1. Introduction
The discovery of ALK and ROS1 gene rearrangements in nonsmall-cell lung cancer (NSCLC) patients 2007 identified two new genetically distinct tumor populations with the potential to be effectively targeted with specific therapies [Citation1,Citation2]. Crizotinib, first-in class MET, ALK, and ROS1 inhibitor, had shown marked antitumor activity either in ALK- and ROS1-addicted NSCLC expansion cohorts from the pivotal phase I study (PROFILE 1001) and in subsequent phase II e III trials (, ), thus determining the conditional approval for the treatment of ALK- (in 2011) and ROS1-driven (in 2016) NSCLC. Evidence of activity of crizotinib in MET-dependent NSCLC are currently opening new fascinating research and clinical perspectives.
Figure 1. Activity (Overall response rate, according to RECIST 1.1 Criteria) of crizotinib for oncogene-addicted NSCLC (studies conducted exclusively in Asian patients are excluded)
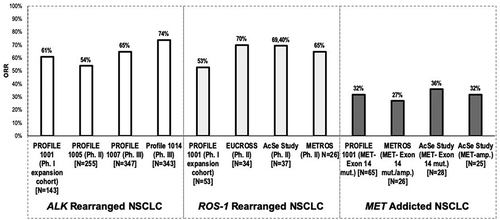
Table 1. Prospective trials using crizotinib for oncogene-addicted NSCLC (studies conducted exclusively in Asian patients are excluded)
2. Crizotinib for treatment of ALK-rearranged NSCLC
In the original phase I trial (PROFILE 1001) crizotinib was initially developed to target cMET in patients with various types of solid advanced cancer. Due to the promising clinical activity in two patients with ALK-positive NSCLC enrolled during the dose escalation phase, a featured cohort was expanded: in 119 evaluable patients, the objective response rate (ORR) was 61% [Citation3]. Similar results (ORR 54%) were obtained from 136 pretreated patients (≥1 previous chemotherapy line) in the phase II trial (PROFILE 1005). Based on these findings, crizotinib was granted the accelerated approval by the Food and Drug Administration (FDA) and European Medical Agency (EMA) and entered in clinical practice. Afterward, crizotinib confirmed superior head-to-head activity compared to chemotherapy in both second (PROFILE 1007) and first line (PROFILE 1014) settings [Citation2]. Unfortunately, since crizotinib introduction in daily routine, it has become apparent that the vast majority of patients will invariably: (1) relapse due acquired drug resistance or (2) experience central nervous system (CNS) progression due also to low drug penetration through blood-brain barrier. Next-generation ALK tyrosine kinase inhibitors (TKIs) have made significant strides toward overcoming some of these limitations, particularly in their potency for targeting a greater spectrum of secondary resistance mutations and by increasing CNS penetration [Citation2,Citation4]. In this regard, in the head-to-head ALEX phase III trial, alectinib improved median PFS in overall population (34.8 months (95% CI 17.7–NR) vs 10.9 m (95% CI 9.1–12.9; HR 0.43, 95% CI 0.32–0.58; p < 0.0001), also considering subgroups of patients with baseline CNS metastases (25.4 months vs 7.4 months, respectively; HR 0.37 95%CI 0.23–0.58) and in those without (38.6 vs 14.8 months, respectively, HR 0.46, 95% CI 0.31–0.68). OS data remain immature (events: 32%; stratified HR 0.69, 95% CI 0.47–1.02); the 4-year OS rate was 64.5% (95% CI 55.6–73.4) with alectinib vs 52.2% (95% CI 42.6–64.8) with crizotinib [Citation5]. Brigatinib has also shown superior efficacy over crizotinib in the ALTA-1 L trial in ALK-inhibitors naïve patients, reporting HR for PFS of 0.49 (95% CI 0.33 to 0.74; p < 0.001), PFS rates at 1 year of 67% and 43%, response rates of 71% and 60%, and intracranial response rates of 78% and 29%, respectively [Citation6]. Lorlatinib, a third-generation inhibitor of ALK and ROS1 tyrosine kinases, was designed to overcome major limitations of earlier ALK inhibitors and it is active against acquired resistance mutations such as ALK G1202 R, which represents a common mechanism of resistance to first generation inhibitors. In a phase II global study, patients were enrolled into six different single-arm expansion cohorts, according to previous treatments. Among ALK-positive patients, the objective response was 90% for treatment-naive patients (30) and 47% for those with at least one previous ALK TKI (n = 198); intracranial responses were seen in 67% treatment-naïve patients and 63% patients pretreated with at least one ALK TKI [Citation7]. The reported superiority in survival outcomes and the absence of new relevant safety concerns lead to a shift to next-generation ALK-TKIs as preferred upfront treatment for ALK+ NSCLC.
