ABSTRACT
Introduction
When estimating the cost-effectiveness or budget impact of chronic obstructive pulmonary disease (COPD) medication, it is common practice to use trial data for clinical inputs. However, such inputs do not always reflect the real-world situation. Previous reviews recognized the need for taking real-world data (medication adherence, comorbidity and adverse drug reactions [ADRs]) into account. Whether recent cost-effectiveness analyses of COPD medication implemented those recommendations is unknown.
Areas covered
The authors reviewed recent economic evaluations of COPD-maintenance treatments focusing on medication adherence, comorbidity and ADRs.
Expert opinion
In most registration trials of COPD treatment, strict inclusion and exclusion criteria are applied. During trials, patient monitoring is well controlled. As such, medication adherence is often higher than seen in less controlled, real-world environments with more heterogeneous characteristics. Additionally, safety data collected in trials may not be widely generalizable due to more comorbidity and polypharmacy in the real-world. Consequently, when merely relying on trial data, the impact of adherence, comorbidity and ADRs on the cost-effectiveness can be underestimated. To overcome these real-world data gaps, use of pragmatic trials and observational studies in addition to strictly controlled trial data is recommended. To catalyze implementation of these real-world issues, reporting checklists should be updated.
1. Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation usually associated with exposure to noxious particles or gases [Citation1]. Worldwide, this chronic condition was responsible for over 3 million deaths in 2015 and it was the third leading cause of death in 2020 [Citation1,Citation2]. Although the disease cannot be cured, effective symptomatic maintenance treatments exist [Citation1–3].
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines four categories of COPD patients, depending on the disease severity (A, B, C and D). Each category requires different pharmacological treatments. For example, patients in the least severe group, i.e. GOLD A patients, need a bronchodilator (short or long-acting), while for GOLD B patients a long-acting muscarinic antagonist (LAMA) or a long-acting beta2-agonist (LABA) is indicated. For GOLD C patients a LAMA is preferred. The most severe patients (GOLD D) are initially advised a LAMA, combined inhaled treatments of LABA/LAMA, or inhaled corticosteroids with LABA (LABA/ICS). Follow-up treatment could involve LABA/LAMA/ICS, azithromycine or treatment with phosphodiesterase 4 (PDE4) inhibitors [Citation1]. All medications can help to reduce disease symptoms and increase quality of life [Citation1,Citation3].
Their route to the market, and availability for the patients is ensured through legislation procedures of drug registration and – reimbursement processes. During the latter process, economic evaluations play an important role. Economic evaluations, such as cost-minimization analysis (CMA), cost-effectiveness analysis (CEA), cost-benefit analysis (CBA) or cost-utilization analysis (CUA) are well established frameworks providing prospective weightage of costs and health benefits while comparing two or more alternatives [Citation4]. In addition to the economic evaluations, budget impact analysis (BIA) shows how the new health intervention will affect the total expenditure of a healthcare system. Together, economic evaluations and BIA represent informative tools for decision makers to decide on reimbursement of medication [Citation5,Citation6]. Hence, inclusion of all relevant parameters for informative decision making is crucial.
Prior reviews examining economic evaluations of pharmacological COPD treatments reported several shortcomings [Citation7–9]. Notably, the most recent systematic review covering evaluations up to 31 December 2015, indicated the incorporation of real-world evidence as one of the most pressing issues that required attention [Citation9]. Such items are also not part of the existing checklists for quality reporting in economic analyses (e.g. CHEERS, QHES, or CHEC) [Citation10–12]. This prompted us to investigate whether recent analyses, published from 2016 onwards, did indeed incorporate real-world evidence. Examples of real-world issues include medication adherence, co-morbidity and adverse drug reactions (ADRs). We conducted a systematic review on recent economic evaluations and BIAs discussing maintenance treatments for COPD, with a focus on medication adherence, co-morbidity and ADRs.
2. Methods
2.1. Protocol and registration
This is a systematic review reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation13]. The protocol of this systematic review has been registered on https://www.crd.york.ac.uk/PROSPERO under the identification number CRD42020167179.
2.2. Eligibility criteria
To be included in this review, studies needed to include COPD patients using maintenance treatments for COPD. The following maintenance treatments were considered: LABA, LAMA, LABA/LAMA, LABA/ICS, LABA/LAMA/ICS and PDE4 inhibitors. Medications used for acute exacerbations, short-acting medications (e.g. short-acting muscarinic antagonist (SAMA) or a short-acting b2-agonist (SABA)), alfa 1-antitrypsin replacement therapy, non-pharmacological treatments or vaccination strategies were not included. Eligible studies had to be full-text publications in a peer-reviewed journal, and having one of the following study – designs: CMA, CEA, CBA, CUA, or BIA. In these analyses, there should always be a comparison between one or more COPD medications and usual care. Partial/non-comparative economic evaluations, evaluations discussing strategies of protection, or conference abstracts were not eligible for this review.
2.3. Information sources and search
Since the previous reviews [Citation7–9] included studies published up to 2015 and our aim was to analyze recent studies, we decided to perform our search for the period between 01.01.2016–01.01.2020, applying no setting or language restrictions. We systematically searched PubMed and Embase following a pre-tested search strategy (Appendix, eTable 1). Notably, we made efforts to optimize the search strategy, e.g. by inclusion of recently registered COPD drugs and expanded our search with BIA terms. Additionally, we performed searches using the key word ‘COPD’ in the following databases indexing economic evaluations: the NHS Economic Evaluation Database (NHS EED) and the Health Economic Evaluations Database and Cost effectiveness analysis (CEA) registry.
2.4. Study selection
The study selection process was performed by two researchers (TF and GZ) working independently. Potential disagreements were resolved through discussion and consulting a third reviewer (JvB) if required. The study selection process followed two steps. First, we scanned title and abstract of each search hit, and assessed adherence to our selection criteria list (). This list was composed of three questions designed to help us identify potentially eligible studies.
Table 1. Study selection criteria
During this process, the online review management tool Rayyan was used [Citation14]. Second, we read the full-texts of each publication to decide on final inclusion.
2.5. Data collection process and data items
The data extraction was performed by the first author, and checked by one more reviewer, using a prior created and piloted data extraction form by the review team. We extracted information regarding: study authors, country and year of publication, type of economic evaluation, study perspective, treatment and its comparator, population characteristics (gender, age), location/geographical info (country), study settings (model-based/trial-based or real-world evidence), time horizon, cost data (difference in costs), currency used, pricing period/date, adherence, ADRs data, co-morbidity, differences in outcomes (quality-adjusted life years [QALYs] gained, life years [LYs] gained) and incremental cost-effectiveness ratio [ICER], sensitivity analyses, funding, authors’ conclusions, and other relevant data that will be identified as significant for inclusion during the article screening.
