ABSTRACT
Introduction
The treatment of rheumatoid arthritis (RA), a chronic, systemic, autoimmune disease, has been greatly advanced by the introduction of biologic disease-modifying antirheumatic drugs (DMARDs); however, many patients still fail to achieve disease remission. Peficitinib, an orally bioavailable inhibitor of the Janus kinase (JAK) receptor family, was approved in Japan in 2019 and Korea in 2020 for the treatment of RA.
Areas covered
This review provides an overview of JAK inhibitors currently marketed or in development; the pharmacodynamics and pharmacokinetics of peficitinib; and the efficacy and safety data for peficitinib from Phase 2b and 3 trials.
Expert opinion
Peficitinib has proven clinical efficacy in Asian patients (Japan, Korea, and Taiwan) with RA who have an inadequate response to conventional DMARDs. In Phase 3 trials, clinical improvements and prevention of joint destruction were demonstrated for both 100 mg and 150 mg once-daily peficitinib versus placebo, and treatment for up to 52 weeks was well tolerated. Safety signals, in particular the increased incidence of herpes zoster-related disease, appeared in line with other JAK inhibitors. Post-launch monitoring will establish the long-term safety and effectiveness of this drug, and further studies are necessary to determine its potential use in non-Asian populations.
1. Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by synovial inflammation, joint destruction, and organ manifestation [Citation1,Citation2]. Methotrexate (MTX), a conventional synthetic disease-modifying antirheumatic drug (csDMARD), is the standard first-line therapy for RA [Citation3] and is often considered an anchor drug both alone and in combination with other medications [Citation4]. In patients with an inadequate response to MTX and/or other csDMARDs, biologic DMARDs (bDMARDs) such as tumor necrosis factor (TNF) inhibitors have proven effective in reducing RA symptoms and slowing disease progression [Citation5,Citation6]. Biologic DMARDs have also proven more effective at preventing structural joint damage compared with csDMARDs [Citation7]. However, there remains a significant unmet need for new, clinically effective therapies [Citation6], as one-third of patients treated with bDMARDs are intolerant to or have an inadequate response to therapy [Citation8].
The Janus kinase (JAK) family of non-receptor protein tyrosine kinases, which includes JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2), is involved in cytokine signal transduction by associating with the intracellular domains of cytokine or growth factor receptors involved in immune responses, inflammatory reactions, and hematopoiesis [Citation9,Citation10]. Increased expression of JAKs and their target substrates, signal transducers and activators of transcription (STATs), is seen in the synovium of patients with RA [Citation11–Citation13]. JAKs are therefore considered to be a promising target for RA treatment [Citation10,Citation14–Citation17].
2. Overview of current JAK inhibitors
Currently approved JAK inhibitors include tofacitinib (licensed in more than 80 countries worldwide [Citation18]), baricitinib (over 55 countries [Citation19]), peficitinib (Japan and Korea [Citation20–Citation22]), and upadacitinib (30 countries [Citation23–Citation25]) [Citation26,Citation27]. Tofacitinib (Xeljanz®, Pfizer [Citation28]) is a potent selective JAK inhibitor that preferentially inhibits JAK1 and JAK3 [Citation26,Citation29]. It is effective in both MTX-naïve patients [Citation30] and those with an inadequate response to MTX and other DMARDs, including TNF inhibitors [Citation31]. Baricitinib (Olumiant™, Eli Lilly [Citation32]), the second drug launched as a JAK inhibitor for RA [Citation33], is predominantly a JAK1/2 inhibitor [Citation34] and Phase 3 trials showed rapid and significant clinical outcomes following baricitinib treatment [Citation35]. Peficitinib (ASP015K, Smyraf®, Astellas Pharma), a pan-JAK inhibitor, has been approved for clinical use in Japan and Korea [Citation20–Citation22] and is in late-stage clinical development as an RA treatment in other Asian countries () [Citation40,Citation41]. Upadacitinib (ABT-494, Rinvoq™, AbbVie [Citation23]) is a selective JAK1 inhibitor [Citation42,Citation43]. It has demonstrated efficacy as both combination therapy and monotherapy in clinical trials [Citation44–Citation46] and has been approved in the US, Japan, and the European Union (including the UK) [Citation23–Citation25].
Other oral JAK inhibitors that have completed Phase 2 or Phase 3 trials include filgotinib and decernotinib. Filgotinib (GLPG0634, Gilead) is a selective JAK1 inhibitor that has shown clinical efficacy during Phase 3 trials in RA [Citation47–Citation49]. A Marketing Authorization Application for filgotinib for the treatment of adults with RA is now under evaluation by the European Medicines Agency [Citation50], and a New Drug Application has been submitted to the Japanese Ministry of Health, Labor and Welfare [Citation51]. Decernotinib (VX-509, Vertex Pharmaceuticals Incorporated) was a selective JAK3 inhibitor tested for treatment of RA in Phase 2 and 3 studies but has been discontinued due to safety concerns [Citation52].
