1. Introduction
Biliary tract cancer (BTC) is a heterogeneous malignancy with dismal outcome, i.e. mortality rates approximating the incidence of the disease. Although BTC is a relatively rare tumor in the Western World (about 4 new cases per 100.000 population), it represents the second most common primary liver tumor after hepatocellular carcinoma. Besides geographically relevant risk factors such as cholelithiasis (e.g. India, Chile) and liver fluke infestation (e.g. Thailand, China), the major risk factors for the development of BTC include primary sclerosing cholangitis, primary biliary cirrhosis, liver cirrhosis, hepatitis C and congenital malformations [Citation1]. Symptoms of BTC are often unspecific and silent (e.g. painless jaundice, weight loss), the consequence being that diagnosis is often made at an already advanced stage of the malignancy – eliminating the option of potential curative surgery [Citation2].
In their article in Expert Opinion on Pharmacotherapy, Filippi et al. provide a critical and comprehensive overview on the current standards in the treatment of BTC patients and give an outlook of novel and targeted pharmacotherapeutic approaches currently under investigation [Citation3].
As discussed by Filippi [Citation3] and others [Citation1], both methodological aspects and biological characteristics of the disease have prevented clinical progress for over a decade. Moreover, due to the difficulty of recruiting sufficient numbers of patients for controlled trials, validity and expressiveness of these trials is limited by small sample size, retrospective design, monocentricity and other factors. Furthermore, the molecular heterogeneity of the tumors, including great variability in terms of mutations, oncogenic pathway addiction of the cancer cells and inter- and intratumoral differences impede rational patient stratification and solid evaluation of therapeutic approaches. Another level of complexity regarding tumor heterogeneity is based on epigenetic alterations – mediated, for example, by the histone methyltransferase G9a as recently shown – which might, however, represent an alternative therapeutic approach in the future [Citation4]. As reviewed in detail by Filippi et al., the main therapeutic decision and outcome parameter is based on the resectability of the primary tumor [Citation3]. The subsequent clinically relevant step is the selection of the (neo-)adjuvant chemotherapy, whereby the currently ongoing development of targeted and immune checkpoint-based therapies will challenge the established combination of cisplatin and gemcitabine as the ‘gold’ standard chemotherapy for advanced BTC.
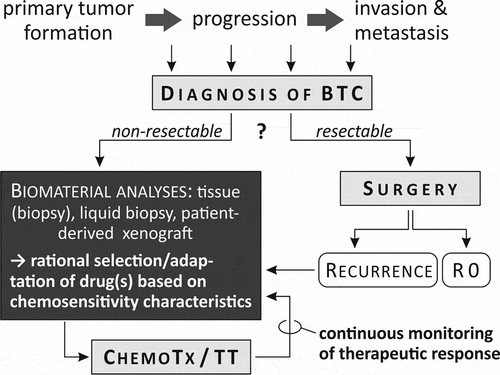
In this respect, a possible ‘bed-side’ chemotherapy testing of patient-derived xenografts will possibly become the standard to evaluate the best chemotherapy in the first-line chemotherapy with further therapeutic options in the second- or third-line chemotherapy as impressively demonstrated by Lampis et al. [Citation5]. In a similar experimental setting, comprehensive molecular investigations such as multiplex gene expression of cancer-relevant factors could be performed in order to identify druggable mutations. To employ such patient-individual approaches is especially relevant for assessment of the mutational spectrum. BTC displays a highly heterogeneous, low frequency and anatomy- and etiology-dependent set of oncogenic mutations: as recently summarized in a systematic review by Roos et al.: the BTC-subtype specific mutations generally affect less than 30% of the investigated subtype cases (mutations overlapping in all BTC subtypes include, e.g. TP53 (~20–30%), ARID1A (6–20%), and KRAS (8–14%)) [Citation6]. Furthermore, integration of whole-genome and epigenome analyses could identify other clinically relevant driver genes, noncoding promoter mutations and structural variants clustering BTCs into four subgroups (fluke-positive BTCs (clusters 1/2) with ERBB2 amplifications and TP53 mutations versus fluke-negative BTAs (clusters 3/4) with high copy-number alterations and PD-1/PD-L2 expression, or epigenetic mutations (IDH1/2, BAP1) and FGFR/PRKA-related gene rearrangements) [Citation7]. Therefore, the timely integration of molecular-pathologic findings of BTC is critical to substantiate a rational decision on the choice of chemo- or targeted therapy for the individual patient.