2.6. Risk of bias/quality assessment
2.6.1. Quality assessment of economic evaluations (CEA, CBA, CUA and CMA)
For assessing the quality and the value of the eligible economic evaluations (CEA, CBA, CUA and CMA), we used the Quality of Health Economic Studies (QHES) tool [Citation12]. The QHES is composed of sixteen questions and was created to provide a quality measurement of health economic evaluations. The QHES scores were categorized in 0–25.0 (category 1), 25.1–50.0 (category 2), 50.1–75.0 (category 3) and 75.1–100 (category 4) with 0 being the lowest score and 100 being the highest score [Citation9]. We are aware of multiple other quality assessment tools yet we chose this tool because it allows us to quantify the outcomes, but also enables comparisons with the results of previous reviews on this topic [Citation9].
2.6.2. Quality assessment of BIA
To assess the quality of BIA reporting, we used a previously developed checklist for a BIA of COPD treatments [Citation15], which was based on the already existing ISPOR guideline on BIA [Citation6], with additional attention for RWE. Each listed criterion was examined with ‘yes, fulfilled’ (accounting for 1 point), ‘no, not fulfilled’ (accounting for 0 points) or ‘partially fulfilled’ (accounting for 0.5 points), depending on how comprehensive the criterion was reported. The total scores were translated into percentages and categorized in 0–25.0 (category 1), 25.1–50.0 (category 2), 50.1–75.0 (category 3) and 75.1–100 (category 4) with 0 being the lowest score and 100 being the highest score. We acknowledge the fact that other studies [Citation16,Citation17] also tried to create checklists based on the ISPOR BIA guidance [Citation6], however these checklists were designed for different medical interventions (e.g. vaccinations) and therefore deemed not suitable for COPD medications.
The QHES and BIA-quality reporting assessments were performed by two reviewers (TF and GZ) independently. Potential disagreements were resolved by consulting a third reviewer (JvB).
2.7. Outcomes
The main outcomes of this review focused on real-world evidence issues, with particular attention to (1) medication adherence, (2) comorbidity and (3) ADRs. Additionally, we assessed the differences in outcomes (QALY gained, LYs gained, and/or other study outcomes) and ICERs associated with the results of the economic evaluation, and the outcomes of main interest in the BIAs.
3. Results
3.1. Study selection
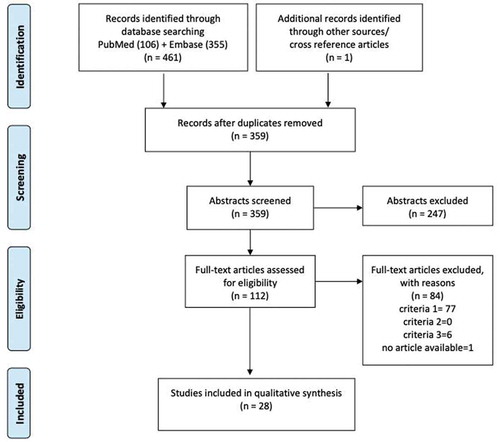
The search strategy yielded 461 articles to which one cross-reference was added. The additional databases (NHS EED and CEA) did not include articles in the time-range of our interest. After removing 102 duplicates, the remaining 359 were screened based on titles and abstracts. This resulted in 247 exclusions, while 112 were further examined using full-text review. Of those, 77 articles did not meet the predefined format of economic evaluation, in 6 the intervention was not maintenance treatment for COPD, and one study had no full-text available. Finally, 28 articles [Citation15,Citation18–44] were eligible for inclusion in this review. The selection process is illustrated in using the PRISMA flow diagram.
Figure 1. PRISMA flow diagram. This diagram shows the study selection process in steps, starting from database search, then removing duplicates, selecting by abstract screening, and full-text screening. The listed criteria for eliminating the full-text articles concern the following questions: (1) Is the article an economic evaluation? (2) Is the disease COPD? (3) Is the intervention pharmacological maintenance treatment for COPD?
3.2. General study characteristics
The general study characteristics were mined from the data-extraction form and are presented in . Detailed study characteristics and references are provided in the Appendix (eTable 2).
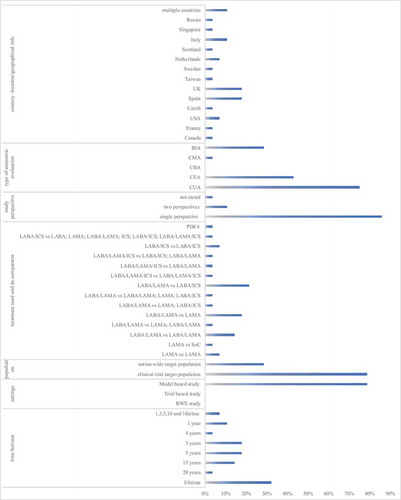
Figure 2. General study characteristics of recent economic evaluations of pharmacological COPD treatments. This figure shows the percentage of studies (out of the 28 reviewed articles), with particular general study characteristics. CMA Cost-Minimization Analysis; CEA Cost-Effectiveness Analysis; CBA Cost-Benefit Analysis; CUA Cost-Utilization Analysis; BIA Budget Impact Analysis; RWE real-world evidence; LAMA long-acting muscarinic antagonist; LABA long-acting beta2-agonist; ICS inhaled corticosteroids; PDE4 phosphodiesterase 4
3.2.1. Country/location, geographical info
Twenty-five out of the 28 studies (89%) were economic evaluations or BIAs conducted in a single country/region: Canada [Citation18], France [Citation19], USA [Citation20,Citation27], Czech Republic [Citation23], Spain [Citation22,Citation34,Citation37–39], UK [Citation21,Citation24,Citation28,Citation35,Citation43], Taiwan [Citation25], Sweden [Citation26], the Netherlands [Citation15,Citation29], Scotland [Citation30], Italy [Citation32,Citation40,Citation41], Singapore [Citation36] and Russia [Citation44]. Three studies included multiple countries [Citation31,Citation33,Citation42].
3.2.2. Type of economic evaluation and study perspective
Twenty-two studies [Citation18–37,Citation43,Citation44] explored COPD treatment cost-effectiveness (79%), of which two [Citation29,Citation44] performed a BIA as well. Six studies [Citation15,Citation38–42] conducted BIA only. Within the economic evaluations, the majority of studies followed the format of CUA (75%), in 12 articles (43%) the CEAs were combined with CUAs and lastly, one CMA was performed (3.6%). Twenty-seven studies [Citation15,Citation18–32,Citation34–44] reported the study perspective. The national health care payer’s perspective was the most commonly used perspective (20 articles) [Citation15,Citation18,Citation21–25,Citation27–30,Citation32,Citation34,Citation35,Citation38–42,Citation44]. Four articles [Citation20,Citation26,Citation31,Citation36] stated using a payer’s perspective in their analyses. All those articles accounted for direct costs only. There were also articles that combined the health care payer’s perspective with a societal – [Citation19] to explore broader costs, or a personal social services perspective [Citation28,Citation43].
