ABSTRACT
Background
With limited real-world insulin glargine 300 U/mL (Gla-300) data among Japanese patients with type 1 diabetes mellitus (T1DM) available, the authors describe its effectiveness and safety in Japanese T1DM patients switching to Gla-300.
Research design and methods
X-STAR was a 12-month prospective, observational, post-marketing study in Japanese patients with diabetes mellitus from 2015 to 2018: insulin-experienced T1DM patients initiating Gla-300 were analyzed.
Results
Of 774 patients, mean (±standard deviation) HbA1c (%) and fasting plasma glucose (mg/dL) decreased from 8.27 ± 1.55 to 8.15 ± 1.35 (by −0.12 ± 1.30 [p = 0.013]) and 167.9 ± 92.6 to 153.9 ± 70.9 (by −13.9 ± 103.8 [p = 0.067]) from baseline to month 12, respectively. A total of 16.3% achieved HbA1c <7.0% at month 12. Gla-300 dose increased by 1.13 ± 3.18 U/day (0.02 ± 0.05 U/kg/day) (p < 0.001), with a + 0.22 ± 2.70 (p = 0.037) body-weight change (kg) from baseline 60.83 ± 12.81 to 12-month 61.06 ± 12.89. Adverse drug reactions (ADRs) and serious ADRs occurred in 9.82% and 0.78% of the patients, respectively. Hypoglycemia was the most common ADR (9.30%). In total, 88.9% adhered to Gla-300 administration schedules, whereas <40% adhered to exercise and dietary instructions, respectively.
Conclusions
Gla-300 showed no unprecedented safety concerns for insulin-experienced T1DM patients in Japanese clinical settings. Our results provide insights into strategies for blunted Gla-300 up-titration dose, despite insufficient HbA1c control and lifestyle modification.
1. Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune disease that causes the destruction of pancreatic β-cells and absolute insulin deficiency [Citation1]. In Japan, approximately 100,000 to 140,000 persons are estimated to have T1DM [Citation2]. The mainstay therapy for patients with T1DM is exogenous basal and prandial insulin administration to mimic the physiological insulin profile [Citation3]. The tight, near normal glycemic control achieved by intensive insulin therapy was demonstrated to prevent or slow the development of microvascular and macrovascular complications related to hyperglycemia [Citation4]. However, it is difficult for persons with T1DM to achieve ‘normal’ glycemic control with current insulin therapy, a finding common among countries across the world, including the US [Citation5] and the UK [Citation6]. Despite the continuous development of numerous insulin types, insulin regimens, and delivery devices, glycemic control of patients with T1DM remains suboptimal and essentially unchanged. In Japan, the mean HbA1c level in patients with T1DM was 7.8% [Citation7,Citation8], with 25.3% of patients achieving an HbA1c <7.0% [Citation7]. Previous studies have suggested that glycemic control is hindered by insufficient insulin titration owing to fears of insulin-induced hypoglycemia [Citation9] and low or non-adherence to insulin therapy [Citation10]. To achieve any improvements in glycemic control in patients with T1DM, the above issues must be addressed.
Since 2015, insulin glargine 300 U/mL (Gla-300 [Toujeo® in the United States and Europe; Lantus® XR in Japan]), a second-generation basal insulin analog, is available for the management of T1DM or type 2 diabetes mellitus (T2DM) in Japan. Gla-300 is characterized by a smoother pharmacokinetic/pharmacodynamic profile with lower variability than the first generation basal insulin analog, glargine 100 U/mL (Gla-100) [Citation11]. EDITION 4 and EDITION JP 1 (in Japan), which were phase 3 randomized controlled trials comprising patients with T1DM, revealed that Gla-300 is as effective as Gla-100 at reducing HbA1c and has lower hypoglycemia risk, particularly nocturnal hypoglycemia [Citation12,Citation13].
Although real-world data on the use of Gla-300 in T1DM are limited, the SPARTA trial, a retrospective, observational study in the UK, recently demonstrated that Gla-300 administered to patients with T1DM was associated with a significant reduction in HbA1c and no increase in hypoglycemia [Citation14]. The issue of an unacceptably low insulin titration was also revealed in the trial. Although most patients were switched to Gla-300 because they had failed to achieve optimal glycemic control with previous insulin therapy (HbA1c >9.0% at baseline), a very blunted (by less than 2 U/day) up-titration of the insulin Gla-300 dose was demonstrated, thereby limiting its potential effectiveness.
