ABSTRACT
Introduction
Recent advances in anti-diabetic medications and glucose monitoring have led to a paradigm shift in diabetes care. Newer anti-diabetic medications such as DPP-4 inhibitors, GLP-1 receptor agonists (GLP-1RAs), and SGLT2 inhibitors have enabled optimal glycemic control to be achieved without increasing the risk of hypoglycemia and weight gain. Treatment with GLP-1RAs and SGLT2 inhibitors has been demonstrated to improve cardiorenal outcomes, positioning these agents as the mainstay of treatment for patients with type 2 diabetes (T2DM). The development of these newer agents has also prompted a paradigm shift in the concept of T2DM, highlighting the importance of beta cell dysfunction in the pathophysiology of T2DM.
Areas covered
Recent advances in pharmacotherapy for diabetes are summarized with a focus on the role of incretin-based drugs and SGLT2 inhibitors. The importance of a paradigm shift from a glucose-centric to a beta cell-centric concept of T2DM is also discussed, given from an Asian perspective.
Expert opinion
Management of T2DM including lifestyle modification as well as pharmacotherapy should be focused on reducing beta cell workload, to preserve functional beta cell mass. A paradigm shift from a glucose-centric to a beta cell-centric concept of T2DM enhances the implementation of person-centered diabetes care.
1. Introduction
During the last two decades, a number of new drugs and devices have been developed, and the treatment of type 2 diabetes (T2DM) has been dramatically changed. Since the U.K. Prospective Diabetes Study (UKPDS) established the importance of intensive glycemic control to prevent diabetic complications [Citation1,Citation2], increased risk of hypoglycemia and weight gain have, however, been major barriers in glucose-lowering therapy using sulfonylureas (SU) and insulin.
Since the launch of incretin-based drugs in 2005, dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs) have made a paradigm shift in diabetes care. While the low risk of hypoglycemia and beneficial effect on weight are major advantages of these drugs, the development of incretin drugs, which primarily act through improving beta cell function [Citation3], has also led to a paradigm shift in the concept of T2DM, revealing beta cell dysfunction as the fundamental pathogenesis of T2DM.
The subsequent launch of sodium glucose cotransporter 2 (SGLT2) inhibitors has also made a great impact on the management of patients with T2DM. The improvement of cardiovascular (CV) outcomes by treatment with SGLT2 inhibitors and GLP-1RAs shown in CV outcome trials (CVOTs) has now led to a change in the guidelines, positioning these two agents in the center of pharmacotherapy for T2DM [Citation4].
From this perspective, this paper reviews the updated pharmacotherapeutic management of T2DM and discusses the future direction of diabetes care.
1.1. Search strategy and selection criteria
The PubMed database was searched for articles published in English up to March 2020, using the key search terms ‘type 2 diabetes’, ‘treatment’, ‘pathophysiology’ and ‘beta cell’. Reference lists of articles identified by this search strategy along with manually selected articles known to the author were also searched.
2. Recent advances in diabetes care
2.1. Pathophysiology of T2DM: insulin resistance or beta cell dysfunction?
T2DM is characterized by insulin resistance and beta cell dysfunction. However, it has long been argued which plays a predominant role in the pathogenesis of T2DM. In contrast to type 1 diabetes (T1DM), patients with T2DM are characterized by obesity, insulin resistance and hyperinsulinemia, implying that T2DM is a problem of reduced insulin action rather than reduced insulin secretion. On the other hand, most Japanese patients with T2DM are not obese, i.e., BMI ≥30 kg/m2, and mean BMI of Japanese patients with T2DM is approximately 25 kg/m2 [Citation5]. This implies that most Japanese patients with T2DM are not characterized by insulin resistance but rather insulin deficiency.
In fact, the American Diabetes Association (ADA) had defined T2DM as ‘ranging from predominantly insulin resistance with relative insulin deficiency to predominantly an insulin secretory defect with insulin resistance’ until recently [Citation6], and a similar definition of T2DM is also currently used in Japan [Citation7]. This implies that there are two independent components of T2DM, i.e., beta cell dysfunction and insulin resistance.
However, current evidence suggests that T2DM never develops unless beta cells fail to compensate insulin resistance, meaning that beta cell dysfunction is a prerequisite of T2DM [Citation8–Citation10]. Histological studies have revealed that beta cell mass (BCM) is reduced by 30–65% in patients with T2DM, and reduced by 15–50% even in those with prediabetes [Citation11–Citation16]. The ADA has recently revised the definition of T2DM to ‘due to a progressive loss of adequate beta cell insulin secretion frequently on the background of insulin resistance’ [Citation17]. This revision implies that insulin resistance and beta cell dysfunction are not two different aspects of T2DM, but rather the cause and consequence of T2DM, and emphasizes the integration of these two factors into one pathogenesis underlying T2DM [Citation18].
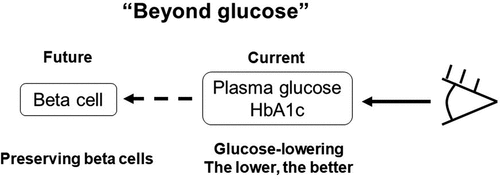
These recent changes in the concept of T2DM should affect the management of T2DM. Therefore, I here propose a ‘beta cell-centric’ rather than a ‘glucose-centric’ concept of T2DM [Citation19] (). Based on this concept, the current issues and future direction of management of T2DM are discussed below.
2.2. From DCCT to ACCORD trial
In 1993, the Diabetes Control and Complications Trial (DCCT) showed that intensive glycose-lowering therapy with basal-bolus insulin or continuous subcutaneous insulin infusion (CSII) reduced the risk of development and/or progression of microvascular complications in patients with T1DM compared with conventional therapy (average HbA1c during the study: 9% vs. 7%) [Citation20]. Subsequently, the UKPDS and Kumamoto study have shown that intensive glucose-lowering therapy with an SU, insulin or metformin also prevents the development and/or progression of microvascular complications compared with conventional therapy in patients with T2DM (average HbA1c during the study: 8% vs. 7%) [Citation1,Citation21,Citation22]. These studies established the importance of glucose-lowering therapy for the prevention of diabetic complications and have contributed to setting the goal for glycemic control as HbA1c < 7% in patients with diabetes in most of the guidelines [Citation4,Citation23,Citation24]. However, the significant reduction in risk of development of complications was mainly observed only for microvascular, but not macrovascular complications. Thus, there remains the question of whether glucose-lowering therapy effectively reduces the risk of macrovascular complications.
In 2008, three randomized controlled trials (RCTs), the ACCORD, ADVANCE, and VADT trials, which examined the efficacy of more intensive glucose-lowering therapy with a HbA1c goal of <6.0–6.5% on CV outcomes compared to standard therapy in patients with T2DM, have been reported [Citation25–Citation27]. However, all these studies have failed to show improvement of CV outcomes. Moreover, the ACCORD study was prematurely discontinued because of increased mortality in the intensive therapy group. Epidemiological studies have also shown a U- or J-shaped curve of the association between HbA1c and mortality in patients with T2DM [Citation28,Citation29].