3.2.3. Pharmacological treatment
Various maintenance treatments for COPD were assessed using different comparators (). LAMA (N = 3 studies) was mostly compared with another LAMA or with usual care [Citation24,Citation33,Citation41]. LABA/LAMA was the most analyzed treatment between 2016–2020 (N = 18) and was compared with another LABA/LAMA [Citation21,Citation28,Citation37,Citation44] or to LAMA [Citation19,Citation29,Citation30,Citation32,Citation34], LABA/ICS [Citation23,Citation26,Citation31,Citation36,Citation40], LABA/LAMA or LAMA [Citation27], LAMA or LABA/ICS [Citation25], LABA/LAMA or LAMA or LABA/ICS [Citation15].
Table 2. Pharmacological COPD treatments compared within the studies
Three articles assessed the fixed-dose combination LABA/LAMA/ICS (‘triple therapy’) with free combination LABA/LAMA/ICS [Citation21], separate triple combinations containing LABA/ICS or LABA/LAMA [Citation18], and with LABA/LAMA [Citation35]. Three articles compared LABA/ICS treatment, i.e. budesonide/formoterol delivered through different inhalators [Citation38,Citation42], or comparing it with LABA, LAMA, LABA/LAMA, ICS, and LABA/ICS [Citation39]. One study compared the PDE4 inhibitor roflumilast added to LABA/LAMA/ICS with LABA/LAMA/ICS alone in patients with severe to very severe COPD, FEV1 less than 50% predicted, symptoms of chronic bronchitis and at least 2 exacerbations per year [Citation43].
3.2.4. Study population
Most studies used populations from trials to populate their models [Citation18–37,Citation43,Citation44]. In BIAs, nation-wide estimations were used [Citation15,Citation29,Citation38–42,Citation44].
3.2.5. Study settings, model cycle length and time horizon
Twenty-two studies were model-based [Citation18–37,Citation43,Citation44], while no studies were real-world or trial-based. Within the economic evaluations that reported the length of the model cycle, the lengths varied between one month [Citation28–30,Citation37,Citation43], three months [Citation20], six months [Citation23,Citation25,Citation31,Citation36] and up to one year [Citation18,Citation21,Citation22,Citation27,Citation35,Citation44]. Some studies [Citation19,Citation24,Citation26,Citation32–34] did not specify the duration of the model cycles.
Regarding the time horizon, a lifetime was most common [Citation18,Citation21,Citation23,Citation24,Citation31,Citation33,Citation35,Citation36,Citation43]. The lifetime horizon was sometimes combined with shorter horizons of e.g. ten, five, and/or three and one year [Citation25,Citation26].
3.3. Real-world outcomes
The inclusion of the real-world outcomes, medication adherence, comorbidities and ADRs are summarized in and further specified in sections 3.3.1 to 3.3.3.
Figure 3. Inclusion of real-world outcomes in recent economic evaluations of COPD treatment (N = 28). ADR-Adverse Drug Reaction; This figure shows the percentage of studies (out of the 28 reviewed articles), reflecting the characteristics of the main review outcomes, first displaying the percentage of studies accounting for the real-world parameter (adherence, comorbidity and occurrence of ADR), secondly, the way of inclusion, and lastly the inclusion per adherence proportion, comorbidity type or ADR
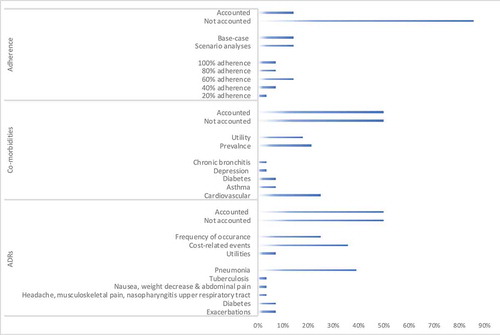
3.3.1. Medication adherence
None of the economic evaluations (CEA, CBA, CUA and CMA) accounted for the effects of medication adherence on the cost-effectiveness. However, Wilson et al. [Citation27] discussed medication adherence as a factor that is more likely to impact separate LABA plus LAMA treatment versus fixed-dose combination treatment.
Unlike the economic evaluations (CEA, CBA, CUA and CMA), half of the BIAs [Citation15,Citation29,Citation38,Citation39], showed the effect of medication adherence on the budget impact. Three BIAs [Citation40,Citation42,Citation44] did not consider medication adherence, and one [Citation41] made an assumption regarding equal effects of adherence in the comparator group. Generally, lower adherence decreased the impact of the COPD drug on the total budget impact. The reviewed studies used different base-case scenarios. For example, in one study [Citation39], 100% adherence to budesonide/formoterol was used in the base-case scenario, and in scenarios with 80% and 60% medication adherence, costs decreased with 12% and 24% respectively. Another study [Citation38] used 40% adherence in their base-case scenario. A mitigation to 20% or increase to 60% adherence impacted the Spanish health budget with €3.1 and €9.4 million respectively [Citation38]. In the Netherlands, two BIAs of LABA/LAMA accounted for adherence. One considered approximately 60% adherence in the base-case scenario by using real-world data in the estimation of the annual costs per treatment [Citation15]. The other one explored the effects in scenarios with 40%, 60%, 80%, and 100% medication adherence affecting the budget with €1.7, €2.6, €3.4 and €4.3 million, respectively [Citation29].
3.3.2. Comorbidity
Half of the evaluated studies accounted for co-morbidities [Citation15,Citation18,Citation19,Citation21–25,Citation31,Citation34–36,Citation42,Citation43]. Among those studies, cardiovascular comorbidity was most often included [Citation18,Citation19,Citation21,Citation22,Citation24,Citation34,Citation35]. Studies also considered the effects of asthma [Citation19,Citation42], diabetes [Citation15,Citation19], depression [Citation19] or chronic bronchitis [Citation43]. Besides the comorbidity prevalence, there were studies that reported effects of comorbidities on patient utilities.
The prevalence of cardiovascular comorbidity varied between 26–69%. Lowest cardiovascular comorbidity prevalence was reported in two Spanish studies comparing the effects of umeclidinium bromide/vilanterol, olodaterol/tiotropium (26%) [Citation22] and tiotropium (29%) [Citation34]. Of note, the cardiovascular disorders were defined as any cardiac disorder, coronary artery disease, myocardial infarction, arrhythmia, congestive heart failure or cerebrovascular accident [Citation22]. When comparing LAMA monotherapies umeclidinium, tiotropium and glycopyrronium in the UK, relatively high comorbidity rates were applied (i.e. 64% and 68% cardiovascular, and 87% and 89% other comorbidities, respectively) [Citation24]. Two analyses of triple therapy, one from Canada [Citation18] and one from the UK [Citation35], included similar rates of cardiovascular comorbidity, i.e. 40–44%. In contrast, for the same triple therapy in another UK study [Citation21] a much higher percentage (68–69%) of cardiovascular comorbidity was applied, as well as high percentages of other comorbidities (87–89%).
