood pressure (0.83%, n = 25); abnormal hepatic function (0.70%, n = 21); and inadequate control of diabetes mellitus (0.67%, n = 20) ().
Table 2. Summary of adverse events observed in ≥5 patients
The incidence of ADRs in patients aged ≥65 years was 2.86% (44/1539), whereas patients <65 years had an incidence of 2.52% (37/1467). Hypoglycemia occurred in 0.40% (n = 12) of patients, and this incidence rate was broadly similar across all concomitant antidiabetic subgroups except for the insulin products subgroup, in which 0.58% (n = 4) of patients experienced hypoglycemia. ADRs as defined in the RMP and stratified by concomitant antidiabetic agents are shown in . Overall, there were 42 RMP-related ADRs in 1.36% (n = 41) of patients. When stratified by concomitant antidiabetic agents, the incidence of RMP-related ADRs was 1.89% (n = 13) in the insulin products subgroup, 1.65% (n = 15) in the Met <1000 mg/day subgroup, 1.06% (n = 7) in the Met ≥1000 mg/day subgroup, 1.52% (n = 25) in the BG subgroup, 1.22% (n = 3) in the glinide subgroup, 1.19% (n = 5) in the TZD subgroup, and 1.01% (n = 8) in the αGI subgroup (). The mean ± SD changes in AST and ALT levels over time are shown in Figure S1. AST changed from 26.4 ± 14.6 IU/L at baseline to 26.0 ± 14.4 IU/L at the endpoint, whereas ALT changed from 29.4 ± 23.2 IU/L at baseline to 27.1 ± 19.4 IU/L at the endpoint.
Table 3. Summary of risk management plan-related ADRs
3.3. Efficacy
The mean ± SD changes in HbA1c over time in the overall efficacy analysis population and subgroups stratified by concomitant antidiabetic agents are shown in . The mean ± SD HbA1c decreased from 8.03 ± 1.42% to 7.27 ± 1.20% at the endpoint for the overall efficacy analysis population. The change in HbA1c from baseline to endpoint was −0.76% for all patients (). Furthermore, there were similarities in the reduction in HbA1c when stratified by concomitant BG (−0.74%), TZD (−0.65%), αGI (−0.72), glinide (−0.68%), and insulin (−0.66%) treatment. In the Met ≥1000 mg/day subgroup, there was a change of −0.64%, whereas in the Met <1000 mg/day subgroup, there was a change of −0.81%.
Figure 2. Changes in HbA1c over time from baseline to endpoint
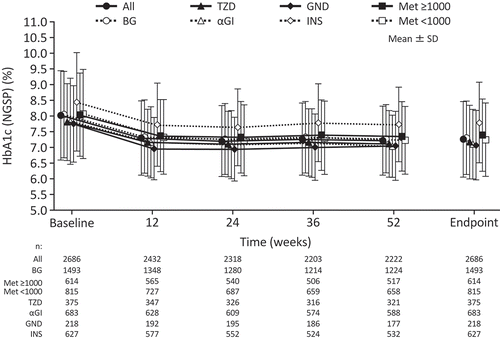
Table 4. Change in HbA1c from baseline stratified by concomitant antidiabetic medication
The changes in HbA1c stratified by responder characteristics including concomitant drugs, sex, age, BMI, and prior treatment with DPP-4 inhibitor are shown in . The mean changes in Hb1Ac, when stratified by the presence or absence of prior treatment with DPP-4 inhibitors, were −0.41% [95% CI: −0.55 to −0.27] and −0.79% [95% CI: −0.84 to −0.74], respectively. When stratified by age (<65 or ≥65 years), the mean changes in HbA1c were −0.85% [95% CI: −0.93 to −0.78] and −0.67% [95% CI: −0.72 to −0.61], respectively. Differences in sex and BMI had no particular effect on the mean change in HbA1c. When stratified by disease duration, the mean ± SD HbA1c at last measurement was 7.14 ± 1.18%, 7.27 ± 1.22%, and 7.44 ± 1.20% in patients with a disease duration of <5 years, 5 to <10 years, and ≥10 years, respectively (Table S2).
Table 5. Mean change in HbA1c over time stratified by patient characteristics
Blood glucose control status in the overall patient population and stratified by concomitant treatment subgroups is shown in Table S3. The overall blood glucose control response rate (including status described as either ‘excellent’ or ‘good’) was 54.72% (n = 1645), while the overall non-response rate (status described as ‘inadequate/poor’, ‘bad’, or ‘not assessable’) was 45.28% (n = 1361). When stratified by concomitant antidiabetic agent, the response rates were similar for BG (52.80%), TZD (55.61%), αGI (59.57%), and glinide (60.82%) treatments. In the Met ≥1000 mg/day subgroup, there was a response rate of 47.42%, whereas in the Met <1000 mg/day subgroup, there was a response rate of 56.42%.