From these results, the importance of hypoglycemia in the management of diabetes has emerged. In fact, in the ACCORD study, the incidence of overall hypoglycemia as well as severe hypoglycemia was significantly higher in the intensive therapy group during the study [Citation25]. Hypoglycemia has been shown to associate with activation of sympathetic nerves, inflammation, arrythmia and increased coagulation, which may lead to increased risk of CV events and mortality [Citation30]. To date, the causality of hypoglycemia, especially severe hypoglycemia, in CV risk remains uncertain. However, an association between severe hypoglycemia and subsequent risk of CV events and mortality has been similarly observed, irrespective of glucose-lowering strategies [Citation31,Citation32].
2.3. Metformin as first-line therapy for T2DM
Metformin was first marketed in the 1950s. However, because an increased risk of lactic acidosis with fenformin has been reported, the use of biguanides diminished until the 1990s [Citation33]. In the 1990s, clinical studies showed the glucose-lowering efficacy of metformin in patients with T2DM [Citation34], and UKPDS showed that intensive therapy with metformin reduced the incidence of diabetic complications and mortality compared with conventional therapy in obese patients with T2DM [Citation2,Citation21], moving metformin into the spotlight.
With this accumulating evidence since UKPDS, the ADA and the European Association for the Study of Diabetes (EASD) published a consensus statement on the management of T2DM in 2009 [Citation35]. In this statement, metformin has been positioned as the first-line therapy for T2DM, as well as lifestyle modification. Since this statement, currently, most guidelines also recommend metformin as first-line therapy [Citation4,Citation24], except in Japan where individualized selection of medication based on the pathophysiology of T2DM is recommended [Citation23].
2.4. Issues in pharmacotherapy for T2DM
Issues in pharmacotherapy for T2DM that should be considered when selecting anti-diabetic agents include hypoglycemia, weight gain, iatrogenic hyperinsulinemia (especially in peripheral arteries), postprandial hyperglycemia, other adverse effects specific to the medication, cost, and route of administration (oral or injection). In addition to hypoglycemia, weight gain is a major adverse effect of glucose-lowering medications (). Weight gain is generally associated with treatment with SUs, insulin and thiazolidinediones (TZD). Significant weight gain was also observed in the intensive therapy group in the ACCORD study [Citation25].
Table 1. Comparison among glucose-lowering agents with respect to glucose-lowering efficacy, risk of hypoglycemia, effect on weight, and beta cell protective effect.
Hypoglycemia, weight gain, acceleration of peripheral hyperinsulinemia, and postprandial hyperglycemia are caused by inappropriate, non-physiological insulin replacement by anti-diabetic medications [Citation36]. Therefore, recovery of physiological insulin secretion is important to overcome these issues in the management of T2DM, reminding us that beta cell dysfunction is a major issue in the treatment of T2DM.
Weight gain associated with therapy with TZDs is due to the insulin-sensitizing effects of TZD on adipose tissue and also due to fluid retention. Therefore, TZDs are contraindicated in patients with heart failure [Citation37]. Although TZDs are expected to have anti-atherosclerotic effects [Citation38,Citation39], the possibility of increased risk of myocardial infarction with rosiglitazone has been reported as a caution [Citation40].
2.5. Importance of multifaceted management of CV risk factors
While more strict glycemic control with increased hypoglycemia and weight gain failed to show an improvement of CV outcomes, efficacy of multifactorial management of CV risk factors on CV outcomes in patients with T2DM has been reported. The Steno-2 trial has shown that intensive control of several CV risk factors including HbA1c, blood pressure, and lipid profile in patients with T2DM and albuminuria reduced composite CV events by 53% during 7.8 years of follow-up [Citation41]. A follow up observation study has shown a reduction in CV and all-cause mortality [Citation42].
The J-DOIT3 trial also examined the efficacy of a multifaceted approach to improve CV outcomes in Japanese patients with T2DM [Citation43]. In this study, intensified multifactorial intervention for control of glucose, blood pressure and lipids reduced composite CV events by 24% after adjustment for baseline risk factors during a median follow up of 8.5 years.
2.6. Cardiovascular outcomes trials (CVOTs)
The possibility of increased risk of cardiovascular events with the use of glucose-lowering agents has had a great impact on clinical practice. Since the possibility of increased CV risk with treatment with rosiglitazone was reported, the use of rosiglitazone and another TZD, pioglitazone, has markedly decreased, and rosiglitazone has been removed from the market in several countries [Citation44].
Based on this, the U.S. Food and Drug Administration (FDA) have mandated pharmaceutical companies to test CV safety of new anti-diabetic agents since 2008 [Citation45]. Pharmaceutical companies need to show a 95%CI of <1.3 for 3-point major adverse cardiovascular events (MACE) in phase 3 trials or conduct CVOTs to test non-inferiority compared with placebo on the basis of standard therapy after the approval of new anti-diabetic agents.
Since the change in FDA regulations, a number of CVOTs have been reported with new anti-diabetic agents such as DPP-4 inhibitors, SGLT2 inhibitors, and GLP-1RAs, which resulted in opening a new era in therapy for T2DM.
3. New era in treatment of type 2 diabetes
3.1. Incretin-based drugs
The first incretin-related drug approved in Japan was sitagliptin, a DPP-4 inhibitor, in 2009, followed by approval of the first GLP-1RA, liraglutide, in 2010. Currently, nine DPP-4 inhibitors including two-weekly drugs (sitagliptin, vildagliptin, alogliptin, linagliptin, teneligliptin, anagliptin, saxagliptin, trelagliptin and omarigliptin) and six GLP-1RAs including three-weekly injections (exenatide, extended-release exenatide, liraglutide, lixisenatide, dulaglutide, and semaglutide) are available in Japan.
DPP-4 inhibitors enhance endogenous incretin (GLP-1 and glucose-dependent insulinotropic polypeptide; GIP) effects by inhibiting the incretin-degrading enzyme, DPP-4 [Citation46]. As a result, DPP-4 inhibitors increase insulin secretion in a glucose-dependent manner. DPP-4 inhibitors also suppress glucagon secretion except under a hypoglycemic condition. The risk of hypoglycemia and weight gain is low with treatment with DPP-4 inhibitors. Therefore, DPP-4 inhibitors are a unique class of drugs which restore islet function, a core deficit of T2DM, by direct and indirect mechanisms, as shown in .
Figure 2. Plausible mechanisms of beta cell preservation by incretin drugs. Incretin drugs may preserve and/or improve functional beta cell mass through indirect and direct effects.