The comorbidities were related to utilities in model cycles. Such calculations showed comorbidity affecting the utility on average with −0.01 per year [Citation23]. To incorporate Spanish disutilities, 0.0074 was used for cardiovascular – and other comorbidities vs no comorbidity [Citation34]. In a study performed in Singapore, such calculations were done under the assumption of four fixed concomitant diseases per person [Citation36]. Comorbidity was also one of the covariates for calculating utilities in studies performed in Taiwan [Citation25], Canada, France, Italy and Portugal [Citation31].
3.3.3. Adverse drug reactions
The inclusion of ADRs was mostly done in terms of mentioning the frequency of their occurrence [Citation19,Citation25–27,Citation31,Citation40,Citation43], but also the costs related to such events [Citation15,Citation20,Citation23,Citation26,Citation27,Citation30,Citation31,Citation36,Citation37,Citation40] were included as well as their impact on utilities [Citation30,Citation37].
Studies analyzing bronchodilators compared to ICS-containing treatments accounted for pneumonia events [Citation15,Citation19,Citation20,Citation23,Citation26,Citation30,Citation31,Citation36,Citation37,Citation40,Citation43] but also for tuberculosis [Citation40], exacerbations [Citation15,Citation26], diabetes [Citation15], headache, musculoskeletal pain, nasopharyngitis and upper respiratory tract infections [Citation27]. Investigated treatments showed lower ADR-related ratios, e.g. comparing indacaterol/glycopyrronium with salmeterol/fluticasone FDC (0.847 versus 1.05) [Citation25]. Generally, LABA/ICS treatments accounted for more pneumonia-related hospitalization [Citation31] and higher pneumonia and tuberculosis incidence [Citation40].
In one study comparing bronchodilators, the difference in incidence of ADRs when comparing LABA/LAMA with LAMA was not considered significant (difference of 0.001 in pneumonia events) [Citation19]. In another study, umeclidinium bromide/vilanterol caused more headache and nasopharyngitis, and less musculoskeletal pain and upper respiratory tract infections than tiotropium [Citation27]. In analyses of PDE4 treatment, authors reported that patients experienced pneumonia, diarrhea, nausea, weight decrease, and abdominal pain [Citation43].
ADR-related costs mostly concerned direct costs for ADR treatment or hospitalizations due to ADRs. This was particularly emphasized when accounting for pneumonia-related costs [Citation20,Citation23,Citation26,Citation30,Citation36,Citation37,Citation40]. Such costs varied between countries, for example in Canada these were €5104, in France €5848, in Italy €2693 and in Portugal €1565 [Citation31]. In one study, the pneumonia costs accounted for 52% of the total costs per patient over lifetime [Citation36]. Last but not least, two studies assumed that ADRs affected the utilities, particularly those due to pneumonia [Citation30,Citation37].
3.4. Incremental outcomes and budget impact
Overall, all economic analyses (CEA, CBA, CUA, and CMA) indicated favorable cost-effectiveness of the investigated treatments [Citation18–36,Citation42–44]. Some studies showed dominance when the investigated treatment was LABA/LAMA [Citation21,Citation27,Citation28,Citation31,Citation36] or LAMA [Citation24,Citation33]. Furthermore, all but one study [Citation23] were industry funded. The number of QALYs gained did not exceed 0.25 (per patient over the base-case horizon), except for one study [Citation35] reporting higher QALY gains of 0.492 per patient over a lifetime, while patients receiving fluticasone furoate/umeclidinium/vilanterol versus budesonide/formoterol in the UK.
The ICER values varied between countries and were dependent on the compared treatments. When comparing ‘triple therapy’ to LABA/ICS, ICERs approximated £3,000 to £6,000 in the UK [Citation21], to almost C$19,000 in Canada [Citation18]. The ICERs were lower in the comparison of ‘triple therapy’ to LABA/LAMA, showing values of £3,000 in the UK [Citation35] to around C$14,000 in Canada [Citation18]. When LABA/LAMA was compared to LAMA, the ICER was under €10,000, in the range of €3,000-€7,000 [Citation19,Citation25,Citation29,Citation30,Citation32] except for one study with an ICER of over €20,000 [Citation34]. Some articles showed the same effectiveness between the investigated LAMA/LABA treatment and the comparator treatment resulting in equal ICERs [Citation27,Citation28,Citation37]. In Sweden and Taiwan, LABA/LAMA treatment compared to LABA/ICS gave ICERs of €9,000 and 15,000 USD, while in the USA it exceeded 100,000 USD [Citation20]. The ICERs of monotherapies (LAMA vs LAMA) had relatively low values, varying between €1,000 and €5,000 [Citation24,Citation33]. The evaluation of roflumilast maintenance treatment added on to triple inhaled therapy showed an ICER of £25,000.
Among the BIAs, the investigated treatments affected the national budgets with between €1 – and €8 – million per year. More precisely, the annual LABA/LAMA impact vs – LAMA was almost €1 – million [Citation29], – vs ICS-containing-treatments €17 million [Citation15], – vs LABA/ICS toward €6 – million [Citation40] and vs another LABA/LAMA treatment €8 – million [Citation44]. In an article evaluating LAMA as addition to standard care, the annual impact on the budget was almost €200 – million [Citation41]. LABA/ICS compared to existing ones impacted the annual budgets in Germany, Italy, Sweden and UK with €33-, €7-, €12 – and €5 – million respectively [Citation42], and €1.5 – million in Spain [Citation38]. Another Spanish study comparing LABA/ICS to all other available usual care treatments showed annual savings of €350,000 [Citation39].
3.5. Risk of bias within studies
3.5.1. Risk of bias among the economic evaluations
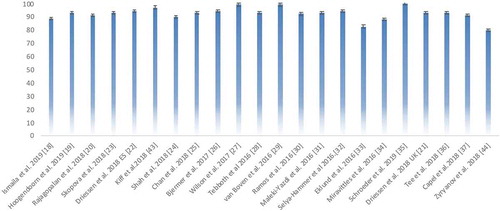
Overall, the included economic evaluations (CEA, CUA, CMA, CBA) reflected no quality issues according to the QHES checklist. However, risk of bias was poorly reported. All studies [Citation18–37,Citation43,Citation44] had a clear study objective, used good sources for their estimates, and those including subgroup analyses had properly prespecified this in their methods [Citation26,Citation30,Citation32,Citation33,Citation35,Citation43]. All studies [Citation18–37,Citation43,Citation44] properly performed incremental analyses, and addressed costs, data extraction, outcome validation, model structure, and provided conclusions and funding statements. The total QHES score per article is provided in showing that all studies were labeled as category 4 (as defined in the Methods section). Detailed scores per study and QHES item are provided in the Appendix (eTable 3 and eFigure 1).