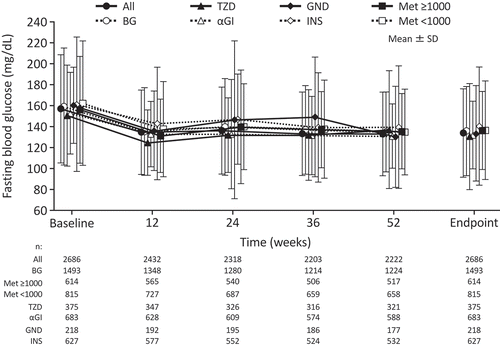
The mean changes in FBG over time in overall patients and subgroups stratified by concomitant antidiabetic agents are shown in . Overall, the baseline FBG was 157.1 ± 51.6 mg/dL and at the endpoint was 133.9 ± 42.2 mg/dL. Similar reductions in FBG over the study period were observed for each subgroup.
4. Discussion
This study evaluated the real-world safety and efficacy data on vildagliptin therapy in combination with OADs in more than 3000 Japanese patients with T2DM for up to 52 weeks. This study demonstrated that vildagliptin was well tolerated and effective, regardless of concomitant OADs (including high-dose Met) or insulin.
Patient characteristics in this study, including mean age and BMI, were similar to the 56,997 T2DM patients registered by the Japan Diabetes Clinical Data Management Study Group (JDDM) in 2013 when this study started [Citation11]. Although HbA1c levels were higher in this study in comparison to the JDDM registry (8.03% vs. 6.96%), the improvement and change in HbA1c was similar to a previous Japanese clinical trial [Citation8]. The incidence of drug-related hypoglycemia in this study was 0.40%, which is in line with the previous Japanese trial [Citation8].
In the safety analysis, the incidence rate of AEs was similar across all concomitant antidiabetic subgroups except for those patients administered SGLT2s. The SGLT2s were approved in 2014 in Japan (after the start date of this study), and as only 28 patients receiving vildagliptin in combination with SGLT2s were enrolled into this study, it was not possible to perform comparisons with other concomitant antidiabetic agents. The incidence rates of AEs in the high-dose Met (≥1000 mg/day) group were similar to those in the Met <1000 mg/day group (12.92% and 12.62%, respectively). Additionally, there were no differences observed in the incidence rates of gastrointestinal AEs such as anorexia, nausea, vomiting, diarrhea, dyspepsia, constipation, abdominal pain, or feeling bloated. The GUARD study [Citation12] showed that vildagliptin therapy over 24 weeks was well tolerated, regardless of concomitant Met administration. Similarly, a small Japanese trial that investigated the up-titration of Met in patients receiving vildagliptin therapy found no tolerability issues with patients receiving up to 1000 mg/day Met [Citation13], which also confirmed the findings of a previous study conducted in Japan [Citation14]. Thus, based on these reports and the results of the present study, the tolerability of vildagliptin plus Met ≥1000 mg/day has been confirmed.
The present study included 97 patients with heart failure. In the VIVIDD study that evaluated the safety of vildagliptin in patients with heart failure and reduced ejection fraction, an increase in left ventricular volumes was observed in the vildagliptin group compared with the placebo group; however no major effect on left ventricular ejection fraction (LVEF) was observed [Citation15]. In the present study, the effects on LVEF and left ventricular volumes in patients with T2DM and heart failure were not evaluated. However, ADRs related to heart failure were not recorded, which supports the safety of vildagliptin in the real-world clinical setting.
The incidence rate of ADRs in elderly (≥65 years) and non-elderly (<65 years) patients were 2.86% and 2.52%, respectively, and this difference was not statistically significant. Hypoglycemia related to the administration of vildagliptin was confirmed in 12 patients, with 11 of these patients classified as elderly (≥65 years). These ADRs were determined to be non-serious and were all shown to be resolved or improved over time. However, most of these patients were receiving additional hypoglycemic drugs and therefore it is unknown if elderly patients are more susceptible to hypoglycemia.
Other important risks identified in the RMP included hepatotoxicity as defined by increased transaminase levels and drug-induced hepatitis. In this study, the incidence of hepatotoxicity was 0.23% (7/3006 patients), which is in line with the safety data for vildagliptin [Citation8]. The Japanese package insert describes hepatic dysfunction as an RMP-related ADR that is rare with increases of AST or ALT (≥3× upper limit of normal on two consecutive measurements) reported to have an incidence of 0.2% for 50-mg once-daily vildagliptin and 0.3% for 50-mg twice-daily vildagliptin [Citation16]. In this study, AST and ALT levels did not change over 52 weeks of treatment. Furthermore, the RMP identifies the development of acute pancreatitis as a known, albeit rare, risk of vildagliptin use. In this study, acute pancreatitis was observed at a lower incidence (0.20%, 6/3006 patients) than in the EDGE study, where the incidence of acute pancreatitis was reported to be 0.65% [Citation9]. Overall, based on the RMP-related ADR data, patients receiving vildagliptin were not found to be at particularly high risk of any of the ADRs listed in the RMP.