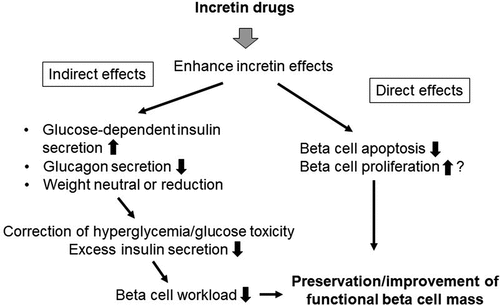
Since DPP-4 inhibitors enhance insulin secretion in a glucose-dependent manner, beta cell workload may not necessarily be increased when hyperglycemia is ameliorated. On the other hand, since SUs increase insulin secretion in a glucose-independent manner, treatment with SUs results in increased risk of hypoglycemia and weight gain. Also, SUs increase beta cell workload irrespective of plasma glucose level and, thereby, often result in secondary failure, probably due to beta cell overwork and exhaustion. Superior glycemic durability of a DPP-4 inhibitor, alogliptin, compared with a SU, glipizide, was shown in the ENDURE trial [Citation47].
GLP-1RAs also enhance GLP-1 action, leading to improved islet function similarly, but to a greater extent than that with DPP-4 inhibitors, resulting in greater glucose-lowering efficacy [Citation3]. Furthermore, in contrast to DPP-4 inhibitors, administration of GLP-1RAs activates the GLP-1 signal at a supraphysiological level, which delays gastric emptying and induces satiety. Thus, GLP-1RAs promote weight loss as well as greater HbA1c reduction compared with DPP-4 inhibitors. In 2014, the FDA approved a higher dose of liraglutide (3 mg/day) for the treatment of obesity [Citation48].
3.2. Combination of insulin and incretin drugs
Incretin drugs have revolutionized the concept of insulin therapy. Insulin therapy aims to reproduce physiological insulin secretion. However, incretin drugs have the potential to overcome the disadvantages of insulin therapy, as shown in , and the combination of insulin and incretin drugs has been shown to achieve optimal glycemic control without increasing the risk of hypoglycemia and weight gain compared with insulin therapy alone [Citation49,Citation50].
Table 2. Advantages and disadvantages of insulin and incretin-based drugs (DPP-4 inhibitors and GLP-1RAs).
The greater efficacy of a combination of incretin drugs and insulin compared with insulin therapy alone has had a great impact on the field of diabetes, since multiple daily injection (MDI) or basal-bolus insulin therapy has been believed to be the ‘best’ available therapy to achieve optimal glycemic control [Citation50,Citation51]. Subcutaneous insulin administration inevitably results in a similar plasma insulin concentration in the portal vein and peripheral arteries, inducing relative hyperinsulinemia in peripheral tissues, while plasma insulin concentration is higher in the portal vein than in peripheral arteries in a physiological condition. Therefore, insulin therapy is now considered to ‘support’ rather than ‘replace’ endogenous insulin secretion through reducing beta cell workload, and concomitant non-insulin treatment that improves endogenous insulin secretion should be considered to reduce the need for exogenous insulin and improve glycemic control without an increase in the risk of hypoglycemia and weight gain. Currently, the ADA/EASD recommends GLP-1RAs as the first injection therapy instead of basal insulin in patients with T2DM [Citation4].
3.3. Paradigm shift in diabetes care after launch of incretin drugs
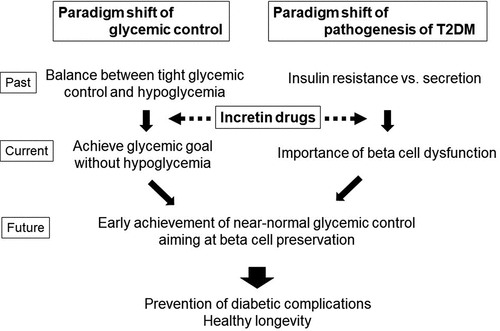
During the decade since incretin-related drugs were first marketed in Japan, the concept of treatment of T2DM has also markedly changed. Before the approval of incretin-related drugs, intensive glycemic control using a SU or insulin was inevitably counter-balanced by the increased risk of hypoglycemia. However, the glucose-dependent insulin secretion induced by incretin drugs has made it possible to achieve near-normal glycemic control without increasing the risk of hypoglycemia, leading to a paradigm shift in the treatment of T2DM (). Not only diabetic complications, but also hypoglycemia and weight gain reduce the quality of life (QOL) of patients with T2DM. Treatment with incretin drugs has been shown to improve treatment satisfaction and QOL in patients with T2DM [Citation52,Citation53]. The increase in treatment options, including these newer agents, has led to the generation of a new concept, ‘patient-centered’ diabetes care, aiming to improve not only hyperglycemia but also QOL in individual patients, stated by the ADA/EASD in 2012 [Citation54].
3.4. SGLT2 inhibitors
The first SGLT2 inhibitor in Japan, ipragliflozin, was approved in 2014, and currently six SGLT2 inhibitors are available in Japan (ipragliflozin, dapagliflozin, canagliflozin, empagliflozin, luseogliflozin, and tofogliflozin). SGLT2 inhibitors lower plasma glucose level by increasing urinary glucose excretion. In addition, SGLT2 inhibitors reduce body weight and improve blood pressure, serum lipid profile, fatty liver, and hyperuricemia [Citation55].
In 2015, the results of the EMPA-REG outcome study, the first CVOT with an SGLT2 inhibitor, were presented at the EASD meeting in Barcelona. The study showed not only non-inferiority but also superiority for CV outcomes with treatment with empagliflozin compared with placebo added to standard therapy in patients with T2DM and prior CV events [Citation56]. The study showed a 14% reduction in 3-point MACE with empagliflozin during 3.1 years of follow up. This was primarily attributable to the 38% reduction in CV deaths, and the risk of hospitalization for heart failure was reduced by 35% by therapy with empagliflozin.
Subsequently, other CVOTs with SGLT2 inhibitors including canagliflozin and dapagliflozin have shown a similar reduction in 3-point MACE and incidence of heart failure hospitalization in patients with T2DM [Citation57–Citation59]. Improvement of renal outcomes was also shown in these studies. Although meta-analysis of CVOTs with SGLT2 inhibitors showed a reduction in heart failure hospitalization and improvement of renal outcomes in patients with T2DM, improvement of CV outcomes was only seen in patients with T2DM and prior CV events [Citation60]. On the other hand, real-world data showed that use of SGLT2 inhibitors was associated with improvement of CV outcomes in T2DM patients both with and without prior CV events [Citation61–Citation63].
Since the EMPA-REG outcome study, various mechanisms have been proposed to explain the improvement of CV outcomes with SGLT2 inhibitors. Although osmotic diuresis and natriuresis seem to play an important role in the improvement of CV outcomes by therapy with SGLT2 inhibitors, other mechanisms including ketogenesis, erythropoiesis, and inhibition of the Na+/H+ exchanger have been proposed [Citation55,Citation64].