3. Crizotinib for treatment of ROS-1-rearranged NSCLC
Considering the close homology between the ALK and ROS1 tyrosine kinase domains, crizotinib was also explored as ROS-1 fusion protein inhibitor, showing up to five times greater potency in the suppression of ROS1 activity and downstream signaling than what had been observed in ALK-rearranged tumors [Citation8]. In the phase I PROFILE 1001, 53 ROS1-positive NSCLC patients, determined by FISH, were enrolled: the objective response rate (ORR) was 72% (95% CI 58%–83%), including six confirmed complete responses, with median duration of response (DoR) of 24.7 months (95% CI, 15.2–45.3); median PFS was 19.3 months (95% CI, 15.2–39.1) and median OS was 51.4 months (95% CI, 29.3 – not reached) and survival probabilities at 12, 24, 36, and 48 months were 79%, 67%, 53%, and 51%, respectively. These results mirror those observed in ALK+ NSCLC and are comparable to what reported in subsequent prospective trials, where response rates yielded 70%–80% [Citation9]. At present, crizotinib has a well-established role in ROS1-positive NSCLC and is worldwide available. However, development of acquired resistance to crizotinib in ROS1-rearranged tumors, mainly due to the appearance of G2032 R secondary mutation, and intracranial progression related to low CNS drug penetration, poses a serious clinical challenge and several new generation ROS-1 inhibitors (lorlatinib, entrectinib) are currently investigating in phase I–II trials [Citation10–13]. However, despite significant activity in crizotinib-resistent patients and likely better intracranial activity, any newer compound has shown significant improvement of the crizotinib PFS in first line setting.
4. Crizotinib for treatment of MET-deregulated NSCLC
Aberrant MET activation by exon 14 mutations (MET-Exon 14 mut.) or gene amplification (MET-amp.) occurs in 3–4% of NSCLCs and it is associated with unfavorable prognosis [Citation14]. The prospective results of expansion cohort of the PROFILE 1001 including MET-Exon 14 mut. NSCLCs were released recently [Citation15]. In this pivotal dataset, the majority of patients had adenocarcinoma histology (84%) and had received ≥1 previous lines of treatment for advanced disease (62%). Among 65 evaluable patients, crizotinib achieved an ORR of 32% (95% CI, 21–45%), a median duration of response of 9.1 months, and a median PFS of 7.3 months. Objective responses were observed independently by splice-site region and mutation type of the MET exon 14 alteration, concurrent increased MET copy number or the detection of a MET exon 14 alteration in circulating tumor DNA.
Other two national-level prospective studies were conducted in MET-deregulated NSCLC, investigating both MET-Exon 14 mut. and MET-amp. patients. The Italian METROS trial [Citation10] enrolled patients with MET-Exon 14 mut. tumors and patients with intermediate or high levels of MET amplification defined as a ratio MET/centromere 7 (MET/CEP7) of > 2.2 – < 5 or > 5, respectively. All patients included had progressed after at least one previous chemotherapy line and MET alterations were retrospectively confirmed centrally by FISH (MET-ampl) and NGS (MET-Exon 14 mut.). Objective response rate was 27%, median PFS was 4.4 months (95% 122 CI 3.0–5.8) and median OS was 5.4 months (95% CI 15.2‒30.3). No difference in any clinical end-point was observed between MET amplified and Exon14 mutated patients; interestingly, any response was observed among the five patients with cooccurrence of a second gene alteration.
Similarly, the French AcSè study [Citation11] enrolled heavily-pretreated patients with tumors having amplified MET (cut off ≥ 6 gene copies) and Exon 14 MET mutations; in this trial samples with an increase in the number of copies of c-MET were secondly classified by the c-MET copies per centromere ratio: high polysomy (<1,8 cMET/centromere), low (≥1,8-≤2,2), intermediate (>2,2-<5,0), and high amplifications (≥5,0). In order to assess crizotinib efficacy, ORR after two cycles was adopted as primary endpoint; disease control rate at two and four cycles, best overall response rate (BOR), progression-free survival (PFS) and overall survival (OS) were secondary endpoints.
In the c-MET-≥6 copies cohort (25 patients), the ORR after two cycles was low (16%) and numerous tumor responses were observed beyond two cycles (BOR 32%, disease control rate at four cycles was 52%); in the exploratory analysis, the c-MET-amplification level (high/intermediate versus low polysomy) was associated with BOR (P = 0.04) and potentially predictive of higher response rates. The median PFS was 3.2 months (95% CI 1.9–3.7) and the median OS was 7.7 months (95% CI 4.6–15.7). In the c-MET-mutation cohort (28 patients), the ORR at two cycles was 10.7% (95% CI 2.3%- 28.2%), not sufficient for further evaluation according to sample-size statistical plan. However, improved response rates were also observed beyond the two first cycles: BOR during treatment was 36% (95% CI 21–54%) and the disease control rate at four cycles was 39% (95% CI 21.5–59.4%). The median PFS was 2.4 months (95% CI 1.6–5.9) and the median OS was 8.1 months (95% CI 4.1–12.7).