The X-STAR study (Lantus® XR post-marketing surveillance) was a 12-month post-marketing study of Gla-300 in patients with diabetes mellitus in Japan. Here, we report the safety and effectiveness of Gla-300 in patients with T1DM in a real-world clinical setting. Furthermore, we sought to expand our understanding of the clinical care provided to patients with T1DM in Japan.
2. Patients and Methods
2.1. Study design and patients
The X-STAR study was a 12-month prospective, observational, post-marketing study of Gla-300 from December 2015 to August 2018; it included patients with T1DM or T2DM who were newly prescribed Gla-300. In the present analysis, patients with T1DM who had been receiving insulin were selectively analyzed.
The X-STAR study was conducted in accordance with the pharmaceutical affairs law and the ministerial ordinance of Good Post-Marketing Study Practice (GPSP) in Japan. Under GPSP regulations, obtaining approval from an ethical committee or informed consent from patients is not mandatory. Eligible patients were enrolled at participating medical institutions under a contract with Sanofi K.K. (Tokyo, Japan) and followed-up for 12 months. These patients were also centrally enrolled within 14 days from the first Gla-300 prescription. Thereafter, anonymized data were entered into the electronic data-capturing system. Attending physicians adjusted the starting and subsequent dose of Gla-300 by their discretion based on the Summary Product Characteristics of Gla-300, symptoms, and laboratory findings.
2.2. Data collection and assessments
In this analysis, baseline demographics and clinical characteristics included age, sex, duration of diabetes, body weight, height, complications, and details on prior medication. Data regarding Gla-300 treatment, such as the injection time and concomitant use of other antidiabetic medications (type and dose), were also collected. Gla-300 dose was monitored at months 1 (Day 22 to 28), 3 (Day 78 to 84), 6 (Day 141 to 168), 9 (Day 225 to 252), and 12 (Day 337 to 364).
Adherence to Gla-300 administration (adherent [≥75%], sometimes non-adherent [50% to <75%], non-adherent [<50%], and unknown) and instructions on exercise and diet modifications (not instructed, adherent [≥75%], sometimes non-adherent [50% to <75%], non-adherent [<50%], and unknown) were estimated by the attending physicians.
HbA1c (the National Glycohemoglobin Standardization Program [NGSP] values), fasting plasma glucose (FPG; laboratory or self-monitored plasma glucose [SMPG] values), and body weight were measured before Gla-300 initiation (baseline) and at months 1 (±4 weeks), 3 (−4 to +6 weeks), 6 (±6 weeks), 9 (±6 weeks), and 12 (±6 weeks). At baseline, the latest data (i.e., within 8 weeks before Gla-300 initiation) were used. These data were stratified by age (<16, 16–<65, 65–<75, and ≥75 years) or type of insulin administered before treatment with Gla-300 (Gla-100, degludec, and detemir).
Adverse drug reactions (ADRs), including abnormal variations in laboratory test parameters, were analyzed during the observation period. If hypoglycemia and other ADRs were observed, these events were reported by the attending physicians. ADRs were classified according to the Medical Dictionary for Regulatory Activities/Japanese version (MedDRA/J Ver.20.1). Hypoglycemia was reported according to the manual issued by the Minister of Health, Labor, and Welfare of Japan [Citation15]. Severe hypoglycemia was defined as an event that required assistance from another person. Nocturnal hypoglycemia was defined as hypoglycemia that occurred during the night (0:00–5:59 AM); these data were stratified by the timing of the Gla-300 injection (morning, lunch, dinner, etc.), as defined prior to this study initiation and specified in our study protocol.
2.3. Statistical analysis
Data are expressed as the mean ± standard deviation (SD) for continuous variables or as the number and proportion of patients in each category for categorical data. To calculate the Gla-300 dose in U/kg/day, the body weight at baseline was used for the dose before baseline and the latest body weight data were used for the dose after baseline. The last observation carried forward (LOCF) approach was used to insert missing values; it was referred to as month 12 (LOCF). To compare the data retrieved at month 12 (LOCF) versus those at baseline, the paired t-test was used. All analyses were performed using SAS software release 9.4 (SAS Institute, Inc., Cary, NC, USA). The significance level was defined as a two-sided p-value <0.05.