3. Introduction to peficitinib
3.1. Pharmacodynamics
In vitro kinase assays show that peficitinib is a pan-JAK inhibitor, inhibiting JAK activity with half maximal inhibitory concentrations (IC50) of 3.9 nM (JAK1), 5.0 nM (JAK2), 0.7 nM (JAK3), and 4.8 nM (TYK2) [Citation29]. Peficitinib prevented IL-2-induced human T-cell proliferation, which involves JAK1/3, with 14-fold greater potency than against erythropoietin-induced proliferation of the human erythroleukemia cell line TF-1, which involves JAK2 activity [Citation53]. This lower inhibition of JAK2 compared with JAK1/3 may reduce the incidence of adverse hematopoietic effects associated with JAK2 inhibition [Citation9,Citation54].
Peficitinib inhibited IL-2-induced human T-cell proliferation (IC50 18 nM) and STAT5 phosphorylation (IC50 127 nM) in a concentration-dependent manner [Citation29,Citation53]. In a rat model of adjuvant arthritis, peficitinib administered prophylactically (at the same time as the adjuvant) dose-dependently suppressed both paw swelling and bone destruction, which were almost completely ameliorated at the highest dose (30 mg/kg). When used therapeutically in established disease, peficitinib suppressed paw swelling in a dose-dependent manner, improved body weight, and significantly reduced radiological bone destruction score [Citation29].
Box 1. Drug summary.
Peficitinib inhibited IL-2-induced human T-cell proliferation (IC50 18 nM) and STAT5 phosphorylation (IC50 127 nM) in a concentration-dependent manner [Citation29,Citation50]. In a rat model of adjuvant arthritis, peficitinib administered prophylactically (at the same time as the adjuvant) dose-dependently suppressed both paw swelling and bone destruction, which were almost completely ameliorated at the highest dose (30 mg/kg). When used therapeutically in established disease, peficitinib suppressed paw swelling in a dose-dependent manner, improved body weight, and significantly reduced radiological bone destruction score [Citation29].
3.2. Pharmacokinetics and metabolism
3.2.1. Peficitinib exposure
At the time of writing, there were few published pharmacokinetic studies and most pharmacokinetic data are therefore derived from publicly available documents.
Peficitinib is absorbed rapidly: after a single dose under fasting conditions, median time to maximum plasma concentration (tmax) was 1.0–1.8 h [Citation55]. The maximum plasma concentration (Cmax) and area under the plasma concentration–time curve (AUC) both showed dose proportionality [Citation55]. Peficitinib can be taken at any time of day but is administered with a meal [Citation20]. After a single oral dose of peficitinib 150 mg was administered in healthy Japanese adults (18 subjects), fed-state administration resulted in a 56.4% increase in Cmax and a 36.8% increase in the area under the plasma concentration–time curve from time zero to time of last measurable concentration (AUClast), compared with fasted-state administration [Citation20]. Following multiple oral doses of peficitinib 150 mg once daily in healthy Japanese adults (24 subjects) after a meal, peficitinib plasma concentrations reached a steady state on day 3; steady-state Cmax and area under the plasma concentration–time curve over 24 h (AUC24 h) were 613.2 ng/mL and 2643 ng·h/mL, respectively; and the median (min, max) tmax was 3.0 h (1.5, 4.0) [Citation56]. The accumulation ratio in the steady-state to post single-dose administration was 1.2 [Citation20].
An in vitro study showed that the binding rate of peficitinib to plasma proteins, of which the main protein was albumin, was 72.8–75.2% [Citation20]. Peficitinib metabolism is mediated mainly by sulfotransferase 2A1 and nicotinamide N-methyltransferase [Citation57]. After a single oral administration of 14C-labeled peficitinib to healthy Caucasian subjects, the area under the plasma concentration–time curve from time 0 to infinity (AUCinf) of metabolites accounted for 67% of total radioactivity in plasma, and approximately 50% of the dose was excreted as metabolites [Citation57]. In urine, 14% of the peficitinib dose was excreted as the unchanged form [Citation57]. While peficitinib is mostly cleared by metabolism, peficitinib is also cleared by other mechanisms (renal and possibly biliary excretion) [Citation57].