3.5.2. Risk of bias among the BIAs
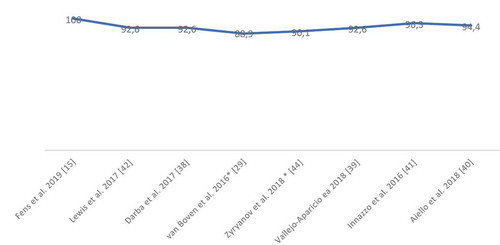
With regards to the reporting quality of the BIAs, all studies had high scores (). The two studies [Citation29,Citation44] that included the BIA in a broader economic analysis did not always clearly describe all reporting items and did not present all results visually. The detailed scores are provided in the Appendix (eTable 4).
4. Discussion
4.1. Summary of evidence
In this review, we have provided an overview of twenty-eight economic evaluations and BIAs of COPD pharmacological maintenance treatments, published over the last four years, with a focus on incorporation of real-world parameters. Of all COPD treatments, LABA/LAMA was the most frequently investigated treatment. The vast majority of the studies performed model-based analyses, used a national healthcare payers’ perspective and a lifetime horizon. While medication adherence was predominantly incorporated in BIAs, comorbidity was more often a parameter influencing the utilities in the determination of the cost-effectiveness. In half of the reviewed articles ADRs were included and their impact was mostly reflected by increased pneumonia-related hospitalizations.
4.2. Interpretation
Previous reviews investigating economic evaluations of COPD maintenance treatments reported ‘consistency of study methodology’ as a remaining problem over the past years [Citation7–9]. In 2012, the need for standard comparators, longer trials, common structures for COPD models, using standard outcome measures, as well as inclusion of greater diversity of COPD populations were stressed as areas for improvement [Citation8]. Some of these issues remained a challenge according to the more recent 2016 review of COPD maintenance treatments. Additionally, the 2016 review highlighted new directions for improvement of economic evaluations on COPD, such as the need for incorporation of real-world evidence [Citation9].
In economic analyses of COPD treatments, we should bear in mind the complexity of this chronic disease, which is often accompanied with comorbidities. While non-adherence is a general challenge, polypharmacy, ADRs and comorbidities can lead to further non-adherence to the prescribed treatment [Citation45]. Some factors that influence treatment adherence in COPD patients include the treatment regimen, patient behavior [Citation46] or device (inhaler) [Citation47,Citation48]. Regarding treatment regimen, a real-world study showed that adherence is higher with once-daily treatments, than with twice-daily treatments [Citation45]. In order to improve adherence, collaboration between patients and healthcare professionals and better patient education is required [Citation49]. Notably, enhancing adherence in patients with COPD has been shown to be cost-effective [Citation50,Citation51]. Contrasting to this evidence, the economic evaluations identified in this review did not consider the effects of medication adherence at all. On the one hand, one can imagine that clinical trial data does not provide sufficient data on adherence in the real-world. Indeed, at time of market access, real-world data is not always available for the latest treatments. On the other hand, BIAs seem to better recognize the need and importance of its inclusion. Effects of non-adherence were typically explored using adherence rates of 20–100%, and showing that lower adherence results in lower impact on total health budgets. These conclusions are also applicable to economic evaluations (CEA, CUA, CBA and CMA). Therefore, we emphasize the need of real-world evidence-based economic evaluations for better capturing the impact of medication adherence. In doing so, it will bring more realistic estimations for decision makers.
Comorbidities and ADRs may also contribute to medication non-adherence and as such to differential health outcomes [Citation52]. Patients with COPD are often suffering from various comorbidities [Citation53]. For example, a review showed that COPD is frequently associated with an increased risk for cardiovascular disease [Citation54]. Indeed, among our included studies, cardiovascular comorbidity was the most prominent. Patient characteristics related to a treatment group (in our review most frequently LABA/LAMA treatments) may be responsible for including this particular comorbidity. We should note that disease severity may also impact the prevalence of comorbidities. A recent real-world study [Citation55] reported the highest percentage of comorbidities among COPD patients of group D followed by group B. Taking into account that over 80% of COPD patients has accompanied comorbidity [Citation55,Citation56], these effects cannot be ignored. Besides the health impact, comorbidities are also causing an economic impact [Citation57], as shown in some of the articles included in this review [Citation23,Citation25,Citation31,Citation35,Citation36]. Researchers emphasized the importance of integration of multiple co-morbidities into the development of COPD guidelines [Citation58]. However, there are concerns regarding medication interactions while treating COPD and comorbidity (e.g. LABA or ICS-containing treatment against cardiovascular safety) [Citation59].
Patient safety, alongside with efficacy are the core elements for allowing and maintaining a medicine on the market [Citation60]. Even though the existing medicines comply with the regulatory norms of safety, ADRs can still occur. COPD maintenance treatments, single or dual bronchodilators, may potentially cause cardiovascular events [Citation61], while ICS-containing treatments may provoke pneumonia, dysregulated diabetes control, osteoporosis, oropharyngeal candidiasis, pharyngeal discomfort, cough and hoarseness [Citation62–65]. As pneumonia events are most commonly considered into the reviewed studies, it is of note that pneumonia as well as tuberculosis as ADR can be caused by prolonged and extended corticosteroid use. However, articles reporting pneumonia as ADR were not only comparing ICS-containing treatments, but also bronchodilators. As ADRs can arise from any of the investigated COPD treatments, the reviewed studies did not always consider their effect on the study outcomes. The frequency of ADR occurrence, its impact on costs and utility loss related to ADRs were all reported. Costs were caused by medication treatment, physicians consultation or hospitalization. Yet, no comprehensive reporting applied. Some studies that did not include the effects of ADRs hypothesized that the compared treatments had similar safety profiles. Such deliberation is not always correct and even within COPD medication of the same class (e.g. ICS) differences in safety profiles exist. This urges us to emphasize that also model-based studies should aim to better capture the impact of potential ADRs. Importantly, ignoring the impact of ADRs on the cost-effectiveness or budget-impact could lead to both over – or underestimation. One final observation deserves a comment. Thus far, health economic evidence for COPD pharmacotherapy comes almost exclusively from high-income countries, while the studied real-world issues stretch way beyond these countries and may be of even more importance in low – and middle income countries (LMIC) [Citation66]. Further efforts should be made to fill these real-world economic evidence gaps also in LMIC settings.
4.3. Strengths and limitations
Our study was performed adhering to the PRISMA guidelines for reporting systematic reviews and the study protocol was pre-published at PROSPERO. We developed a structured and comprehensive search strategy and explored various databases. The search was not limited to a particular language, allowing an Italian and a Russian article to enter our review. Furthermore, the search was expanded to include BIA (not considered in previous reviews). Lastly, all research activities were performed independently using two reviewers, to avoid reporting bias.