3.5. Anti-diabetic agents to improve CV outcomes
Since the EMPA-REG outcome trial was reported, the LEADER trial, the second CVOT with GLP-1RAs, was reported in 2016 [Citation65]. This trial also showed superiority of treatment with liraglutide for CV outcomes compared with standard therapy. In the LEADER trial, treatment with liraglutide reduced the incidence of 3-point MACE by 13% during a median follow up of 3.8 years in T2DM patients at high risk of CVD. Improvement of renal outcomes in the liraglutide group has also been shown in the LEADER trial. Subsequent CVOTs with other GLP-1RAs such as semaglutide [Citation66], albiglutide [Citation67] and dulaglutide [Citation68] have shown similar beneficial effects on CV outcomes compared with standard therapy, and meta-analysis confirmed the improvement of CV and renal outcomes by treatment with GLP-1RAs in patients with T2DM [Citation69], while some GLP-1RAs such as lixisenatide and extended-release exenatide failed to show improvement of CV outcomes [Citation70,Citation71]. Oral semaglutide has also been developed [Citation72] and is currently under application for approval in Japan. GLP-1RAs may delay the progression of atherosclerosis, although the mechanisms by which GLP-1RAs improve CV outcomes remain to be elucidated [Citation3,Citation55]. On the other hand, CVOTs of treatment with DPP-4 inhibitors have shown only non-inferiority for CV outcomes, i.e., CV safety, but not superiority [Citation73–Citation77].
The findings of these CVOTs have led to a change in the current guidelines, and the ADA/EASD guidelines now position GLP-1RAs and SGLT2 inhibitors as the mainstay of the management of T2DM [Citation4].
3.6. Advances in glucose monitoring
In addition to the development of new anti-diabetic drugs, new technologies for glucose monitoring have been recently developed. Especially, the development of continuous glucose monitoring (CGM) has greatly changed the management of diabetes. CGM has made it possible to precisely evaluate the daily glycemic profile and glycemic variability (GV) [Citation78,Citation79]. As a result, a number of reported studies have shown an association between GV and oxidative stress, inflammation, atherosclerosis, and cognitive dysfunction [Citation80–Citation83].
A major component of GV in patients with T2DM is postprandial glycemic excursion. Therefore, management of postprandial glycemic excursion is assumed to be important to prevent diabetic complications [Citation78]. However, therapies aiming to control postprandial hyperglycemia have failed to improve CV outcomes in RCTs [Citation84–Citation86].
Nonetheless, another important aspect in managing GV is prevention of hypoglycemia [Citation78]. Patients with greater GV are at risk of hypoglycemia, both of which are also associated with beta cell dysfunction [Citation87,Citation88]. Thus, optimal control of postprandial glycemic excursion will result in reduction of the risk of hypoglycemia in patients with T2DM.
The use of CGM provides more precise and objective evaluation of GV. Currently, among various GV indices, coefficient of variance (CV) is recommended as a marker of GV [Citation89]. Recently, time in range (TIR) has also been proposed as a new glycemic indicator [Citation89,Citation90]. With the advances in glucose monitoring, glycemic control is now expected to not only lower HbA1c, but also increase TIR without hypoglycemia [Citation90]. Anti-diabetic agents should be selected to achieve this goal.
3.7. Conclusions
Recent advances in anti-diabetic medications such as incretin-based drugs and SGLT2 inhibitors and glucose monitoring using CGM have led to a paradigm shift in diabetes care. These advances have also prompted a paradigm shift in the concept of T2DM. In the following section, the importance of a novel concept, the beta cell-centric concept of T2DM, is discussed from the Asian perspective.
4. Expert opinion
4.1. From ‘glucose-centric’ to ‘beta cell-centric’ concept
As described above, T2DM never develops without beta cell dysfunction, meaning that beta cell dysfunction is an essential factor in the development of T2DM [Citation8,Citation10]. This also indicates that beta cell dysfunction is not the consequence, but the cause of T2DM [Citation18]. Therefore, I propose a ‘beta cell-centric’ rather than a ‘glucose-centric’ concept of T2DM ()[Citation19]. Although T2DM is a heterogenous disease and subclassification of T2DM has been recently proposed [Citation91], the core concept of the disease remains important to facilitate precision medicine.
Table 3. Expected contributions of beta cell-centric concept in management of T2DM.
4.2. Beta cell overwork
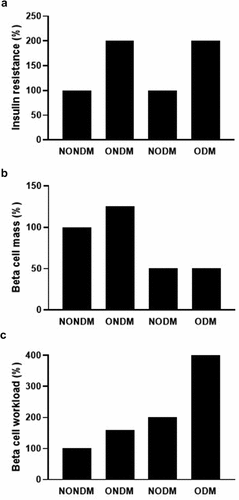
Recent evidence has shown that beta cell function and mass, referred to as functional BCM, are already reduced in patients with prediabetes [Citation8,Citation9,Citation16]. Although the mechanisms by which functional BCM decreases before the development of T2DM remain unclear, various mechanisms have been proposed such as oxidative stress, endoplasmic reticulum (ER) stress, mitochondrial dysfunction, autophagy dysfunction, amyloid toxicity, inflammatory cytokines, and glucolipotoxicity [Citation8]. Rather than a single mechanism, several mechanisms may be involved in this process. However, recent studies have shown that the change in BCM in response to insulin resistance is only modest in humans, indicating that the workload of individual beta cells secreting insulin is easily increased by the condition of insulin resistance in humans ()[Citation8]. This leads to the hypothesis that beta cell overwork is the cause of beta cell dysfunction in T2DM [Citation18]. Asians are known to be at risk of T2DM with lower BMI compared with other ethnicities [Citation92,Citation93]. This may be explained by the smaller increase in BCM in response to obesity in Asians compared with Caucasians [Citation94–Citation96]. Intrauterine environment as well as genetic imprinting may also affect inter-individual difference in BCM [Citation97,Citation98]. It has also been reported that Asians show abdominal fat accumulation with lower BMI compared with Caucasians [Citation99–Citation101], suggesting that insulin resistance is already present with low BMI in Asians, and contributes to increasing beta cell workload [Citation102], although insulin sensitivity is generally higher in Asians when directly compared with Caucasians [Citation103,Citation104].
Figure 4. Hypothetical models of beta cell workload. For example, compared to subjects without obesity and diabetes (NONDM, reference), those with obesity and T2DM (ODM) are assumed to have greater insulin resistance (two-fold) but reduced beta cells (50%), resulting in a 4-fold increase in workload of individual beta cells, which could be attributable to progression of beta cell failure. This model indicates greater beta cell workload even in those with T2DM but without obesity (NODM), due to reduced beta cells, suggesting the importance of reducing beta cell workload in those with T2DM, irrespective of the presence or absence of obesity. This model also indicates greater beta cell workload in those with obesity but without diabetes (ONDM), due to limited beta cell expansion in response to obesity in humans, suggesting the importance of reducing beta cell workload in obese individuals without diabetes to prevent the development of T2DM.