3. Results
3.1. Patients
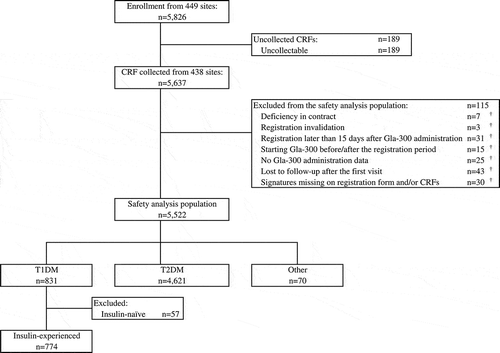
In total, 5,826 patients with diabetes mellitus to whom Gla-300 was newly administered were enrolled at 449 institutions. Of the 5,522 patients included in the safety analysis population, 774 with T1DM who were already administered insulin therapy were analyzed to elucidate the safety and effectiveness of Gla-300 ().
Figure 1. Participant disposition CRF: case report form; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.†Reasons for exclusion are duplicated
The demographic and clinical characteristics of patients at baseline are shown in . In total, 49.6% of patients were males; the mean age was 53.0 ± 17.1 years. Patients were diagnosed with autoimmune (65.0%) or idiopathic (35.0%) T1DM and had a mean diabetes duration of 14.7 ± 11.2 years. Mean body weight was 60.6 ± 12.8 kg. Further, 44.8% of patients had diabetic complications at baseline, including retinopathy (26.6%), nephropathy (24.9%), and neuropathy (27.8%). Hypoglycemia was reported in 257 (33.2%) patients within the 3 months before Gla-300 initiation. Most patients (89.9%) began Gla-300 therapy because of insufficient glycemic control.
Table 1. Participant demographic and clinical characteristics at baseline
Before the initiation of Gla-300 therapy, 95.9% of patients with T1DM were prescribed long-acting (basal) insulins, such as Gla-100, degludec, and detemir (). At baseline, rapid-acting insulins were prescribed concomitantly with Gla-300 to 93.3% of patients. Some patients were administered regular insulin (3.3%) or premix insulin (0.7%). α-Glucosidase inhibitors (9.7%) were identified as the most commonly prescribed oral antidiabetic drugs (OADs). In fact, 5.6% of patients received Gla-300 alone (i.e., without other insulins or OADs). Basal-bolus therapy was the most common insulin regimen administered to 698 (90.2%) patients at baseline and 705 (91.1%) patients at month 12 (LOCF) ().
Table 2. Antidiabetic medication use before and when starting Gla-300
Table 3. Regimens when starting Gla-300 (at baseline) and at 12-month LOCF
3.2. Insulin dose
Before initiating Gla-300 administration, the mean doses of basal, bolus (regular or rapid) and total insulin were 14.98 ± 9.85 U/day (0.24 ± 0.14 U/kg/day), 25.37 ± 17.12 U/day (0.42 ± 0.24 U/kg/day), 37.52 ± 23.49 U/day (0.62 ± 0.33 U/kg/day), respectively. During the observation period, the mean doses of Gla-300 were 15.02 ± 9.78 U/day (0.25 ± 0.14 U/kg/day) at baseline and 16.15 ± 10.58 U/day (0.26 ± 0.15 U/kg/day) at month 12 (LOCF), with a change of +1.13 ± 3.18 U/day (+0.02 ± 0.05 U/kg/day) (p < 0.001). The mean doses of bolus insulin were 25.41 ± 17.31 U/day (0.42 ± 0.24 U/kg/day) at baseline and 25.26 ± 16.98 U/day (0.41 ± 0.23 U/kg/day) at month 12 (LOCF), with a change of −0.15 ± 5.46 U/day (−0.01 ± 0.09 U/kg/day) (p = 0.468). The mean doses of total insulin were 38.10 ± 23.67 U/day (0.63 ± 0.33 U/kg/day) at baseline and 39.18 ± 23.98 (0.65 ± 0.32 U/kg/day) at month 12 (LOCF), with a change of +1.07 ± 6.87 U/day (+0.01 ± 0.11 U/kg/day) (p < 0.001)
3.3. Adherence to the instructions
Of the 774 patients, 633 (88.9%) were adherent (≥75%) to the Gla-300 administration (), whereas 231 (32.4%) and 279 (39.2%) were adherent (≥75%) to the exercise and dietary instructions, respectively. Furthermore, 27.2% and 16.7% of patients did not receive the exercise and dietary instructions, respectively.