3.2.2. Interaction studies
As patients with RA often require combination DMARD therapy, usually with MTX, drug interaction studies were key to establishing peficitinib as a viable therapeutic option for RA. Co-administration of peficitinib with MTX did not affect the pharmacokinetic profile of either drug in a patient setting [Citation58].
Peficitinib is a substrate of P-glycoprotein and co-administration of peficitinib with the P-glycoprotein inhibitor verapamil was found to increase mean peficitinib Cmax by 39% and mean AUCinf by 27% [Citation59]; however, peficitinib co-administration with verapamil was generally well tolerated [Citation59], and the increase in exposure is considered not clinically significant. Peficitinib has also been found to inhibit the organic anion transporting polypeptide 1B1 (OATP1B1), a hepatic uptake membrane transporter, and peficitinib co-administration with the OATP1B1 substrate rosuvastatin led to a 15% increase in rosuvastatin Cmax and 18% increase in AUC; however, these differences were not considered clinically meaningful [Citation60]. Co-administration of peficitinib with the CYP3A substrate midazolam increased the Cmax of midazolam by 13% and the AUC by 37% [Citation20]; however, this change may also not be clinically relevant.
Phase 1 studies have been conducted to assess the impact of renal and hepatic impairment on peficitinib pharmacokinetics in Japanese subjects. Following a single dose of peficitinib 150 mg, the AUCinf geometric mean ratios (GMR) in subjects with mild, moderate, and severe renal impairment versus subjects with normal renal function were 0.87 (90% confidence interval [CI] 0.61–1.25), 0.83 (90% CI 0.58–1.19), and 1.09 (90% CI 0.74–1.60), respectively [Citation61]. Thus, peficitinib plasma concentration does not appear to be significantly affected by impaired renal function.
In a comparison of subjects with moderate hepatic impairment versus subjects with normal hepatic function, receiving a single dose of peficitinib 150 mg, the AUCinf GMR was 1.92 (90% CI 1.39–2.66) [Citation62]. A reduced peficitinib dose of 50 mg once daily is therefore recommended in patients with moderate hepatic impairment [Citation20]. However, the AUCinf was not markedly different between subjects with mild hepatic impairment and those with normal hepatic function (GMR: 1.19 [90% CI 0.86–1.64]) [Citation62]. Mean terminal elimination half-life (t½) (10.43–11.16) was comparable between subjects with normal hepatic function and those with mild or moderate hepatic impairment [Citation62]. Peficitinib has not been studied in subjects with severe hepatic impairment.
3.3. Clinical efficacy
3.3.1. Phase 2 trials
Two global, randomized, placebo-controlled, Phase 2b trials, RA21 and RA22, were conducted to evaluate the safety and efficacy of peficitinib versus placebo [Citation63,Citation64]. In the RA21 study, 378 patients with an inadequate response to prior MTX across 43 sites in North America, Latin America, and Europe received peficitinib 25 mg, 50 mg, 100 mg, 150 mg, or placebo once daily, in combination with MTX, for 12 weeks () [Citation63]. However, differences versus placebo for the primary endpoint, American College of Rheumatology (ACR)20 response at Week 12, were not statistically significant with peficitinib 25 mg, 100 mg, or 150 mg, partly due to unexpectedly high placebo responses (75.0%) in patients from Latin America [Citation63].
Table 1. Phase 2 and 3 studies evaluating the clinical efficacy of peficitinib in patients with RA.
The RA22 study, conducted at 41 sites in North America, Mexico, and Europe, included 289 patients with an inadequate response to prior DMARDs who received peficitinib 25 mg, 50 mg, 100 mg, 150 mg, or placebo once daily, alone or in combination with DMARDs, for 12 weeks () [Citation64]. Response rates for the primary endpoint, ACR20 at Week 12, were significantly different versus placebo in the peficitinib 100 mg and 150 mg groups [Citation64].
A third Phase 2b trial conducted in Japan, RAJ1, randomized 281 patients not receiving current DMARDs to receive peficitinib monotherapy (25 mg, 50 mg, 100 mg, or 150 mg) or placebo once daily for 12 weeks [Citation36] (). Response rates for the primary efficacy variable, ACR20 at Week 12/early termination (ET), were 10.7%, 23.6%, 31.6%, 54.5%, and 65.5% in the placebo and peficitinib 25 mg, 50 mg, 100 mg, and 150 mg groups, respectively, and were significantly different versus placebo with peficitinib 50 mg, 100 mg, and 150 mg.