Additionally, constipation, hunger, and asthenia have been identified as safety concerns in pre-approval clinical trials with reported incidences of 3.2% (36/1128), 3.0% (34/1128), and 2.0% (22/1128), respectively [Citation10]. In this study, constipation (0.47%, 14/3006) had a much lower incidence rate than previously reported, and neither hunger nor asthenia were observed. Throughout the observation period, no other clinically significant abnormalities were observed in laboratory parameter values; therefore, the safety of vildagliptin was confirmed in any OAD combination.
Regarding efficacy, the changes in HbA1c and FBG levels decreased over time until Week 12. Thereafter, these parameters remained at a lower level than baseline until Week 52, and there were no clear differences observed among the different OAD combination subgroups. Even though these results were obtained in a real-world clinical setting, they were consistent with a previous Japanese clinical trial [Citation8]. Two other Japanese studies evaluating vildagliptin, used either in combination with insulin [Citation17] or as add-on to insulin and other OADs [Citation18], also showed significant improvements in HbA1c over 12 weeks. Although the study designs (randomized, placebo-controlled) differ from the present study, all three studies are consistent in terms of improvement in HbA1c over time.
Efficacy parameters were comparable in both the high-dose Met ≥1000 mg/day and Met <1000 mg/day groups and a statistically significant reduction in HbA1c was observed after 52 weeks in both groups (p < 0.0001). These data were similar to a randomized double-blind controlled trial in Japanese diabetic patients on a stable Met daily dose of either 500 or 1000 mg [Citation14]. Furthermore, there were no clear differences in the efficacy of vildagliptin when stratified by age or BMI, which supports previous observations that no dose adjustment is required according to these patient characteristics [Citation19]. The efficacy of vildagliptin in combination with Met, in terms of reduction in HbA1c, was comparable with observational trials such as GUARD [Citation12] and GALATA [Citation20], although differences in the background of patients and the study designs preclude direct comparisons.
In the present study, despite the difference in baseline HbA1c between patients aged <65 years and those aged ≥65 years, a reduction in HbA1c was confirmed in both groups. In this study, comparison between patient populations aged <65 years and ≥65 years was not performed. In another report, pooled data from five randomized controlled trials with vildagliptin showed that patients aged ≥65 years had a slightly greater HbA1c reduction compared with younger patients [Citation21]; however, with the exception of vildagliptin, studies of other DPP-4 inhibitors in Japan have reported improvements in HbA1c across all three groups of patients aged <65, 65–74, and ≥75 years [Citation22,Citation23]. The efficacy of vildagliptin was confirmed both in Japanese patients aged <65 years and in those ≥65 years. However, comparisons of efficacy by race and class of DPP-4 inhibitor were not assessed in this study. When stratifying the efficacy data by duration of diabetes, it was found that longer disease duration was associated with higher HbA1c levels after 52 weeks of treatment. Therefore, this suggests that treatment should be started as early as possible to ensure the greatest therapeutic efficacy. Furthermore, the reduction in HbA1c was observed to be greater in patients who were not pre-treated with a DPP-4 inhibitor. However, no statistical test was performed to compare these results.
This study is limited by the absence of a control group for comparison. Moreover, in addition to starting vildagliptin, data regarding changes in other OADs during the study period were not collected. Furthermore, in the real-world clinical setting it cannot be ruled out that the data collected are attributable to the exposure to vildagliptin or the natural course of disease or other unknown factors.
5. Conclusions
This study evaluated the real-world safety and efficacy data from over 3000 Japanese patients who received vildagliptin in combination with other non-SU OADs or insulin, over a period of 52 weeks. Overall, our results show that vildagliptin combination therapy with other OADs including high-dose Met or insulin appeared to be well tolerated and effective over 52 weeks in a real-world clinical setting in Japan.
Declaration of interest
All authors are employees of Novartis Pharma K.K.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
TH was involved in data interpretation, discussion, and revising and drafting the manuscript; HM was involved in data interpretation, discussion, and revising the manuscript; YS was involved in the study concept and design, data interpretation, discussion, and revising the manuscript; TT was involved in data interpretation, discussion, and revising the manuscript; IT was involved in data interpretation, discussion, performing statistical analyses and revising the manuscript; and NO was involved in data interpretation, discussion, and revising the manuscript.
Supplemental Material
Download Zip (103.7 KB)Acknowledgments
The authors would like to thank James Graham, PhD, of Edanz Medical Writing for providing medical writing assistance, which was funded by Novartis Pharma K.K. through EMC K.K. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Supplementary Material
Supplemental data for this article can be acessed here.