Dysfunctional beta cells eventually lead to beta cell death through induced apoptosis or necrosis [Citation11,Citation105,Citation106]. Other studies have suggested that beta cell dedifferentiation and/or transdifferentiation to other endocrine cells are also involved in reduced BCM in T2DM [Citation107]. ‘Karoshi’ is a Japanese term meaning death due to overwork. We can call beta cell death due to overwork ‘beta cell karoshi’, which would make it easier for the general population to understand the pathophysiology of T2DM [Citation108].
4.3. Importance of beta cell rest
If beta cell overwork and subsequent ‘beta cell karoshi’ is the cause of T2DM, reducing beta cell workload or beta cell rest should be the primary target of treatment of T2DM. The ADOPT study examined the glycemic durability of monotherapy among SU, metformin, and TZD in patients with newly diagnosed T2DM [Citation109]. This study showed superior glycemic durability with metformin and TZD, which reduce beta cell workload compared with SU, which increases beta cell workload, consistent with the importance of beta cell rest, as also supported by rodent studies [Citation110].
Currently, most guidelines recommend metformin as the first-line medication for T2DM [Citation4,Citation24]. In Japan, although the current guidelines do not state it is ‘the first line drug’ and rather emphasize individualized treatment options based on the pathophysiology of T2DM [Citation23], metformin should be considered as the first-line drug also in Japanese despite the lower BMI in this population compared with other ethnicities such as Caucasians, aiming at reducing beta cell workload as much as possible. Metformin has been shown to effectively improve glycemic control irrespective of the presence or absence of obesity [Citation33]. Metformin has also been shown to improve beta cell function irrespective of obesity in the Asian population [Citation111].
4.4. Early combination therapy
The fact that functional BCM is already reduced by ~50% or even more in people with prediabetes indicates the need for early intensive care to prevent further reduction in functional BCM and worsening glucose metabolism. Weight loss results in the improvement of insulin sensitivity and reduction in beta cell workload. Indeed, the marked weight loss after metabolic surgery is strongly associated with diabetes remission [Citation112,Citation113]. A recent study also showed that weight reduction by caloric restriction in patients with newly diagnosed T2DM resulted in diabetes remission [Citation114].
The ADA/EASD recommend that metformin, as well as lifestyle modification, should be started in patients with newly diagnosed T2DM [Citation4]. After metformin, stepwise intensification of anti-diabetic medication is recommended if the glycemic goal for the patient is not achieved. Recently, the VERIFY study has been conducted to examine the glycemic durability of early combination therapy of metformin and vildagliptin, a DPP-4 inhibitor, in patients with newly diagnosed T2DM [Citation115]. In the VERIFY study, early combination therapy resulted in better glycemic durability during 5 years of follow up compared with metformin monotherapy, indicating a delay in disease progression. These findings highlight the importance of early treatment intensification aiming at beta cell preservation to delay the progression of T2DM.
A fixed-dose combination tablet has been shown to improve patients’ adherence to medication [Citation116]. Currently, fixed-dose combinations of basal insulin and GLP-1RAs are also available [Citation117]. The use of fixed-dose combination drugs is expected to foster early intensification and prevent clinical inertia.
4.5. Implementing person-centered diabetes care
Lifestyle modification, including reducing excess calorie and carbohydrate intake and physical inactivity, is the core of treatment of T2DM. However, it is often difficult to continue a healthy lifestyle lifelong. Correct understanding of T2DM will be the initial step to improve patients’ adherence to lifestyle modification.
The paradigm shift from a ‘glucose-centric’ to a ‘beta cell-centric’ concept of T2DM emphasizes the importance of preservation of beta cells rather than lowering HbA1c. In the glucose-centric concept, patients often become overly anxious about the up and down fluctuations of plasma glucose and HbA1 c levels. Patients and physicians also primarily judge the adherence to lifestyle modification by HbA1c level. However, as T2DM is a progressive disease, HbA1c level will often worsen over time, which may induce a feeling of ineffectiveness and depression, and cause patients to discontinue treatment.
In contrast, with the beta cell-centric concept, since patients understand the progressive nature of the disease, they may act to delay the progression of disease irrespective of their HbA1c level. Also, this may change the relationship between patients and healthcare professionals from ‘confrontation’ to ‘alliance’, fighting together to protect beta cells. This concept also helps patients to realize the importance of early treatment to preserve beta cells, also preventing clinical inertia. Finally, this concept will emphasize the importance of prevention of T2DM to the general population, endorsing advocacy of patients with T2DM.
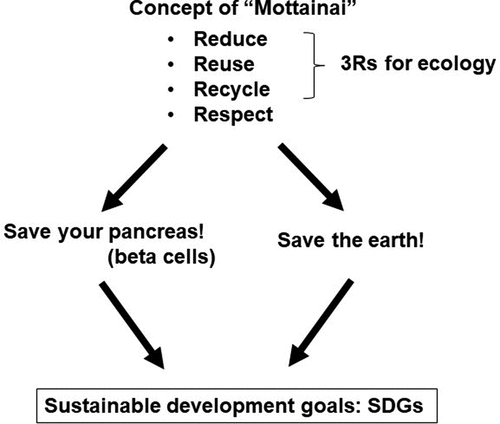
In 2015, Sustainable Development Goals (SDGs) were proposed by the United Nations. SDGs consist of 17 goals and 169 targets, including reinforcement of human health and ecology of the earth [Citation118]. ‘Mottainai’ is a Japanese term which contains ‘3Rs’ for ecology, i.e., reduce, reuse and recycle, and also ‘respect’ as the 4th ‘R’ in its meaning [Citation119]. Wangari Maathai (1940–2011), a Kenyan ecologist and Nobel Peace Prize winner, was impressed by this Japanese word and proposed making it a global concept to fight against the waste of resources, promoting the MOTTAINAI Campaign in 2005 to spread this concept both within Japan and internationally.
The concept of ecology may be synchronized with the concept of diabetes care. ‘Save the earth’ can be rephrased as ‘Save your pancreas (beta cells)’. We need to ‘reduce’ beta cell workload by pursuing a healthy lifestyle with ‘respect’ for our body. The beta cell-centric concept of T2DM helps people recognize beta cells as a limited and precious resource in our body, as an organ with a total weight of only approximately 1 g that may be lost by overuse. Maintaining a healthy lifestyle may also foster awareness of environmental issues and promote an eco-friendly society. In other words, we need to change ourselves to change the world. Integrating the more common and global goals in diabetes care will motivate people to adhere to ‘sustainable’ individual lifestyle modification, eventually leading to the achievement of SDGs. Recognition of the global goals will change the way of thinking to one that lifestyle modification is not only for patients with T2DM but also for the general population, and sharing this concept among people with and without diabetes will possibly diminish the ‘stigma’ of diabetes and increase advocacy of person-centered diabetes care ().