3.3.2. Phase 3 studies
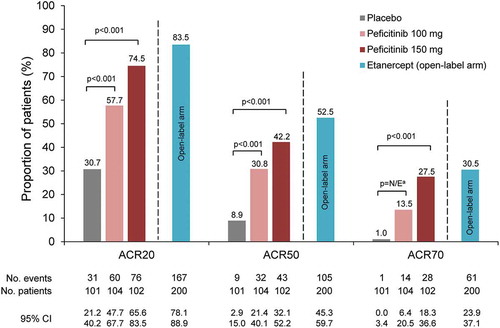
In the 52-week, randomized, double-blind RAJ3 trial (NCT02308163) conducted in Japan, Korea, and Taiwan, peficitinib (100 mg and 150 mg once daily), alone or in combination with DMARDs, was evaluated against placebo or open-label etanercept (50 mg once weekly for safety comparison) in patients with active RA (n = 507) who had an inadequate response to prior DMARDs () [Citation37]. The primary efficacy endpoint of ACR20 response rate at Week 12/ET was significantly higher in the peficitinib 100 mg (57.7%) and 150 mg (74.5%) groups versus placebo (30.7%) (p < 0.001) ( and ). Subgroup analyses indicated that both peficitinib doses produced numerically higher ACR20 response rates than placebo regardless of concomitant DMARD use; however, these subgroup analyses were not powered for statistical comparisons [Citation37]. In addition, it was difficult to compare peficitinib with etanercept, as etanercept was an open-label treatment and not included in statistical comparisons versus the other treatment arms [Citation37].
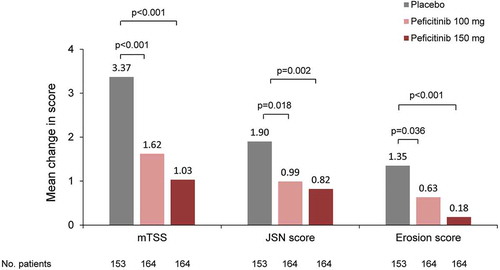
The 52-week, randomized, double-blind, placebo-controlled RAJ4 trial (NCT02305849; n = 518) evaluated peficitinib (100 mg or 150 mg once daily) in combination with MTX in Japanese patients who had an inadequate response to MTX () [Citation38]. The primary endpoint of ACR20 response rate at Week 12/ET was significantly higher in the peficitinib 100 mg (58.6%) and 150 mg (64.4%) groups versus placebo (21.8%) (p < 0.001) [Citation38]. The co-primary endpoint, change from baseline in van der Heijde-modified total Sharp score (mTSS) at Week 28/ET, was significantly lower in the peficitinib 100 mg (1.62) and peficitinib 150 mg (1.03) groups compared with placebo (3.37) (p < 0.001) () [Citation38]. Peficitinib 100 mg and 150 mg prevented joint destruction more effectively than placebo () [Citation38].
3.4. Safety and tolerability
In the Phase 2b RAJ1 trial, the total incidence of treatment-emergent adverse events (TEAEs) was similar in the placebo group (64.3%) and combined peficitinib groups (64.0%) () [Citation36]. Seven patients experienced serious TEAEs, two of which (spontaneous abortion in the peficitinib 25 mg group and cholecystitis in the peficitinib 100 mg group) were deemed to be related to the study drug [Citation36]. Herpes zoster occurred in four patients (two each in the peficitinib 25 mg and 100 mg groups), while malignancy was not observed in any of the peficitinib groups [Citation36].
Table 2. Summary of TEAEs in RAJ1, RAJ3, and RAJ4.
In the Phase 3 trials, the percentage of patients who had TEAEs during the first 12 weeks of both studies was similar for both peficitinib doses compared with placebo or with open-label etanercept: 55.3% and 55.5% of peficitinib-treated patients in RAJ3 and RAJ4, respectively, versus 53.5% (RAJ3) and 49.4% (RAJ4) of those receiving placebo () [Citation37–Citation38]. The incidence of serious infections and herpes zoster-related disease was higher in the peficitinib groups than in the placebo group in both studies; however, no dose-dependency was observed for serious infections, herpes zoster-related disease, or malignancies (). Although there are concerns of an increased risk of thromboembolic events with JAK inhibitors [Citation66], no thromboembolic events were reported in these trials [Citation37–Citation38]. Reassuringly, peficitinib was also associated with an increase in hemoglobin levels in both the RAJ3 and RAJ4 trials [Citation37–Citation38], instead of a decrease in hemoglobin that has been attributed to JAK2 inhibition; this increase is likely to be due to its anti–inflammatory effect [Citation67].
Table 3. Summary of adverse events of special interest in RAJ3 and RAJ4 from Week 0 to Week 52.