Another central question is linked to the frequently observed chemotherapy resistance with, again, significant implications for the patients’ prognosis. Known mechanisms of chemoresistance involve a heterogeneous set of proteins expressed during initiation, progression and metastasation of tumors, providing BTC cells with selective escape mechanism from the cytotoxic effects of cytostatic drugs [Citation8]. Therefore, the following questions have to be addressed to improve the therapy of BTC: (i) Which parameters define chemoresistance? (ii) How to identify factors of chemoresistance (individual factors, source of material)? and (iii) Which (drug-based) approaches are effective in either prevention of development of chemoresistance and/or reversal. In general, overcoming chemoresistance and knowledge about the cancer’s drug sensitivity represent key steps for choosing and improving the most appropriate and efficient chemotherapy. For example, based on in vitro data using BTC cell lines, the sensitivity to chemotherapeutic agents and targeted drugs is not related to the histological type of BTC, whereas molecular factors such as expression of multi-drug resistant proteins or microRNAs play a key role in determining drug resistance [Citation5,Citation9]. Furthermore, gene expression analysis of gemcitabine-treated BTC cell lines could predict the gemcitabine resistance via the upregulation of ribonucleotide reductase 2b [Citation10]. Therefore, it is essential to test chemosensitivity or chemoresistance before and continuously during the chemotherapy. Another important question is, which biomaterial should be used for molecular tests on chemosensitivity. The obvious approach is analyzing tumor tissue. However, such approach requires invasive procedures to gather the tumor material. In situ investigations using a high-throughput fourier transform infrared microspectroscopy on the other hand could possibly discriminate the chemotherapeutic response of BTCs with very high sensitivity and specificity as already shown [Citation11]. New approaches use enrichment technologies and are based on more easily accessible material (e.g. blood): such liquid biopsies analyze, e.g. circulating tumor cells, tumor-derived cell-free DNA or exosomes (extracellular vesicles) and could simplify the access to the chemotherapy-relevant information the next years [Citation12]. Alternatively, patient-derived xenografts were successfully used to guide the selection of chemotherapy regimens for gallbladder cancer, thus providing a proof-of-principle for individualized therapeutic decisions. Such approach would also take into account the heterogeneity (and different chemosensitivity) of each patient’s tumor [Citation13]. Different (adjuvant) strategies have been investigated in experimental settings in order to sensitize (biliary) cancer cells toward chemotherapy. For example, NRF2 inhibition resulted in sensitizing BTC cells toward chemotherapy both in vitro and in vivo [Citation14,Citation15]. Another recent example exploits interferences with metabolic pathways to modify and increase the chemoresponsiveness of cancer cells [Citation16]. Furthermore, combined bile acids like cisplatin-ursodeoxycholic acid derivative (named Bamet-UD2) could be used as Trojan Horses, showing promising inhibition of tumor growth in vitro and in vivo [Citation8]. Moreover, as recently demonstrated, adenoviral vectors encoding human organic cation transporter type 1 could improve chemosensitization and consecutively the response of BTC to sorafenib [Citation17]. In summary, BTC is a highly chemoresistant tumor, limiting the efficiency of current treatments. Based on the current knowledge, the development of chemoresistance of BTC is a highly dynamic and complex process, involving general alterations (e.g. somatic mutations, epigenome) and/or alterations of concrete factors. Consequently, it is still demanding to execute experimental studies and clinical trials which cover all these mentioned areas of pharmacological and pharmacodynamic issues to develop effective therapeutic options for BTCs and to essentially improve the outcome of this still dismal tumor entity.