4.6. Summary and future directions: All roads lead to beta cells
This narrative review summarizes recent advances in anti-diabetic medications and glucose monitoring technologies and their impact on diabetes care. Along with these advances, a paradigm shift in diabetes management and the concept of T2DM has emerged. Now it is considered that beta cell deficit is the common pathogenesis of both T1DM and T2DM, and T2DM is characterized by beta cell dysfunction caused by insulin resistance increasing beta cell workload. The management of T2DM should therefore aim to reduce beta cell workload and restore functional BCM ().
Figure 6. Updated management strategy for T2DM (modified from [Citation8]). T2DM is characterized by progressive loss of functional beta cell mass (BCM) on a background of insulin resistance. Therefore, treatment of T2DM should focus on aiming at reducing beta cell workload to maintain optimal glycemic control. Lifestyle modification and weight loss remain the most fundamental and important therapy to improve insulin resistance. Pharmacotherapy should also be initiated to reduce beta cell workload, and thereby, metformin, SGLT2 inhibitors, and α-glucosidase inhibitors are preferred if tolerated. GLP-1RAs and SGLT2 inhibitors are expected to improve CV outcomes, and their use should be considered in those at high risk of CVD and those with heart failure and/or chronic kidney disease. DPP-4 inhibitors are expected to improve beta cell function, and better glycemic durability with initial combination therapy of a DPP-4 inhibitor with metformin compared with metformin monotherapy has been reported. Selection of the treatment option should be individualized according to a patient-centered approach. * Cardiorenal benefits have been shown in CVOTs.
![Figure 6. Updated management strategy for T2DM (modified from [Citation8]). T2DM is characterized by progressive loss of functional beta cell mass (BCM) on a background of insulin resistance. Therefore, treatment of T2DM should focus on aiming at reducing beta cell workload to maintain optimal glycemic control. Lifestyle modification and weight loss remain the most fundamental and important therapy to improve insulin resistance. Pharmacotherapy should also be initiated to reduce beta cell workload, and thereby, metformin, SGLT2 inhibitors, and α-glucosidase inhibitors are preferred if tolerated. GLP-1RAs and SGLT2 inhibitors are expected to improve CV outcomes, and their use should be considered in those at high risk of CVD and those with heart failure and/or chronic kidney disease. DPP-4 inhibitors are expected to improve beta cell function, and better glycemic durability with initial combination therapy of a DPP-4 inhibitor with metformin compared with metformin monotherapy has been reported. Selection of the treatment option should be individualized according to a patient-centered approach. * Cardiorenal benefits have been shown in CVOTs.](/cms/asset/2bcabd29-4603-48d9-bd24-21710f7aee8f/ieop_a_1776262_f0006_b.gif)
Newer anti-diabetic agents such as DPP-4 inhibitors, GLP-1RAs, and SGLT2 inhibitors improve beta cell function and lower HbA1c without increasing the risk of hypoglycemia and weight gain. Further, GLP-1RAs and SGLT2 inhibitors induce weight loss and have been shown to improve CV outcomes. Therefore, in addition to metformin, GLP-1RAs, and SGLT2 inhibitors should be preferentially considered especially for patients with T2DM at risk of CV disease.
Also, considering beta cell deficit and the progressive nature of the disease, early intensification of treatment should be considered. This may be more important for younger patients whose glycemic control and diabetic complications worsen more progressively [Citation120–Citation122]. Use of a fixed-dose combination tablet or injection is useful not only to improve adherence to therapy, but also to introduce early treatment intensification and prevent clinical inertia.
Further development of anti-diabetic agents such as oral GLP-1RAs [Citation123], imeglimin [Citation124] and dual agonists including GLP-1-GIP [Citation125], GLP-1-glucagon [Citation126], GLP-1-peptide YY [Citation127] and GLP-1-amylin [Citation128] is also expected to improve beta cell function directly and/or indirectly through weight loss and improve glycemic control.
Nevertheless, to preserve functional BCM, lifestyle modification remains the most fundamental and important therapy for patients with T2DM. Integration of diabetes care into the global goals, SDGs, may change people’s daily behavior and motivate both those with and without T2DM to maintain a ‘healthy’ lifestyle lifelong. The beta cell-centric concept of T2DM is therefore useful to change our ideas and improve the management of T2DM, fostering ‘person-centered’ diabetes care. Pharmacotherapy then should be used aiming to help patients lose weight with lifestyle modification.
Article highlights
Recent advances in anti-diabetic medications such as DPP-4 inhibitors, GLP-1 receptor agonists and SGLT2 inhibitors have led to a paradigm shift in diabetes care, and also prompted a paradigm shift in the concept of type 2 diabetes (T2DM), highlighting the importance of beta cell dysfunction in the pathophysiology of T2DM.
Recent studies have revealed that functional beta cell mass is reduced in patients with T2DM irrespective of the presence or absence of obesity, resulting in increased workload of residual beta cells.
Management of T2DM including lifestyle modification as well as anti-diabetic medications should therefore be focused on reducing beta cell workload, to preserve functional beta cell mass.
The paradigm shift from a glucose-centric to a beta cell-centric concept of T2DM enhances the implementation of person-centered diabetes care.
This box summarizes key points contained in the article.
Declaration of interest
Y Saisho has received honoraria from Sumitomo Dainippon Pharma Co., Ltd., Japan, Novartis Pharma K.K., Japan, Takeda Pharma Co., Ltd., Japan and Merck Sharp and Dohme K.K., Japan. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
We thank Wendy Gray (London, UK) for editing the manuscript.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- UK Prospective Diabetes. Study (UKPDS) Group: intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853..
- Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589..
- Holst JJ. Incretin therapy for diabetes mellitus type 2. Curr Opin Endocrinol Diabetes Obes. 2020;27(1):2–10.
- Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European association for the study of diabetes (EASD). Diabetologia. 2020;63(2):221–228.
- Yokoyama H, Oishi M, Takamura H, et al. Large-scale survey of rates of achieving targets for blood glucose, blood pressure, and lipids and prevalence of complications in type 2 diabetes (JDDM 40). BMJ Open Diabetes Res Care. 2016;4(1):e000294.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement_1):S81–S90.
- Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes M, Seino Y, Nanjo K, Tajima N, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010;1(5):212–228.
- Saisho Y. Beta cell dysfunction: its critical role in prevention and management of type 2 diabetes. World J Diabetes. 2015;6(1):109–124.
- DeFronzo RA, Abdul-Ghani MA. Preservation of beta-cell function: the key to diabetes prevention. J Clin Endocrinol Metab. 2011;96(8):2354–2366.
- Weir GC, Gaglia J, Bonner-Weir S. Inadequate beta-cell mass is essential for the pathogenesis of type 2 diabetes. Lancet Diabetes Endocrinol. 2020;8(3):249–256.
- Butler AE, Janson J, Bonner-Weir S, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110..