3.5. Regulatory affairs
Peficitinib was approved as an RA treatment in Japan in March 2019 [Citation20,Citation68] and in Korea in January 2020 [Citation21,Citation22]. The worldwide rights except for Japan were initially licensed to Janssen Biotech in October 2012 and were transferred to Astellas Pharma in December 2014 [Citation68]. A further study of peficitinib for the treatment of patients with RA and an inadequate response or intolerance to MTX is ongoing in China, Taiwan, and Korea (CNA3, NCT03660059; ).
4. Conclusion
Peficitinib is an oral pan-JAK inhibitor with proven clinical efficacy in Asian patients with RA who have had an inadequate response to MTX and/or other conventional DMARDs. In pivotal Phase 3 studies, ACR20 was achieved by up to 75% of patients treated with peficitinib, and prevention of joint destruction was demonstrated for both 100 mg and 150 mg once-daily doses versus placebo. Across the peficitinib safety database, there is no clear signal that the safety profile of peficitinib differs from those of other JAK inhibitors.
5. Expert opinion
Peficitinib may prove a valuable addition to the treatment options for Asian patients with RA who have not responded to conventional therapies. The guidance in Japan for dosing in adults recommends 150 mg orally once daily after a meal, which can be reduced to 100 mg once daily depending on the patient’s condition [Citation20]. However, laboratory data, such as hepatic function parameters and neutrophil count, and patient characteristics, such as age, will determine the initial dose of peficitinib for individual patients. The safety profile of peficitinib appears similar to that of other JAK inhibitors; the incidence of herpes zoster-related disease, although approximately doubled with peficitinib compared with etanercept in the Phase 3 RAJ3 trial, remained almost within the range previously observed with tofacitinib (in Japanese/Korean populations [Citation69]) and baricitinib (in Japanese/Korean/Taiwanese populations [Citation70]). Of note, the Shingrix® vaccine was approved for use in Japan in 2018 [Citation71], which may prove a useful measure to mitigate the risk of herpes zoster-related disease.
It is interesting to note that peficitinib performed better in clinical trials of patients in Asian countries [Citation36–Citation38] compared with global trials [Citation63,Citation64]. In the Kivitz et al. study, which recruited patients across North America, Latin America, and Europe with moderate-to-severe RA and an inadequate response to MTX, statistically significant treatment differences versus placebo were only observed for peficitinib 50 mg and there was a high placebo response, particularly among patients from Latin America [Citation63]. Since peficitinib exposure is greater in Japanese versus Caucasian subjects [Citation60] and Asian patients tend to have a lower body weight compared with their American and European counterparts [Citation72], a peficitinib dose of 150 mg may be sufficient for patients from Asia but it remains to be seen if the same or higher dose is required for patients from other regions. Of note, body weight was not a covariate in the population pharmacokinetic analysis of peficitinib [Citation56]; thus, the cause of the reduced efficacy of peficitinib 150 mg in Caucasian patients remains unclear. Further studies in non-Asian patients are required to establish the most effective dose in these populations.
To complement the clinical trial data available in Japan and East Asia, post-marketing surveillance of peficitinib has already been initiated (from July 2019 in Japan), and acquisition of real-world safety data following market launch is expected to enrich what is already known about this drug.
Article Highlights
Peficitinib, an inhibitor of the Janus kinase (JAK) family of signal transducers, has been approved in Japan and Korea for the treatment of rheumatoid arthritis (RA).
In two randomized, placebo-controlled, Phase 3 trials in Asia, significant clinical improvements and prevention of joint destruction were seen with peficitinib 100 mg and 150 mg once daily, compared with placebo.
The incidence of treatment-emergent adverse events was similar between peficitinib, placebo, and etanercept.
The incidence of serious infections and herpes zoster-related disease was higher with peficitinib than with placebo; however, no dose dependency was observed for serious infections, herpes zoster-related disease, or malignancies. Rates of herpes zoster-related disease were almost within the range seen with other JAK inhibitors in Asian populations.
Further studies and post-launch data are expected to confirm the safety and effectiveness of peficitinib in long-term clinical use.
Declaration of interest
Y Tanaka has received speaking fees and/or honoraria from Daiichi Sankyo, Astellas, Eli Lilly, Chugai, AbbVie, Pfizer, YL Biologics, Bristol-Myers Squibb, Mitsubishi-Tanabe, Novartis, Eisai, Takeda, Janssen, and Teijin, and research grants from Asahi Kasei, Mitsubishi Tanabe, Bristol-Myers Squibb, Eisai, Chugai, Takeda, Sanofi, UCB, Daiichi Sankyo, and Ono. Meanwhile, H Izutsu is an employee of Astellas Pharma Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
T Patrick and I Easthope of Cello Health MedErgy (Europe) are acknowledged for their medical writing support.