This review also has several limitations. For assessing the quality of the economic evaluations, we used the QHES instrument. On the one hand, this tool is useful to identify if studies reported a particular issue but on the other hand, it does not fully elaborate on the quality of performance and did not address all real-world issues of interest. Therefore, solely relying on the final QHES-scores per study should be done with caution. Moreover, since some questions were too rigid, we applied our own interpretation and division of the points per question. However, we used this instrument to align with the previous reviews, and as such to allow for easier interpretation. Since no instrument is available to assess the quality of reporting of BIAs, we used a self-designed checklist [Citation15], which was based on the ISPOR BIA-guidance [Citation6]. These results should also be interpreted with caution, since no grades scores were assigned per item. Development of a BIA-checklist accounting for different scoring per item is recommended.
4.4. Conclusion
Recent pharmacological maintenance treatments for COPD patients reflect favorable economic outcomes and budget impact. Yet, recent pharmacoeconomic evaluations (CEA, CBA, CUA and CMA) and BIAs lack comprehensive inclusion of medication adherence, ADRs and co-morbidity. To better reflect real-world cost implications, inclusion of these issues is required.
5. Expert opinion
Over the last decade, we have seen little innovation in the development of truly novel pharmacologic COPD treatments. Notably, looking at the international GOLD guidelines, we are still relying on three major classes of long-acting medications including long-acting beta2 agonists, long-acting muscarinic antagonists and inhaled corticosteroids. Most of the recent efforts have focused on combining these three treatments in fixed-dose combinations of two or three of these three classes. Additionally, multiple novel inhaler devices to administer these pharmacologic treatments have been developed. As such, clinical improvement as well as cost-effectiveness compared to current ‘usual care’ for COPD may be more and more difficult to demonstrate. Therefore, when focusing on differences in cost-effectiveness, the devil may be in the detail, e.g. slightly less ADRs or slightly higher adherence, leading on the long-term to better patient outcomes and reduction in hospital admissions. For this reason, placing more emphasis on the inclusion of these apparent ‘details’ of COPD treatment that do however play an important role in the real-world, needs to be considered. We argue that it is time to move away from the traditional paradigm of merely relying on randomized controlled trial evidence when assessing cost-effectiveness.
Why so? In most registration trials of COPD treatment, strict inclusion – and exclusion criteria are applied. This results in fairly homogeneous patient populations and straightforward interpretation of the findings. To further minimize inter-patient variability, during the trial, patient instructions and monitoring are well controlled. As such, adherence to treatments and inhaler technique are often higher than seen in less controlled, real-world environments with more heterogeneity in patient characteristics. Especially patients with COPD, which often entail elderly populations, frequently suffer from comorbidities such as cardiovascular, psychiatric and rheumatologic diseases. These diseases may not only impact the course of COPD itself, but also the ability to correctly follow a lifelong daily medication regimen. For the inhaled therapies that are often applied in COPD, this includes the right inhaler use that requires good hand-mouth coordination and sufficient inspiratory flow capacity. In trials, poor ability to use an inhaler is often an exclusion criterion yet in the real-world this a very common phenomenon with over 70% of patients having difficulties using an inhaler properly. Additionally, safety data collected in trials may therefore not be widely generalizable due to more comorbidity, poorer adherence, poorer inhaler technique and more polypharmacy in the real-world. A relevant example here is the occurrence of pneumonia and tuberculosis which are often not recognized as ADRs of COPD treatment but rather as comorbidities. However, also prolonged and extended ICS use could lead to these pneumonia and (reactivation of) tuberculosis. Yet, most clinical trials are not long enough to capture all these effects of prolonged ICS use.
Consequently, when merely relying on trial data, the impact of adherence, comorbidity and ADRs on the cost-effectiveness can be underestimated. To overcome these real-world data gaps in economic analyses of COPD medication, use of pragmatic trials and observational studies in addition to strictly controlled trial data is recommended. Moreover, to catalyze implementation of these real-world issues, reporting checklists such as CHEERS, QHES, or CHEC should incorporate items on the inclusion of the adherence, ADRs and comorbidity. Thinking more pragmatically, we should always make a trade-off between internal and external validity when designing our cost-effectiveness and budget impact studies. Notably, budget impact models for COPD tend to more closely capture issues seen in the real-world by including nationwide population parameters and drug use patterns and would therefore reflect better external validity. On the other hand, pharmacoeconomic models (CUA, CEA, CMA and CBA) stay closer to clinical trial populations and their drug effects and do therefore have better internal validity.
Lastly, we should realize that most pharmacoeconomic models for COPD aim to estimate cost-effectiveness as good as they can, however there remain estimations where garbage in means garbage out. A critical look at the totality of evidence, including both clinical trial and real-world data remains therefore essential.
Article highlights
Real-world issues such as medication adherence, co-morbidities and adverse drug-reactions are not comprehensively included in recent economic evaluations and budget impact analyses of COPD maintenance treatments.
Medication adherence is predominantly incorporated in budget impact analyses of COPD maintenance treatments, and neglected in most economic evaluations.
Comorbidities within economic evaluations of COPD maintenance treatments mostly influence the utilities underlying the cost-effectiveness estimates.
The inclusion of adverse drug reactions among economic evaluations of COPD maintenance treatments varied depending on the pharmacological group of the studied medicines, yet the effects of pneumonia-related events were most often reported.
When merely relying on trial data, the impact of medication adherence, comorbidities and adverse drug reactions on the cost-effectiveness is often inadequately covered, hence, use of pragmatic trials and observational studies in addition to strictly controlled trial data is recommended.
There is a need for updating the guidelines and reporting checklists for economic evaluations and budget impact analyses to incorporate items on the inclusion of real-world issues such as medication adherence, comorbidities and adverse drug reactions.
Declaration of interest
J van Boven has received funding from the European Commission to Chair the European Network to Advance Best practices & technoLogy on medication adherencE (COST Action CA19132, ENABLE). His institution has received consultancy fees, speaking fees, and/or research grants from AstraZeneca, Boehringer Ingelheim, Chiesi, eLucid mHealth, Menarini, Novartis, Teva and Trudell Medical, all unrelated to this study. M Postma has received consultancy fees, speaking fees, and/or research grants from AstraZeneca, GlaxoSmithKline, Merck Sharp & Dohme, Sanofi, Boehringer Ingelheim, Pfizer, Biomarin, Johnson & Johnson, Merck & Co and Sequiris, all unrelated to this study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Global Initiative for Chronic Obstructive Lung Disease [Internet]. Global initiative for chronic obstructive lung disease - GOLD. [cited 2020 Sept 29]. Available from: https://goldcopd.org/
- Chronic obstructive pulmonary disease (COPD). [cited 2020 Sept 29]. Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)
- WHO | COPD: Definition. WHO; [cited 2020 Sept 29]. Available from: https://www.who.int/respiratory/copd/definition/en/
- van Boven JFM, van de Hei SJ, Sadatsafavi M. Making sense of cost-effectiveness analyses in respiratory medicine: a practical guide for non-health economists. Eur Respir J. 2019;53(3):1801816.
- Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes. Oxford University Press; 2015.
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis—principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17:5–14.
- Starkie HJ, Briggs AH, Chambers MG. Pharmacoeconomics in COPD: lessons for the future. Int J Chron Obstruct Pulmon Dis. 2008;3:71–88.
- Rutten-van Mölken MPMH, Goossens LMA. Cost effectiveness of pharmacological maintenance treatment for chronic obstructive pulmonary disease: a review of the evidence and methodological issues. Pharmacoeconomics. 2012;30:271–302.
- van der Schans S, Goossens LMA, Boland MRS, et al. Systematic review and quality appraisal of cost-effectiveness analyses of pharmacologic maintenance treatment for chronic obstructive pulmonary disease: methodological considerations and recommendations. Pharmacoeconomics. 2017;35:43–63.
- Evers S, Goossens M, de Vet H, et al. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care. 2005;21:240–245.
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ. 2013;346:f1049-f1049.
- Ofman JJ, Sullivan SD, Neumann PJ, et al. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manag Care Pharm. 2003;9:53–61.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
- Rayyan QCRI, The systematic reviews web app. [cited 2020 Jul 15]. Available from: https://rayyan.qcri.org/welcome
- Fens T, van der Pol S, Kocks JWH, et al. Economic impact of reducing inappropriate inhaled corticosteroids use in patients with chronic obstructive pulmonary disease: ISPOR’s guidance on budget impact in practice. Value Health. 2019;22:1092–1101.
- Carvalho N, Jit M, Cox S, et al. Capturing budget impact considerations within economic evaluations: a systematic review of economic evaluations of rotavirus vaccine in low- and middle-income countries and a proposed assessment framework. Pharmacoeconomics. 2018;36:79–90.
- Loze PM, Nasciben LB, Sartori AMC, et al. Vaccines are different: a systematic review of budget impact analyses of vaccines. Vaccine. 2017;35:2781–2793.
- Ismaila AS, Risebrough N, Schroeder M, et al. Cost-effectiveness of once-daily single-inhaler triple therapy in COPD: the IMPACT trial. Int J Chron Obstruct Pulmon Dis. 2019;14:2681–2695.
- Hoogendoorn M, Corro Ramos I, Baldwin M, et al. Long-term cost-effectiveness of the fixed-dose combination of tiotropium plus olodaterol based on the DYNAGITO trial results. Int J Chron Obstruct Pulmon Dis. 2019;14:447–456.
- Rajagopalan K, Bloudek L, Marvel J, et al. Cost-effectiveness of twice-daily indacaterol/glycopyrrolate inhalation powder for the treatment of moderate to severe COPD in the US. Int J Chron Obstruct Pulmon Dis. 2018;13:3867–3877.
- Driessen M, Shah D, Risebrough N, et al. Cost-effectiveness of umeclidinium as add-on to ICS/LABA therapy in COPD: A UK perspective. Respir Med. 2018;145:130–137.
- Driessen MT, Whalen J, Seewoodharry Buguth B, et al. Cost-effectiveness analysis of umeclidinium bromide/vilanterol 62. 5/25mcg versus tiotropium/olodaterol 5/5 mcg in symptomatic patients with chronic obstructive pulmonary disease: a Spanish national healthcare system perspective. Respir Res. 2018;19. DOI:10.1186/s12931-018-0916-7.
- Skoupa J, Kasak V, Klimes J, et al. Indacaterol/glycopyrronium versus salmeterol/fluticasone in patients with COPD—a cost-effectiveness analysis in the Czech Republic. Value Health Reg Issues. 2018;16:112–118.
- Shah D, Driessen M, Risebrough N, et al. Cost-effectiveness of umeclidinium compared with tiotropium and glycopyrronium as monotherapy for chronic obstructive pulmonary disease: a UK perspective. Cost Eff Resour Alloc. 2018;16:16.
- Chan M-C, Tan EC-H, Yang M-C. Cost-effectiveness analysis of a fixed-dose combination of indacaterol and glycopyrronium as maintenance treatment for COPD. Int J Chron Obstruct Pulmon Dis. 2018;1079–1088. DOI:10.2147/COPD.S159103
- Bjermer L, van Boven JFM, Costa-Scharplatz M, et al. Indacaterol/glycopyrronium is cost-effective compared to salmeterol/fluticasone in COPD: FLAME-based modelling in a Swedish population. Respir Res. 2017;18:206.
- Wilson MR, Patel JG, Coleman A, et al. Cost-effectiveness analysis of umeclidinium/vilanterol for the management of patients with moderate to very severe COPD using an economic model. Int J Chron Obstruct Pulmon Dis. 2017;12:997–1008.
- Tebboth A, Ternouth A, Gonzalez-Rojas N. UK-specific cost-effectiveness of tiotropium + olodaterol fixed-dose combination versus other LAMA + LABA combinations in patients with COPD. Clinicoecon Outcomes Res. 2016;8:667–674.
- van Boven JF, Kocks JW, Postma MJ. Cost-effectiveness and budget impact of the fixed-dose dual bronchodilator combination tiotropium–olodaterol for patients with COPD in the Netherlands. Int J Chron Obstruct Pulmon Dis. 2016;11:2191–2201.
- Ramos M, Haughney J, Henry N, et al. Cost versus utility of aclidinium bromide 400 µg plus formoterol fumarate dihydrate 12 µg compared to aclidinium bromide 400 µg alone in the management of moderate-to-severe COPD. Clinicoecon Outcomes Res. 2016;8:445–456.
- Reza Maleki-Yazdi M, Molimard M, Keininger DL, et al. Cost effectiveness of the long-acting β2-adrenergic agonist (LABA)/long-acting muscarinic antagonist dual bronchodilator indacaterol/glycopyrronium versus the LABA/inhaled corticosteroid combination salmeterol/fluticasone in patients with chronic obstructive pulmonary disease: analyses conducted for Canada, France, Italy, and Portugal. Appl Health Econ Health Policy. 2016;14:579–594.
- Selya-Hammer C, Gonzalez-Rojas Guix N, Baldwin M, et al. Development of an enhanced health-economic model and cost-effectiveness analysis of tiotropium + olodaterol Respimat® fixed-dose combination for chronic obstructive pulmonary disease patients in Italy. Ther Adv Respir Dis. 2016;10:391–401.
- Eklund O, Afzal F, Borgström F, et al. Cost-effectiveness of tiotropium versus glycopyrronium in moderate to very severe chronic obstructive pulmonary disease in Canada, Spain, Sweden, and the UK. Clinicoecon Outcomes Res. 2016;8:243–252.