2. Expert opinion
As discussed by Filippi [Citation3] in their recent review, the validity of previously conducted clinical trials was limited by factors related to the study design or the recruitment of patients. Therefore, we suggest that the highest level of quality, validity, and expressiveness should be considered in design and preparation of future clinical trials enrolling patients with a disease as rare as BTC. As demonstrated by Jusakul et al. [Citation7], a comprehensive, multicenter, and bio-integrative approach might be most appropriate for BTC by combining whole-genome, targeted/exome, copy-number, gene expression, and DNA methylation information of 489 BTCs from 10 countries. While keeping in mind the limitations of preclinical models, additionally, each clinical trial should be preceded by intensive pre-clinical (in vitro/in vivo) experimentation in order to best substantiate the (novel) therapeutic approach. Such preclinical models similarly need to mirror the biological differences between BTC subtypes, e.g. by inclusion of several different cell lines in case of in vitro studies (e.g. [Citation5]). In addition, within the design of clinical studies, the evident heterogeneity of BTC entities must be taken into account – including formulation of in- and exclusion criteria, guaranteeing comparability of patient subgroups and their homogeneity as well as planning of collection of biological material for ex post analysis of possible prognostically relevant markers for therapeutic response. Recent innovation including patient-derived xenografts successfully exemplify patient-personalized strategies in order to achieve a rational and efficient selection of (chemo-) therapeutic approaches for BTC patients. Therefore, it seems mandatory to include deep molecular analysis of developing chemoresistance during chemotherapy of BTCs in clinical trials to cover all clinical and therapeutic issues, especially for approaches of potential chemosensitization (as summarized in ).
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Tariq NU, McNamara MG, Valle JW. Biliary tract cancers: current knowledge, clinical candidates and future challenges. Cancer Manag Res. 2019;11:2623–2642.
- Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014 Jun 21; 383(9935):2168–2179.
- Filippi R, Lombardi P, Quara V, et al. Pharmacotherapeutic options for biliary tract cancer: current standard of care and new perspectives. Expert Opin Pharmacother. 2019;24:1–17.
- Mayr C, Helm K, Jakab M, et al. The histone methyltransferase G9a: a new therapeutic target in biliary tract cancer. Hum Pathol. 2018;72:117–126.
- Lampis A, Carotenuto P, Vlachogiannis G, et al. MIR21 drives resistance to heat shock protein 90 Inhibition in cholangiocarcinoma. Gastroenterology. 2018 Mar;154(4):1066–1079 e5.
- Roos E, Soer EC, Klompmaker S, et al. Crossing borders: A systematic review with quantitative analysis of genetic mutations of carcinomas of the biliary tract. Crit Rev Oncol Hematol. 2019 Aug;140:8–16.
- Jusakul A, Cutcutache I, Yong CH, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017 Oct;7(10):1116–1135.
- Marin JJG, Lozano E, Herraez E, et al. Chemoresistance and chemosensitization in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018 Apr;1864(4 Pt B):1444–1453.
- Tepsiri N, Chaturat L, Sripa B, et al. Drug sensitivity and drug resistance profiles of human intrahepatic cholangiocarcinoma cell lines. World J Gastroenterol. 2005 May 14;11(18):2748–2753.
- Sato J, Kimura T, Saito T, et al. Gene expression analysis for predicting gemcitabine resistance in human cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2011 Sep;18(5):700–711.
- Wongwattanakul M, Hahnvajanawong C, Tippayawat P, et al. Classification of gemcitabine resistant cholangiocarcinoma cell lines using synchrotron FTIR microspectroscopy. J Biophotonics. 2017 Mar;10(3):367–376.
- De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019 Mar;40(3):172–186.
- Zhan M, Yang RM, Wang H, et al. Guided chemotherapy based on patient-derived mini-xenograft models improves survival of gallbladder carcinoma patients. Cancer Commun (Lond). 2018 Jul 17;38(1):48.
- Samatiwat P, Prawan A, Senggunprai L, et al. Nrf2 inhibition sensitizes cholangiocarcinoma cells to cytotoxic and antiproliferative activities of chemotherapeutic agents. Tumour Biol. 2016 Aug;37(8):11495–11507.
- Zhan M, Wang H, Xu SW, et al. Variants in oxidative stress-related genes affect the chemosensitivity through Nrf2-mediated signaling pathway in biliary tract cancer. EBioMedicine. 2019;48:143–160.
- Gonzalez PS, O’Prey J, Cardaci S, et al. Mannose impairs tumour growth and enhances chemotherapy. Nature. 2018 Nov;563(7733):719–723.
- Lozano E, Macias RIR, Monte MJ, et al. Causes of hOCT1-dependent cholangiocarcinoma resistance to sorafenib and sensitization by tumor-selective gene therapy. Hepatology. 2019 Oct;70(4):1246–1261.