Additional information
Funding
References
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–2038.
- McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389:2328–2337.
- Kameda H, Fujii T, Nakajima A, et al. Japan College of Rheumatology guideline for the use of methotrexate in patients with rheumatoid arthritis. Mod Rheumatol. 2019;29:31–40.
- Kay J, Westhovens R. Methotrexate: the gold standard without standardisation. Ann Rheum Dis. 2009;68:1081–1082.
- O’Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med. 2004;350:2591–2602.
- Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther. 2011;33:679–707.
- Ciubotariu E, Gabay C, Finckh A, The physicians of the Swiss Clinical Quality Management Program for Rheumatoid Arthritis. Joint damage progression in patients with rheumatoid arthritis in clinical remission: do biologics perform better than synthetic antirheumatic drugs? J Rheumatol. 2014;41:1576–1582.
- Yamaoka K, Tanaka Y. Targeting the Janus kinases in rheumatoid arthritis: focus on tofacitinib. Expert Opin Pharmacother. 2014;15:103–113.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287.
- O’Shea JJ, Kontzias A, Yamaoka K, et al. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):ii111–5.
- van der Pouw Kraan TCTM, van Gaalen FA, Kasperkovitz PV, et al. Rheumatoid arthritis is a heterogeneous disease: evidence for differences in the activation of the STAT-1 pathway between rheumatoid tissues. Arthritis Rheum. 2003;48:2132–2145.
- Walker JG, Ahern MJ, Coleman M, et al. Expression of JAK3, STAT1, STAT4, and STAT6 in inflammatory arthritis: unique JAK3 and STAT4 expression in dendritic cells in seropositive rheumatoid arthritis. Ann Rheum Dis. 2006;65:149–156.
- Isomäki P, Junttila I, Vidqvist KL, et al. The activity of JAK-STAT pathways in rheumatoid arthritis: constitutive activation of STAT3 correlates with interleukin 6 levels. Rheumatology (Oxford). 2015;54:1103–1113.
- Tanaka Y. Recent progress and perspective in JAK inhibitors for rheumatoid arthritis: from bench to bedside. J Biochem. 2015;158:173–179.
- Tanaka Y, Maeshima K, Yamaoka K. In vitro and in vivo analysis of a JAK inhibitor in rheumatoid arthritis. Ann Rheum Dis. 2012;71(Suppl 2):i70–4.
- Nakayamada S, Kubo S, Iwata S, et al. Chemical JAK inhibitors for the treatment of rheumatoid arthritis. Expert Opin Pharmacother. 2016;17:2215–2225.
- Tanaka Y. The JAK inhibitors: do they bring a paradigm shift for the management of rheumatic diseases? Rheumatology (Oxford). 2019;58(Suppl 1):i1–3.
- Cohen S, Curtis JR, DeMasi R, et al. Worldwide, 3-year, post-marketing surveillance experience with tofacitinib in rheumatoid arthritis. Rheumatol Ther. 2018;5:283–291.
- van der Heijde D, Schiff M, Tanaka Y, et al. Low rates of radiographic progression of structural joint damage over 2 years of baricitinib treatment in patients with rheumatoid arthritis. RMD Open. 2019;5:e000898.
- Pharmaceuticals and Medical Devices Agency (PMDA) Japan. Smyraf tablets® 50 mg and 100 mg: interview form v5 2020. [cited 2020 Feb 21]. https://www.info.pmda.go.jp/go/interview/1/800126_3999046F1023_1_005_1F.pdf
- Ministry of Food and Drug Safety Korea. Drug details: 50 mg Smyraf (peficitinib hydrobromide) 2020. [cited 2020 Feb 18]. https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetail?itemSeq=202000404
- Ministry of Food and Drug Safety Korea. Drug details: 100 mg Smyraf (peficitinib hydrobromide) 2020. [cited 2020 Feb 18]. https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetail?itemSeq=202000403
- AbbVie Inc. RINVOQTM (upadacitinib) extended-release tablets: prescribing information 2019. [cited 2019 Oct 10]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211675s000lbl.pdf
- Pharmaceuticals and Medical Devices Agency (PMDA) Japan. Deliberation result report: upadacitinib hydrate 7.5 mg, 15 mg. 2019 [cited 2020 Feb 3]. http://www.pmda.go.jp/drugs/2020/P20200120001/112130000_30200AMX00027_A100_1.pdf
- European Medicines Agency (EMA). Assessment Report: Rinvoq (upadacitinib). 2019. https://www.ema.europa.eu/en/documents/assessment-report/Rinvoq-epar-public-assessment-report_en.pdf
- Jegatheeswaran J, Turk M, Pope JE. Comparison of Janus kinase inhibitors in the treatment of rheumatoid arthritis: a systemic literature review. Immunotherapy. 2019;11:737–754.