- Miravitlles M, Gáldiz JB, Huerta A, et al. Cost-effectiveness of combination therapy umeclidinium/vilanterol versus tiotropium in symptomatic COPD Spanish patients. Int J Chron Obstruct Pulmon Dis. 2016;11:123–132.
- Schroeder M, Shah D, Risebrough N, et al. Cost-effectiveness analysis of a single-inhaler triple therapy for patients with advanced chronic obstructive pulmonary disease (COPD) using the FULFIL trial: A UK perspective. Respirat Med. 2019;1:100008.
- Tee A, Chow WL, Burke C, et al. Cost-effectiveness of indacaterol/glycopyrronium in comparison with salmeterol/fluticasone combination for patients with moderate-to-severe chronic obstructive pulmonary disease: a LANTERN population analysis from Singapore. Singapore Med J. 2018;59:383–389.
- Capel M, Mareque M, Álvarez CJ, et al. Cost-effectiveness of fixed-dose combinations therapies for chronic obstructive pulmonary disease treatment. Clin Drug Investig. 2018;38:611–620.
- Darbà J, Ramírez G, García-Rivero JL, et al. Estimating the economic consequences of an increased medication adherence due to a potential improvement in the inhaler technique with Spiromax® compared with Turbuhaler® in patients with moderate-to-severe chronic obstructive pulmonary disease in Spain. Clinicoecon Outcomes Res. 2017;9:127–137.
- Vallejo-Aparicio LA, Peces-Barba G, Gil A, et al. Cost–consequence analysis of fluticasone furoate/vilanterol 92/22 mcg for the management of COPD in the Spanish NHS. Clinicoecon Outcomes Res. 2018;Volume 10:501–510.
- Aiello A, Ritrovato D, Pitotti C. Budget impact model of indacaterol/glycopyrronium in the treatment of chronic obstructive pulmonary disease in Italy based on the FLAME study. Glob Reg Health Technol Assess. 2018:2284240318804808.
- Iannazzo S, Distante C, Corsico AG. Costs of Tiotropium Bromide Delivered via the Respimat® Inhaler in COPD Patients in Italy. Glob Reg Health Technol Assess. 2016;3:GRHTA.5000220. .
- Lewis A, Torvinen S, Dekhuijzen PNR, et al. Budesonide + formoterol delivered via Spiromax® for the management of asthma and COPD: the potential impact on unscheduled healthcare costs of improving inhalation technique compared with Turbuhaler®. Respir Med. 2017;129:179–188.
- Kiff C, Ruiz S, Varol N, et al. Cost-effectiveness of roflumilast as an add-on to triple inhaled therapy vs triple inhaled therapy in patients with severe and very severe COPD associated with chronic bronchitis in the UK. Int J Chron Obstruct Pulmon Dis. 2018;13:2707–2720.
- Зырянов СК, Дьяков ИН. Фармакоэкономическая оценка двойной бронходилатационной терапии у пациентов с хронической обструктивной болезнью легких. Пульмонология. 2018:61–68. Available from https://journal.pulmonology.ru/pulm/article/view/962
- Trudo F, Kern DM, Davis JR, et al. Comparative effectiveness of budesonide/formoterol combination and tiotropium bromide among COPD patients new to these controller treatments. Int J Chron Obstruct Pulmon Dis. 2015;10:2055–2066.
- Rand CS. Patient adherence with COPD therapy. Eur Respir Rev. 2005;14:97–101.
- Dekhuijzen R, Lavorini F, Usmani OS, et al. Addressing the impact and unmet needs of nonadherence in asthma and chronic obstructive pulmonary disease: where do we go from here? J Allergy Clin Immunol. 2018;6:785–793.
- Cushen B, Sulaiman I, Greene G, et al. The clinical impact of different adherence behaviors in patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197:1630–1633.
- van Boven JFM, Ryan D, Eakin MN, et al. Enhancing respiratory medication adherence: the role of health care professionals and cost-effectiveness considerations. J Allergy Clin Immunol Pract. 2016;4:835–846.
- van Boven JFM, Tommelein E, Boussery K, et al. Improving inhaler adherence in patients with chronic obstructive pulmonary disease: a cost-effectiveness analysis. Respir Res. 2014;15:66.
- van Boven JFM, Cushen B, Sulaiman I, et al. Personalising adherence-enhancing interventions using a smart inhaler in patients with COPD: an exploratory cost-effectiveness analysis. NPJ Prim Care Respir Med. 2018;28:1–3.
- Rogliani P, Ora J, Puxeddu E, et al. Adherence to COPD treatment: myth and reality. Respir Med. 2017;129:117–123.
- Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev. 2013;22:454–475.
- Müllerova H, Agusti A, Erqou S, et al. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144:1163–1178.
- Dimitrova M, Kamusheva M, Tachkov K, et al. Cardiovascular co-morbidity in patients with COPD in Bulgaria. Biotechnol Biotechnol Equip. 2020;34:918–924.
- Chetty U, McLean G, Morrison D, et al. Chronic obstructive pulmonary disease and comorbidities: a large cross-sectional study in primary care. Br J Gen Pract. 2017;67:e321–e328.
- Negewo NA, Gibson PG, McDonald VM. COPD and its comorbidities: impact, measurement and mechanisms. Respirology. 2015;20:1160–1171.
- Fabbri LM, Boyd C, Boschetto P, et al. How to integrate multiple comorbidities in guideline development: article 10 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report. Proc Am Thorac Soc. 2012;9:274–281.
- Corsonello A, Antonelli Incalzi R, Pistelli R, et al. Comorbidities of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2011;17(Suppl 1):S21–28.
- EMA. Authorisation of medicines [Internet]. European Medicines Agency; 2018. [cited 2020 Sept 20]. Available from: https://www.ema.europa.eu/en/about-us/what-we-do/authorisation-medicines
- Rogliani P, Matera MG, Ora J, et al. The impact of dual bronchodilation on cardiovascular serious adverse events and mortality in COPD: a quantitative synthesis. Int J Chron Obstruct Pulmon Dis. 2017;12:3469–3485.
- Matera MG, Calzetta L, Puxeddu E, et al. A safety comparison of LABA+LAMA vs LABA+ICS combination therapy for COPD. Expert Opin Drug Saf. 2018;17:509–517.
- van Boven JFM, de Jong-van den Berg LTW, Vegter S. Inhaled corticosteroids and the occurrence of oral candidiasis: a prescription sequence symmetry analysis. Drug Saf. 2013;36:231–236.
- Price D, Yawn B, Brusselle G, et al. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim Care Respir J. 2013;22:92–100.
- Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch Intern Med. 2009;169:219–229.
- Tabyshova A, Hurst JR, Soriano JB, et al. Gaps in COPD guidelines of low- and middle income countries: a systematic scoping review. Chest. 2020. DOI:10.1016/j.chest.2020.09.260.