- Yoon KH, Ko SH, Cho JH, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88(5):2300–2308.
- Sakuraba H, Mizukami H, Yagihashi N, et al. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45(1):85–96.
- Rahier J, Guiot Y, Goebbels RM, et al. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):32–42.
- Meier JJ, Breuer TG, Bonadonna RC, et al. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia. 2012;55(5):1346–1354.
- Inaishi J, Saisho Y, Hirakawa Y, et al. Association of glucose tolerance status with pancreatic beta- and alpha-cell mass in community-based autopsy samples of Japanese individuals: the Hisayama Study. J Diabetes Investig. 2020. 10.1111/jdi.13232
- American Diabetes Association. 2. classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care. 2020;43:S14–S31.
- Saisho Y. Changing the concept of type 2 diabetes: beta cell workload hypothesis revisited. Endocr Metab Immune Disord Drug Targets. 2019;19(2):121–127.
- Saisho Y. How can we develop more effective strategies for type 2 diabetes mellitus prevention? A paradigm shift from a glucose-centric to a beta cell-centric concept of diabetes. EMJ Diabet. 2018;6:46–52.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854–865.
- Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–117.
- Haneda M, Noda M, Origasa H, et al. Japanese clinical practice guideline for diabetes 2016. J Diabetes Investig. 2018;9(3):657–697.
- International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104(1):1–52.
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559.
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
- Reaven PD, Moritz TE, Schwenke DC, et al. Intensive glucose-lowering therapy reduces cardiovascular disease events in veterans affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes. 2009;58(11):2642–2648.
- Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375(9713):481–489.
- Cavero-Redondo I, Peleteiro B, Alvarez-Bueno C, et al. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis. BMJ Open. 2017;7(7):e015949.
- Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33(6):1389–1394.
- Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410–1418.
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340(jan08 1):b4909.
- Saisho Y. Metformin and inflammation: its potential beyond glucose-lowering effect. Endocr Metab Immune Disord Drug Targets. 2015;15(3):196–205.
- DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The multicenter metformin study group. N Engl J Med. 1995;333(9):541–549.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European association for the study of diabetes. Diabetes Care. 2009;32(1):193–203.
- Saisho Y. Importance of beta cell function for the treatment of type 2 diabetes. J Clin Med. 2014;3(3):923–943.
- Chaggar PS, Shaw SM, Williams SG. Review article: thiazolidinediones and heart failure. Diab Vasc Dis Res. 2009;6(3):146–152.
- Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone clinical trial in macrovascular events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289.
- Young LH, Viscoli CM, Curtis JP, et al. Cardiac outcomes after ischemic stroke or transient ischemic attack: effects of pioglitazone in patients with insulin resistance without diabetes mellitus. Circulation. 2017;135(20):1882–1893.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471.
- Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393.
- Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591.
- Ueki K, Sasako T, Okazaki Y, et al., Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 5(12): 951–964. 2017. .
- Nissen SE. The rise and fall of rosiglitazone. Eur Heart J. 2010;31(7):773–776.
- Sharma A, Pagidipati NJ, Califf RM, et al. Impact of regulatory guidance on evaluating cardiovascular risk of new glucose-lowering therapies to treat type 2 diabetes mellitus. Circulation. 2020;141:843–862.
- Gallwitz B. Clinical Use of DPP-4 Inhibitors. Front Endocrinol (Lausanne). 2019;10:389.
- Del Prato S, Camisasca R, Wilson C, et al. Durability of the efficacy and safety of alogliptin compared with glipizide in type 2 diabetes mellitus: a 2-year study. Diabetes Obesity Metab. 2014;16(12):1239–1246.
- Nuffer WA, Trujillo JM. Liraglutide: a new option for the treatment of obesity. Pharmacotherapy. 2015;35(10):926–934.
- Kim YG, Min SH, Hahn S, et al. Efficacy and safety of the addition of a dipeptidyl peptidase-4 inhibitor to insulin therapy in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2016;116:86–95.
- Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384(9961):2228–2234.
- Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of IDegLira versus basal-bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care. 2018;41(5):1009–1016.
- Davies M, Speight J. Patient-reported outcomes in trials of incretin-based therapies in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14(10):882–892.
- Saisho Y. Use of diabetes treatment satisfaction questionnaire in diabetes care: importance of patient-reported outcomes. Int J Environ Res Public Health. 2018;15(5):947.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the american diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care. 2012;35(6):1364–1379.
- Nagahisa T, Saisho Y. Cardiorenal protection: potential of SGLT2 Inhibitors and GLP-1 receptor agonists in the treatment of type 2 diabetes. Diabetes Therapy. 2019;10(5):1733–1752.
- Zinman B, Wanner C, Lachin JM, et al., Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 373(22): 2117–2128. 2015.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;373(7):644–657.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357.
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306.
- Zelniker TA, Wiviott SD, Raz I, et al., SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 393(10166): 31–39. 2019. .
- Kosiborod M, Cavender MA, Fu AZ, et al., Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL Study (Comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors). Circulation. 136(3): 249–259. 2017. .
- Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71(23):2628–2639.
- Birkeland KI, Jorgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5(9):709–717.
- Verma S, JJV: M. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61(10):2108–2117.
- Marso SP, Daniels GH, Brown-Frandsen K, et al., Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 375(4): 311–322. 2016.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844.
- Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–1529.
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130.
- Kristensen SL, Rorth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785.
- Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257.
- Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239.
- Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–851.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326.
- Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242.
- Rosenstock J, Perkovic V, Johansen O, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the carmelina randomized clinical trial. JAMA. 2018;321(1):69–79.
- Rosenstock J, Kahn SE, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322(12):1155–1166.
- Saisho Y. Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int J Mol Sci. 2014;15(10):18381–18406.
- Dandona P. Minimizing glycemic fluctuations in patients with type 2 diabetes: approaches and importance. Diabetes Technol Ther. 2017;19(9):498–506.
- Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687.
- Okada K, Hibi K, Gohbara M, et al. Association between blood glucose variability and coronary plaque instability in patients with acute coronary syndromes. Cardiovasc Diabetol. 2015;14:(1):111.
- Teraguchi I, Imanishi T, Ozaki Y, et al. Impact of glucose fluctuation and monocyte subsets on coronary plaque rupture. Nutr Metab Cardiovasc Dis. 2014;24(3)::309–314.
- Rizzo MR, Marfella R, Barbieri M, et al. Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care. 2010;33(10):2169–2174.
- Raz I, Wilson PW, Strojek K, et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care. 2009;32(3):381–386.
- Holman RR, Haffner SM, McMurray JJ, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1463–1476.
- Holman RR, Coleman RL, Chan JCN, et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(11):877–886.
- Saisho Y, Tanaka C, Tanaka K, et al. Relationships among different glycemic variability indices obtained by continuous glucose monitoring. Prim Care Diabetes. 2015;9(4):290–296.