- Westhovens R. Clinical efficacy of new JAK inhibitors under development. Just more of the same? Rheumatology (Oxford). 2019;58(Suppl 1):i27–33.
- U.S. Food and Drug Administration. XELJANZ® (tofacitinib) tablets: prescribing information. 2018 [cited 2019 Oct 8]. http://labeling.pfizer.com/showlabeling.aspx?id=959
- Ito M, Yamazaki S, Yamagami K, et al. A novel JAK inhibitor, peficitinib, demonstrates potent efficacy in a rat adjuvant-induced arthritis model. J Pharmacol Sci. 2017;133:25–33.
- Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–2386.
- Bird P, Bensen W, El-Zorkany B, et al. Tofacitinib 5 mg twice daily in patients with rheumatoid arthritis and inadequate response to disease-modifying antirheumatic drugs: a comprehensive review of phase 3 efficacy and safety. J Clin Rheumatol. 2019;25:115–126.
- European Medicines Agency (EMA). Olumiant EPAR - product information. 2017 [cited 2019 Feb 24]. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Kubo S, Nakayamada S, Tanaka Y. Baricitinib for the treatment of rheumatoid arthritis and systemic lupus erythematosus: a 2019 update. Expert Rev Clin Immunol. 2019;15:693–700.
- Fridman JS, Scherle PA, Collins R, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol. 2010;184:5298–5307.
- van der Heijde D, Durez P, Schett G, et al. Structural damage progression in patients with early rheumatoid arthritis treated with methotrexate, baricitinib, or baricitinib plus methotrexate based on clinical response in the phase 3 RA-BEGIN study. Clin Rheumatol. 2018;37:2381–2390.
- Takeuchi T, Tanaka Y, Iwasaki M, et al. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis. 2016;75:1057–1064.
- Tanaka Y, Takeuchi T, Tanaka S, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to conventional DMARDs: a randomised, double-blind, placebo-controlled phase III trial (RAJ3). Ann Rheum Dis. 2019;78:1320–1332.
- Takeuchi T, Tanaka Y, Tanaka S, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III randomised, double-blind, placebo-controlled trial (RAJ4) in Japan. Ann Rheum Dis. 2019;78:1305–1319.
- Takeuchi T, Tanaka Y, Tanaka S, et al. Safety and effectiveness of peficitinib (ASP015K) in patients with rheumatoid arthritis: interim data (22.7 months mean peficitinib treatment) from a long-term, open-label extension study in Japan, Korea and Taiwan. Arthritis Res Ther. 2020;22:47.
- Astellas Pharma China Inc. A study to assess safety and efficacy of ASP015K in patients with rheumatoid arthritis (RA) who had an inadequate response or intolerance to methotrexate (MTX) (NCT03660059). 2019 [cited 2020 Feb 18]. https://clinicaltrials.gov/ct2/show/NCT03660059
- Astellas Pharma Inc. A study to continue ASP015K treatment to rheumatoid arthritis patients who completed phase IIb study or phase III study of ASP015K (NCT01638013). 2019. [cited 2020 Feb 18]. https://clinicaltrials.gov/ct2/show/study/NCT01638013
- Parmentier JM, Voss J, Graff C, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018;2:23.
- Choy EH. Clinical significance of Janus kinase inhibitor selectivity. Rheumatology (Oxford). 2019;58:953–962.
- Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:2503–2512.
- Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391:2513–2524.
- Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet. 2019;393:2303–2311.
- Combe B, Kivitz A, Tanaka Y, et al. LB0001 Efficacy and safety of filgotinib for patients with rheumatoid arthritis with inadequate response to methotrexate: FINCH1 primary outcome results. Ann Rheum Dis. 2019;78:77–78.
- Genovese MC, Kalunian K, Gottenberg J-E, et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA. 2019;322:315–325.
- Westhovens R, Rigby W, van der Heijde D, et al. LB0003 Efficacy and safety of filgotinib for patients with rheumatoid arthritis naïve to methotrexate therapy: FINCH3 primary outcome results. Ann Rheum Dis. 2019;78:259–261.
- Gilead Sciences Inc. European Medicines Agency validates Marketing Application for filgotinib for the treatment of rheumatoid arthritis. 2019 [cited 2020 Apr 16]. http://investors.gilead.com/news-releases/news-release-details/european-medicines-agency-validates-marketing-application.