Table 4. Adherence status with treatment instructions during the 12 months after Gla-300 initiation
3.4. Effectiveness
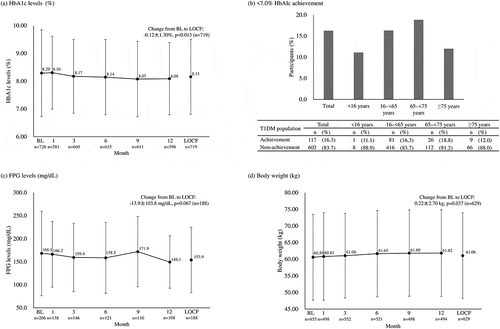
The mean (±SD) HbA1c levels were 8.27 ± 1.55% at baseline and 8.15 ± 1.35% at month 12 (LOCF), with a small but statistically significant change of −0.12 ± 1.30% (n = 719, p = 0.013) (). This change did not differ between the types of insulins administered before the initiation of Gla-300 therapy (Supplemental ). In total, 16.3% (117/719) achieved HbA1c <7.0% at month 12; however, this achievement was consistently low (<20%) across all age subgroups (). The mean FPG levels were 167.9 ± 92.6 mg/dL at baseline and 153.9 ± 70.9 mg/dL at month 12 (LOCF), with a non-significant change of −13.9 ± 103.8 mg/dL (n = 188, p = 0.067) (). The mean body weight was 60.83 ± 12.81 kg at baseline and 61.06 ± 12.89 kg at month 12 (LOCF), with a change of +0.22 ± 2.70 kg (n = 629, p = 0.037) ().
3.5. Safety
The incidence of ADRs, serious ADRs, severe hypoglycemia, and nocturnal hypoglycemia is shown in . ADRs and serious ADRs occurred in 76 (9.82%) and 6 (0.78%) patients, respectively. Furthermore, hypoglycemia was the most commonly observed (n = 72, 9.30%) adverse event in patients. Nocturnal hypoglycemia was identified in the 3 (0.39%) patients administered Gla-300 at night.
Table 5. ADRs, serious ADRs, severe hypoglycemia, and nocturnal hypoglycemia
4. Discussion
The X-STAR study was a 12-month, prospective, observational, post-marketing study of Gla-300 that aimed to assess the effectiveness and safety of Gla-300 in patients with T1DM who were receiving insulin therapy in a real-world clinical setting in Japan. Although the mean HbA1c levels were significantly decreased at 12 months (−0.12%, 8.27% to 8.15%, p = 0.013), only 16.3% of patients achieved an HbA1c <7.0%. Additionally, only a small increase in the Gla-300 dose (+1.13 U/day [+0.02 U/kg/day]) and a marginal increase in weight were observed. Compared to previous randomized controlled trials of Gla-300, there were no unprecedented ADRs from the perspective of safety [Citation12,Citation13].
In this real-world study, the HbA1c reduction over 12 months (−0.12%) was slightly moderate compared with the clinical trial results of Gla-300 in the patients with T1DM including Japanese (−0.20% in EDITION 4 [Citation12], −0.2% in EDITION JP1 [Citation13]) despite comparable baseline HbA1c levels among the studies. This may be attributable to the blunted insulin up-titration observed in this study (+1.13 U/day [+0.02 U/kg/day]. Indeed, the previous EDITION trials, although the comparison is limited to basal insulin results, basal insulin was more up-titrated toward target FPG 80–130 mg/dl over 12 months in the patients using Gla-300 (change: +14.9 U/day [+0.17 U/kg/day] from baseline to month 12 [Citation12], and +6.3 U/day [+0.09 U/kg/day] from baseline to month 12 [LOCF] [Citation13]). Recent interventional studies of Gla-300 in T1D patients with instructed dose-adjustment toward target FPG have shown similar results on HbA1c reduction and up-titration of Gla-300 dose as in EDITION programs [Citation16,Citation17]. These suggest a need for appropriate Gla-300 up-titration with a careful consideration for hypoglycemia risk, to more improve patients’ glycemic control with Gla-300 in a real-world setting.