- Hope SV, Knight BA, Shields BM, et al. Random non-fasting C-peptide testing can identify patients with insulin-treated type 2 diabetes at high risk of hypoglycaemia. Diabetologia. 2018;61(1):66–74.
- Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603.
- Advani A. Positioning time in range in diabetes management. Diabetologia. 2020;63(2):242–252.
- Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–369.
- Hsu WC, Araneta MRG, Kanaya AM, et al. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care. 2015;38(1):150–158.
- Zhu Y, Sidell MA, Arterburn D, et al. Racial/ethnic disparities in the prevalence of diabetes and prediabetes by bmi: patient outcomes research to advance learning (PORTAL) multisite cohort of adults in the U.S. Diabetes Care. 2019;42(12):2211–2219.
- Kou K, Saisho Y, Satoh S, et al. Change in beta-cell mass in Japanese nondiabetic obese individuals. J Clin Endocrinol Metab. 2013;98(9):3724–3730.
- Inaishi J, Saisho Y, Sato S, et al. Effects of obesity and diabetes on alpha- and beta-cell mass in surgically resected human pancreas. J Clin Endocrinol Metab. 2016;101(7):2874–2882.
- Mizukami H, Takahashi K, Inaba W, et al. Age-associated changes of islet endocrine cells and the effects of body mass index in Japanese. J Diabetes Investig. 2014;5:38–47.
- Sasaki H, Saisho Y, Inaishi J, et al. Associations of birthweight and history of childhood obesity with beta cell mass in Japanese adults. Diabetologia. 2020;63(6):1199–1210.
- Le Bacquer O, Kerr-Conte J, Gargani S, et al. TCF7L2 rs7903146 impairs islet function and morphology in non-diabetic individuals. Diabetologia. 2012;55(10):2677–2681.
- Azuma K, Kadowaki T, Cetinel C, et al. Higher liver fat content among Japanese in Japan compared with non-hispanic whites in the United States. Metabolism. 2009;58(8):1200–1207.
- Sugimoto D, Tamura Y, Takeno K, et al. Clinical features of non-obese, apparently healthy japanese men with reduced adipose tissue insulin sensitivity. J Clin Endocrinol Metab. 2019;104(6):2325–2333.
- Hulman A, Simmons RK, Brunner EJ, et al. Trajectories of glycaemia, insulin sensitivity and insulin secretion in South Asian and white individuals before diagnosis of type 2 diabetes: a longitudinal analysis from the Whitehall II cohort study. Diabetologia. 2017;60(7):1252–1260.
- Wang T, Lu J, Shi L, et al. Association of insulin resistance and β-cell dysfunction with incident diabetes among adults in China: a nationwide, population-based, prospective cohort study. Lancet Diabetes Endocrinol. 2019;8(2):115–124.
- Kodama K, Tojjar D, Yamada S, et al. Ethnic differences in the relationship between insulin sensitivity and insulin response: A systematic review and meta-analysis. Diabetes Care. 2013;36(6)::1789–1796.
- Møller JB, Man CD, Overgaard RV, et al. Ethnic differences in insulin sensitivity, β-Cell function, and hepatic extraction between Japanese and caucasians: a minimal model analysis. J Clin Endocrinol Metab. 2014;99(11):4273–4280.
- Jurgens CA, Toukatly MN, Fligner CL, et al. beta-cell loss and beta-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011;178(6):2632–2640.
- Mizukami H, Takahashi K, Inaba W, et al. Involvement of oxidative stress-induced DNA damage, endoplasmic reticulum stress, and autophagy deficits in the decline of beta-cell mass in Japanese type 2 diabetic patients. Diabetes Care. 2014;37(7):1966–1974.
- Accili D. Insulin action research and the future of diabetes treatment: the 2017 banting medal for scientific achievement lecture. Diabetes. 2018;67(9):1701–1709.
- Saisho Y. Prevention of beta cell “karoshi”: a new paradigm for prevention and management of type 2 diabetes. Med Res Archives. 2016;4(6). 10.18103/mra.v4i6.772.
- Kahn SE, Haffner SM, Heise MA, et al., Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 355(23)::2427–2443. 2006.
- Boland BB, Brown C, Boland ML, et al. Pancreatic β-cell rest replenishes insulin secretory capacity and attenuates diabetes in an extreme model of obese type 2 diabetes. Diabetes. 2019;68(1):131–140.
- Bi Y, Tong GY, Yang HJ, et al. The beneficial effect of metformin on β-cell function in non-obese Chinese subjects with newly diagnosed type 2 diabetes. Diabetes Metab Res Rev. 2013;29(8):664–672.
- Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39(6):861–877.
- Cefalu WT, Rubino F, Cummings DE. Metabolic surgery for type 2 diabetes: changing the landscape of diabetes care. Diabetes Care. 2016;39(6):857–860.
- Lean ME, Leslie WS, Barnes AC, et al., Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 391(10120): 541–551. 2018.
- Matthews DR, Paldanius PM, Proot P, et al. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet. 2019;394(10208):1519–1529.
- Hutchins V, Zhang B, Fleurence RL, et al. A systematic review of adherence, treatment satisfaction and costs, in fixed-dose combination regimens in type 2 diabetes. Curr Med Res Opin. 2011;27(6):1157–1168.
- Schlosser R. Fixed-dose and fixed-ratio combination therapies in type 2 diabetes. Can J Diabetes. 2019;43(6):440–444.
- The United Nations. Sustainable Development Goals. [cited 2020 Mar 18]. Available from: https://sustainabledevelopment.un.org/post2015
- Public Relations Office Government of Japan. Highlighting Japan. [cited 2020 Mar 18]. Available from: https://www.gov-online.go.jp/eng/publicity/book/hlj/html/201807/201807_12_en.html
- Consortium R, Investigators RC. Effects of treatment of impaired glucose tolerance or recently diagnosed type 2 diabetes with metformin alone or in combination with insulin glargine on beta-cell function: comparison of responses in youth and adults. Diabetes. 2019;68:1670–1680.
- Dennis JM, Shields BM, Henley WE, et al. Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol. 2019;7(6):442–451.
- Sattar N, Rawshani A, Franzén S, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation. 2019;139(19):2228–2237.
- Hedrington MS, Davis SN. Oral semaglutide for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2019;20(2):133–141.
- Johansson KS, Bronden A, Knop FK, et al. Clinical pharmacology of imeglimin for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2020;1–12. 10.1080/14656566.2020.1729123
- Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180–2193.
- Ambery P, Parker VE, Stumvoll M, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391(10140):2607–2618.
- Svane MS, Jorgensen NB, Bojsen-Moller KN, et al. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int J Obes (Lond). 2016;40(11):1699–1706.
- Boyle CN, Lutz TA, Le Foll C. Amylin - Its role in the homeostatic and hedonic control of eating and recent developments of amylin analogs to treat obesity. Mol Metab. 2018;8:203–210.