5. Expert opinion
Fusion events involving ALK and ROS1 genes are found respectively in 5–7% and 1–2% of NSCLC patients and represent distinct molecular tumor subtypes with specific clinical characteristics and drugs’ vulnerabilities. The discovery of crizotinib clinical activity and its superiority compared to platinum-based chemotherapy opened a new scenario into the field of oncogene-addicted lung cancer and established a new paradigm for the development of next-generation drugs. Indeed, second and third generation ALK/ROS-1 TKIs were mainly designed for overcoming pharmacodynamics and pharmacokinetics limitations of crizotinib, including a greater activity for a larger spectrum of rearranged kinase mutations and improved penetration through the blood-brain barrier. Thanks to these pharmacology developments, new TKIs produced significantly better survival outcomes compared with crizotinib, either considering indirect comparison in single-arm phase I–II trials and either in head-to-head phase III studies. Based on these findings, in clinical practice next generation TKIs have overtaken crizotinib as upfront treatment for ALK-rearranged NSCLCs. In the field of ROS-1 TKI-naïve rearranged tumors, no new compound has yet to improve on crizotinib’s PFS results, maybe due to less impact of intracranial progression in disease history. Consequently, head-to-head trials are required before changing treatment algorithms.
Treatment of MET-addicted tumors is an emerging research field, also considering the primary role these alterations as resistance mechanism to third-generation EGFR TKIs, and the sporadic off-label use of MET-inhibitors in clinical practice for such patients. In the PROFILE 1001 MET-Exon 14 mut cohort, the ORR of crizotinib in pretreated populations is not negligible, although lower when compared to other targeted therapy for oncogene-addicted NSCLC; additionally, median PFS and OS are not revolutionary and do not appear to be strikingly better than those observed with first-line chemoimmunotherapy combinations in unselected NSCLC. Additionally, available survival outcomes from real life populations are very poor, particularly in AcSè trial within which even ROS1-positive patients perform way under expectation. Realistically, dissimilarity of techniques used for molecular testing and different cutoffs adopted for biomarker definition may affect clinical outcomes and produce discordance in actually available results. As MET-deregulated NSCLCs are extremely heterogeneous, many molecular events may likely contribute to a reduced response to crizotinib, including mutation type and splice-site region in exon 14, MET copy number related to high polysomy or true gene amplification and presence of concurrent genomic alterations. Beyond these considerations, clinical efficacy data are present and additional investigations are needed in order to improve biomarker-driven patient selection. However, to date a multitude of other selective (capmatinib, salvolitinib, tepotinib, tivantinib) and unselective (cabozatinib) anti-MET TKIs have been developed and are currently in advanced phases of clinical research.
In conclusion, despite surpassed by more effective drugs, crizotinib represented a landmark treatment advance for ALK/ROS1-addicted NSCLC patients with an otherwise dismal prognosis. Regarding MET-deregulated NSCLC, crizotinib activity paves the way for further research and development of more effective strategies for these subgroups of patients with unfavorable prognosis.
Declaration of interest
E Bria has received speakers’ and travel fees from Merck Sharp and Dohme, AstraZeneca, Celgene, Pfizer Inc, Helsinn, Eli Lilly and Company, Bristol-Myers Squibb, Novartis and Roche. E Bria has also received consultancy fees from Roche and Pfizer as well as institutional research grants from AstraZeneca and Roche. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One referee declares that that their institution receives research support from Pfizer, Genentech, Novartis and Roche. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203.
- Caccese M, Ferrara R, Piloto S, et al. Current and developing therapies for the treatment of non-small cell lung cancer with ALK abnormalities: update and perspectives for clinical practice. Expert Opin Pharmacother. 2016;17:2253–2266.
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–1019.
- Gobbini E, Chiari R, Pizzutillo P, et al. Real-world outcomes according to treatment strategies in ALK-rearranged non-small-cell lung cancer (NSCLC) patients: an Italian retrospective study. Clin Transl Oncol. 2020;22(3):294–301.
- Camidge DR, Dziadziuszko R, Peters S, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol. 2019;14:1233–1243.
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;S0923-7534(20):39796–9. doi:10.1016/j.annonc.2020.04.478
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667.
- Shaw AT, Riely GJ, Bang YJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30:1121–1126.
- Facchinetti F, Rossi G, Bria E, et al. Oncogene addiction in non-small cell lung cancer: focus on ROS1 inhibition. Cancer Treat Rev. 2017;55:83–95.
- Landi L, Chiari R, Tiseo M, et al. Crizotinib in MET -Deregulated or ROS1 -rearranged pretreated non–small cell lung cancer (METROS): a phase II, prospective, multicenter, two-arms trial. Clin Cancer Res. 2019;25:7312–7319.
- Moro-Sibilot D, Cozic N, Pérol M, et al. Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSé phase II trial. Ann Oncol. 2019;30:1985–1991.
- Shaw AT, Solomon BJ, Chiari R, et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Onc. 2019;20:1691–1701.
- Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1–2 trial. Lancet Onc. 2020;21:261–270.
- Pilotto S, Carbognin L, Karachaliou N, et al. Tracking MET de-addiction in lung cancer: A road towards the oncogenic target. Cancer Treat Rev. 2017;60:1–11.
- Drilon A, Jeffrey W, Clark JW, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat Med. 2020;26:47–51.