Over the observation period, the patients in our study were found to have higher HbA1c levels than those reported in the general Japanese population in the JDCP 2 (7.8%) [Citation7] and JDDM (7.82%) studies [Citation8], which included T1DM patients regardless of drug therapy or glycemic control status. Achievement of HbA1c <7.0% was 16.3% in X-STAR and 25.3% in the JDCP study 2 population [Citation7]. The participants of our study who were switched to Gla-300 required a change of treatment because of the need for improved glycemic control (89.9%) or hypoglycemia (12.9%) (). Given the relatively long duration of T1DM (14.7 years), patients who have particular difficulty in achieving the glycemic target were likely included in our study, which may be the reason why the Gla-300 dose was only modestly up-titrated (less than 2 U/day). Although there may be selection bias in our study, we believe the present study provides important insights into the current situation concerning T1DM treatment in Japan.
Similar findings were reported in retrospective observational studies such as SPARTA in the UK [Citation14] and REALITY in Canada [Citation18]. In the SPARTA study, the researchers investigated 300 T1DM patients who were switched to Gla-300. Although the mean HbA1c at baseline was >9.0% and switching was performed to achieve improved glycemic control, the increase in basal insulin dose was only 2 U/day and the mean reduction in HbA1c levels was 0.4%. In the REALITY study, the T1DM patients switching from Gla-100, neutral protamine Hagedorn, or detemir to Gla-300 showed similarly modest HbA1c reduction (−0.17%, from 8.35% to 8.18%) with a subtle basal insulin dose change (from 0.36 U/kg to 0.38 U/kg) during follow-up [Citation18]. On the basis of the findings of these studies, physicians and patients may be balancing the improved glycemic control against the risk of nocturnal hypoglycemia. Unfortunately, the evident reduction in the risk of hypoglycemia, which was reported for Gla-300 [Citation12,Citation13], was not translated in these countries to build a more assertive proposal for its clinical use.
Although Gla-300 could lower fasting plasma glucose with a lower risk of hypoglycemia than Gla-100, it may not be sufficient to achieve the glycemic target. Challenges of predicting the optimal dose of insulin still remain, such as bolus insulin for T1D patients. Traditionally, carbohydrate counting, calculating dose of bolus insulin based on estimation of carbohydrate intake, has been recommended [Citation3]. Recently, it has been suggested that, besides carbohydrate, both protein and fat are also important parameters for predicting postprandial plasma glucose [Citation19,Citation20]. Further improvement of prediction models may help T1D patients achieve glycemic target.
Most patients in this analysis (88.9%) were adherent to the Gla-300 administration. However, adherence to the exercise and diet instructions was relatively low (32.4% and 39.2%, respectively). We also found that 27.2% and 16.7% of patients did not receive the instructions. A balance between insulin dose and diet and exercise is fundamentally important for the management of T1DM [Citation21]. Furthermore, non-adherence to, or omission of, this guidance could have significant adverse effects on patient outcomes and experiences when FPG is not at target, thereby negating the benefits of Gla-300 and obstructing the appropriate titration of the dose of insulin. Our findings strongly suggest that a further comprehensive support, including lifestyle modifications, should be established for patients with T1DM to achieve optimal glycemic control with insulin therapy.
This study had several limitations. As underreporting is commonly observed in post-marketing surveillance studies in which data are collected without strict definitions according to clinical judgment, the incidence of ADRs, including hypoglycemia, may have been underestimated [Citation22,Citation23]. Moreover, because the X-STAR was a single-arm observational study, a comparison of the effectiveness and safety of Gla-300 to alternative therapies could not be performed.
5. Conclusions
Based on the findings of the X-STAR study, a 12-month post-marketing surveillance of Gla-300 use in Japan, Gla-300 has been safely administered as basal insulin to treat Japanese patients with insulin-experienced T1DM. Furthermore, there were no unprecedented safety concerns during the observation period. Collectively, the results of the present study provide insights into the strategies for T1DM treatment currently adopted in clinical practice, which include blunted up-titration of the Gla-300 dose, despite the low achievement of an HbA1c level <7.0% and insufficient lifestyle modification.
Author contributions
M Matsuhisa and Y Terauchi conceived and designed the study, interpreted the data, and critically revised the manuscript. M Odawara, T Hirose, and Y Tanaka interpreted the data and critically revised the manuscript. R Koshida interpreted the data, and drafted and critically revised the manuscript. M Senda conceived and designed the study, participated in data acquisition, analysis, and interpretation, and critically revised the manuscript. All authors approved the final version of the manuscript for publication and agreed to be accountable for the data presented herein.