- Gilead Sciences Inc. Gilead and Galapagos Announce Efficacy and Safety Results of Filgotinib Through 52 Weeks in FINCH 1 and FINCH 3 Studies in Rheumatoid Arthritis. n.d. [cited 2019 Oct 24]. http://investors.gilead.com/news-releases/news-release-details/gilead-and-galapagos-announce-efficacy-and-safety-results
- Bechman K, Yates M, Galloway JB. The new entries in the therapeutic armamentarium: the small molecule JAK inhibitors. Pharmacol Res. 2019;147.
- Hamaguchi H, Amano Y, Moritomo A, et al. Discovery and structural characterization of peficitinib (ASP015K) as a novel and potent JAK inhibitor. Bioorg Med Chem. 2018;26:4971–4983.
- Broxmeyer HE. Erythropoietin: multiple targets, actions, and modifying influences for biological and clinical consideration. J Exp Med. 2013;210:205–208.
- Cao YJ, Sawamoto T, Valluri U, et al. Pharmacokinetics, pharmacodynamics, and safety of ASP015K (peficitinib), a new Janus kinase inhibitor, in healthy subjects. Clin Pharmacol Drug Dev. 2016;5:435–449.
- Astellas Pharma Inc. Peficitinib Common Technical Document - summary of clinical pharmacology studies. [cited 2019 Oct 3]. http://www.pmda.go.jp/drugs/2019/P20190419003/index.html
- Oda K, Cao YJ, Sawamoto T, et al. Human mass balance, metabolite profile and identification of metabolic enzymes of [14C]ASP015K, a novel oral Janus kinase inhibitor. Xenobiotica. 2015;45:887–902.
- Zhu T, Oda K, Valluri U, et al. AB0363 Coadministration of ASP015K, a novel Janus kinase inhibitor, with methotrexate demonstrates tolerability and lack of pharmacokinetic interactions in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:A898.2-A898.
- Zhu T, Howieson C, Wojtkowski T, et al. The effect of verapamil, a P-glycoprotein inhibitor, on the pharmacokinetics of peficitinib, an orally administered, once-daily JAK inhibitor. Clin Pharmacol Drug Dev. 2017;6:548–555.
- Zhu T, Parker B, Wojtkowski T, et al. Drug interactions between peficitinib, an orally administered, once-daily Janus kinase inhibitor, and rosuvastatin in healthy subjects. Clin Pharmacokinet. 2017;56:747–757.
- Miyatake D, Shibata T, Shibata M, et al. Pharmacokinetics and safety of a single oral dose of peficitinib (ASP015K) in Japanese subjects with normal and impaired renal function. Clin Drug Investig. 2020;40:149–159.
- Miyatake D, Shibata T, Toyoshima J, et al. Pharmacokinetics and safety of a single oral dose of peficitinib (ASP015K) in Japanese subjects with normal and impaired hepatic function. Clin Pharmacol Drug Dev. 2019. Epub:[Epub ahead of print]. DOI:10.1002/cpdd.751.
- Kivitz AJ, Gutierrez-Ureña SR, Poiley J, et al. Peficitinib, a JAK inhibitor, in the treatment of moderate-to-severe rheumatoid arthritis in patients with an inadequate response to methotrexate. Arthritis Rheumatol. 2017;69:709–719.
- Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932–942.
- Genovese MC, Greenwald MW, Gutierrez-Ureña SR, et al. Two-year safety and effectiveness of peficitinib in moderate-to-severe rheumatoid arthritis: a phase IIb, open-label extension study. Rheumatol Ther. 2019;6:503–520.
- Scott IC, Hider SL, Scott DL. Thromboembolism with Janus kinase (JAK) inhibitors for rheumatoid arthritis: how real is the risk? Drug Saf. 2018;41:645–653.
- Weiss G, Schett G. Anaemia in inflammatory rheumatic diseases. Nat Rev Rheumatol. 2013;9:205–215.
- Markham A, Keam SJ. Peficitinib: first global approval. Drugs. 2019;79:887–891.
- Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis. 2017;76:1253–1262.
- Chen YC, Yoo DH, Lee CK, et al. Safety of baricitinib in East Asian patients with moderate-to-severe active rheumatoid arthritis: an integrated analysis from clinical trials. Int J Rheum Dis. 2019;23:65–73.
- Pharmaceuticals and Medical Devices Agency (PMDA) Japan. Deliberation result report: Shingrix® vaccine 2018 [cited 2020 Feb 18]. http://www.pmda.go.jp/drugs/2018/P20180329002/342275000_23000AMX00460_A100_1.pdf
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781.