Additional information
Funding
References
- Ahrén B, Landin-Olsson M, Jansson PA, et al. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:2078–2084.
- Utzschneider KM, Tong J, Montgomery B, et al. The dipeptidyl peptidase-4 inhibitor vildagliptin improves beta-cell function and insulin sensitivity in subjects with impaired fasting glucose. Diabetes Care. 2008;31:108–113.
- Bosi E, Camisasca RP, Collober C, et al. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890–895.
- Garber AJ, Schweizer A, Baron MA, et al. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9:166–174.
- Garber AJ, Foley JE, Banerji MA, et al. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab. 2008;10:1047–1056.
- Fonseca V, Baron M, Shao Q, et al. Sustained efficacy and reduced hypoglycemia during one year of treatment with vildagliptin added to insulin in patients with type 2 diabetes mellitus. Horm Metab Res. 2008;40:427–430.
- Kikuchi M, Abe N, Kato M, et al. Vildagliptin dose-dependently improves glycemic control in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;83:233–240.
- Odawara M, Suzuki M, Hamada I, et al. Clinical evaluation of combination therapy with vildagliptin in patients with type 2 diabetes; safety of add-on to metformin, thiazolidine, α-GI or glinide for 52 weeks (in Japanese). J New Rem Clin. 2012;61:2593–2611.
- Mathieu C, Barnett AH, Brath H, et al. Effectiveness and tolerability of second-line therapy with vildagliptin vs. other oral agents in type 2 diabetes: a real-life worldwide observational study (EDGE). Int J Clin Pract. 2013;67:947–956.
- Pharmaceuticals and Medical Devices Agency. Vildagliptin (EQUA) package insert. [cited 2018 Oct 2]. Available from: http://www.info.pmda.go.jp/downfiles/ph/PDF/300242_3969011F1020_1_21.pdf [in Japanese].
- Japan Diabetes Clinical Data Management Study Group. 2013. Available from: http://jddm.jp/data/index-2016.html
- Rosales R, Abou Jaoude E, Al-Arouj M, et al. Clinical effectiveness and safety of vildagliptin in >19 000 patients with type 2 diabetes: the GUARD study. Diabetes Obes Metab. 2015;17:603–607.
- Suzuki L, Kanazawa A, Uzawa H, et al. Safety and efficacy of metformin up-titration in Japanese patients with type 2 diabetes mellitus treated with vildagliptin and low-dose metformin. Expert Opin Pharmacother. 2017;18:1921–1928.
- Odawara M, Hamada I, Suzuki M. Efficacy and safety of vildagliptin as add-on to metformin in Japanese patients with type 2 diabetes mellitus. Diabetes Ther. 2014;5:169–181.
- McMurray JJV, Ponikowski P, Bolli GB, et al. Effects of vildagliptin on ventricular function in patients with type 2 diabetes mellitus and heart failure: a randomized placebo-controlled trial. JACC Heart Fail. 2018;6:8–17.
- European Medicines Agency. Galvus (vildagliptin). Summaries of product characteristics. 2012 [cited 2018 Jul 31] Available from http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000771/WC500020327.pdf
- Hirose T, Suzuki M, Tsumiyama I. Efficacy and safety of vildagliptin as an add-on to insulin with or without metformin in Japanese patients with type 2 diabetes mellitus: a 12-week, double-blind, randomized study. Diabetes Ther. 2015;6:559–571.
- Saito D, Kanazawa A, Shiqhara N, et al. Efficacy and safety of vildagliptin as an add-on therapy in inadequately controlled type 2 diabetes patients treated with basal insulin. J Clin Med Res. 2017;9:193–199.
- He YL, Sabo R, Campestrini J, et al. The effect of age, gender, and body mass index on the pharmacokinetics and pharmacodynamics of vildagliptin in healthy volunteers. Br J Clin Pharmacol. 2008;65:338–346.
- Ayvaz G, Keskin L, Akin F, et al. Real-life safety and efficacy of vildagliptin as add-on to metformin in patients with type 2 diabetes in Turkey–GALATA study. Curr Med Res Opin. 2015;31:623–632.
- Pratley RE, Rosenstock J, Pi-Sunyer FX, et al. Management of type 2 diabetes in treatment-naive elderly patients. Diabetes Care. 2007;30:3017–3022.
- Umezawa S, Kubota A, Maeda H, et al. Two-year assessment of the efficacy and safety of sitagliptin in elderly patients with type 2 diabetes: post hoc analysis of the ASSET-K study. BMC Endocr Disord. 2015;15:34.
- Kadowaki T, Haneda M, Ito H, et al. Safety and efficacy of long-term treatment with teneligliptin: interim analysis of a post-marketing surveillance of more than 10,000 Japanese patients with type 2 diabetes mellitus. Expert Opin Pharmacother. 2018;19:83–91.