Declaration of interest
M Matsuhisa received honoraria from Sanofi K.K., Takeda Pharmaceutical Company, Ltd., Eli Lilly Japan K.K., Mitsubishi Tanabe Pharma Corp., Astellas Pharma Inc., Novo Nordisk Pharma Ltd., and Merck Sharp & Dohme K.K.; research funding from Sysmex Corp., Nissui Pharmaceutical Co., Ltd., and Tokushima Data Service Co. Ltd.; and subsidies or donations from Astellas Pharma Inc., Nippon Boehringer Ingelheim Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corp., Novartis Pharma K.K., Sanofi K.K., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Company, Ltd., Merck Sharp & Dohme K.K., and Ono Pharmaceutical Co., Ltd. M Odawara received honoraria, subsidies, or donations from Novo Nordisk Pharma Ltd., Sanofi K.K., Merck Sharp & Dohme K.K., Ono Pharmaceutical Co., Ltd., Novartis Pharma K.K., Astellas Pharma Inc., AstraZeneca K.K., Kowa Pharmaceutical Co. Ltd., Takeda Pharmaceutical Company, Ltd., Mitsubishi Tanabe Pharma Corp., Eli Lilly Japan K.K., Nippon Boehringer Ingelheim Co., Ltd., and Sumitomo Dainippon Pharma Co., Ltd. T Hirose received honoraria from Sanofi K.K., Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Company, Ltd., Merck Sharp & Dohme K.K., Sumitomo Dainippon Pharma Co., Ltd., Novartis Pharma K.K., Nippon Boehringer Ingelheim Co., Ltd., Ono Pharmaceutical Co., Ltd., AstraZeneca K.K., Mitsubishi Tanabe Pharma Corp., and Kowa Company, Ltd.; research funding from Mitsubishi Tanabe Pharma Corp. and AstraZeneca K.K.; and subsidies or donations from Astellas Pharma Inc., Novartis Pharma K.K., Eli Lilly Japan K.K., MSD K.K., Sanofi K.K., Mitsubishi Tanabe Pharma Corp., Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Company, Ltd., Taisho Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Ono Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Soiken, Inc., and Bayer Yakuhin, Ltd. R Koshida and M Senda are employees of Sanofi K.K. Y Tanaka serves in an advisory role to Top Corp.; and received honoraria from Merck Sharp & Dohme K.K., Kissei Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Astellas Pharma Inc., Takeda Pharmaceutical Company, Ltd., Arkray, Inc., and Sanofi K.K.: collaborative research funding from Nichirei Foods Inc.; and subsidies or donations from Sanofi K.K., Takeda Pharmaceutical Company, Ltd., Merck Sharp & Dohme K.K., Daiichi Sankyo Co., Ltd., Novo Nordisk Pharma Ltd., Kissei Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Arkray, Inc., AstraZeneca K.K., Mitsubishi Tanabe Pharma Corp., Ono Pharmaceutical Co., Ltd., and Astellas Pharma Inc. Y Terauchi received honoraria from Astellas Pharma Inc., AstraZeneca K.K., Daiichi Sankyo Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Eli Lilly Japan K.K., Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corp., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Sanofi K.K., Taisho Toyama Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Company, Ltd.; and research funding or grant from AstraZeneca K.K., Daiichi Sankyo Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Eli Lilly Japan K.K., Merck Sharp & Dohme K.K., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., and Sanofi K.K. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Fig_S1.tiff
Download TIFF Image (1.3 MB)Acknowledgments
The authors thank EP-PharmaLine Co. Ltd for assistance in the monitoring of the post-marketing study, S, Yamane of Post-Authorization Regulatory Studies, Sanofi KK for post-marketing study operational support and CMIC Co. Ltd. for Electronic Data Capture. The authors also utilized statistical analysis and editorial support from Clinical Study Support, Inc, which was funded by Sanofi KK.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Haneda M, Noda M, Origasa H, et al. Japanese clinical practice guideline for diabetes 2016. J Diabetes Investig. 2018;9:657–697.
- Insulin bunpitsu ga kokatsu shita ichigata tonyobyo toha [About type 1 diabetes mellitus with depleted insulin secretion]. [Internet]. Japan:National Institute of Public Health; 2018 [ cited 2020 Jan 24]. Available from: http://dmic.ncgm.go.jp/content/type1_insulin_20180817.pdf. Japanese.
- American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2019. Diabetes Care. 2019;42:S90–S102. 9.
- Nathan DM, Genuth S, et al.; Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986.
- Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38:971–978.
- Idris I, Peng X, He X, et al. The trend of high-dose insulin usage among patients with diabetes in the UK: a retrospective study. Diabetes Ther. 2018;9:2245–2257.
- Nishimura R, Izumi K, Hayashino Y, et al. A large-scale observational study to investigate the current status of diabetes complications and their prevention in Japan: research outline and baseline data for type 1 diabetes-JDCP study 2. Diabetol Int. 2016;7:4–11.
- Kisosyukeisiryo 2017 nendo. [Internet]. Japan: Japan Diabetes Clinical Data Management Study Group (JDDM); 2018 [ cited 2020 Jan 24]. Available from: http://jddm.jp/data/index-2017.html. Japanese.
- Wild D, von Maltzahn R, Brohan E, et al. A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns. 2007;68:10–15.
- Peyrot M, Barnett AH, Meneghini LF, et al. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in insulin therapy study. Diabet Med J Br Diabet Assoc. 2012;29:682–689.
- Becker RHA, Dahmen R, Bergmann K, et al. New insulin glargine 300 units · mL-1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units · mL-1. Diabetes Care. 2015;38:637–643.
- Home PD, Bergenstal RM, Bolli GB, et al. Glycaemic control and hypoglycaemia during 12 months of randomized treatment with insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 1 diabetes (EDITION 4). Diabetes Obes Metab. 2018;20:121–128.
- Matsuhisa M, Koyama M, Cheng X, et al. Sustained glycaemic control and less nocturnal hypoglycaemia with insulin glargine 300U/mL compared with glargine 100U/mL in Japanese adults with type 1 diabetes (EDITION JP 1 randomised 12-month trial including 6-month extension). Diabet Res Clin Pract. 2016;122:133–140.
- Pang T, Bain S, Black R, et al. A multicentre, UK, retrospective, observational study to assess the effectiveness of insulin glargine 300 units/ml in treating people with Type 1 diabetes mellitus in routine clinical practice (SPARTA). Diabet Med. 2019;36:110–119.
- Jutoku hukusayo shikkann betsu taiou manyuaru, teikettou [Manuals for handling disorders due to adverse drug reactions: hypoglycaemia]. [Internet]. Japan: Ministry of Health, Labour and Welfare; 2011 [ cited 2020 Jan 24]. Available from: http://www.info.pmda.go.jp/juutoku/file/jfm1104010.pdf. Japanese
- Mathieu C, Weisnagel SJ, Stella P, et al. Impact of switching from twice-daily basal insulin to once-daily insulin glargine 300 U/mL in people with type 1 diabetes on basal–bolus insulin: phase 4 OPTIMIZE study. Diabetes Therapy. 2020 Feb 1;11(2):495–507.
- Pettus J, Gill J, Paranjape S, et al. Efficacy and safety of a morning injection of insulin glargine 300 units/mL versus insulin glargine 100 units/mL in adult patients with type 1 diabetes: A multicentre, randomized controlled trial using continuous glucose monitoring. Diabetes Obes Metab. 2019;21:1906–1913.
- Abitbol A, Brown RE, Jiandani D, et al. Real-world health outcomes of insulin Glargine 300 U/mL vs Insulin Glargine 100 U/mL in adults with Type 1 and Type 2 diabetes in the Canadian LMC diabetes patient registry: the REALITY study. Can J Diabetes. 2019 Oct;43(7):504–509.e1. .
- Bell KJ, Smart CE, Steil GM, et al. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care. 2015 Jun;38(6):1008–1015. .
- Bell KJ, Fio CZ, Twigg S, et al. Amount and type of dietary fat, postprandial glycemia, and insulin requirements in Type 1 diabetes: a randomized within-subject trial. Diabetes Care. 2020 Jan;43(1):59–66. Epub 2019 Aug 27.
- DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ. 2002;325:746.
- Odawara M, Kadowaki T, Naito Y. Effectiveness and safety of basal supported oral therapy with insulin glargine, in Japanese insulin-naive, type 2 diabetes patients, with or without microvascular complications: subanalysis of the observational, non-interventional, 24-week follow-up add-on Lantus® to oral hypoglycemic agents (ALOHA) study. J Diabetes Complications. 2015;29:127–133.
- Utsunomiya K, Kakiuchi S, Senda M, et al. Safety and effectiveness of tofogliflozin in Japanese patients with type 2 diabetes mellitus: results of 24-month interim analysis of a long-term post-marketing study (J-STEP/LT). J Diabetes Investig. 2020 Mar 30; cited. DOI:10.1111/jdi.13233.