?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.1. Introduction
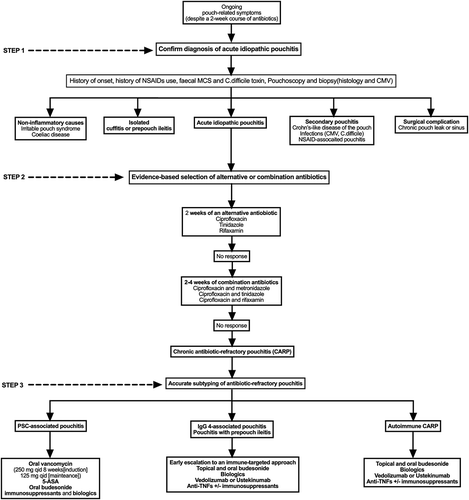
Idiopathic pouchitis is the most common complication of ileal pouch–anal anastomosis, affecting up to 50–60% of ulcerative colitis pouch patients and 15–22% of familial adenomatous polyposis pouch patients 10 years after stoma closure [Citation1,Citation2]. Pouchitis can be clinically classified into three phenotypes: acute antibiotic-responsive (<4 episodes a year), chronic antibiotic-dependent (4 antibiotic-responsive episodes or need for ongoing antibiotics), and chronic antibiotic-refractory pouchitis (CARP)[Citation3]. Secondary causes of pouchitis include infections (Cytomegalovirus [CMV] and Clostridioides difficile), ischemia (asymmetric pouchitis), and nonsteroidal anti-inflammatory drugs (NSAIDs)-associated pouchitis. Second, in patients who have never had a normal pouch function, idiopathic pouchitis needs to be distinguished from a chronic leak or sinus. Thirdly, other inflammatory complications of the pouch such as cuffitis and prepouch ileitis, non-inflammatory complications of the pouch, such as irritable pouch syndrome, can present similarly and need to be ruled out. While most acute episodes of pouchitis respond readily to a finite course of a single antibiotic, up to 20% can be refractory and more challenging to treat. A useful stepwise approach in such patients includes three steps: (i) ensuring this is an episode of acute idiopathic pouchitis, (ii) following an evidence-base selection of alternative or combination antibiotics, and (iii) accurate subtyping of antibiotic refractory pouchitis to allow for a targeted and personalized approach.
Pouch symptoms of increased frequency, urgency and abdominal cramps are not specific to idiopathic pouchitis and can be seen in secondary pouchitis and non-inflammatory pouch complications. Therefore, objective evaluation with pouchoscopy is needed. This is particularly crucial when managing a first episode of pouch dysfunction or when an empiric course of antibiotic fails. In fact, pouchoscopy is considered more cost-effective than an empiric trial of antibiotics for episodes of acute pouchitis [Citation4]. It will help diagnose isolated cuffitis, prepouch ileitis and ischemic pouchitis (asymmetric sharply demarcated inflammation within the pouch body). Furthermore, the degree of pouch body inflammation on pouchoscopy can help distinguish pouchitis from irritable pouch syndrome. Using the Pouchitis Disease Activity Index (PDAI), which consists of symptom (0–6 points), endoscopy (0–6 points), and histology (0–6 points) sub-scores, a PDAI score of ≥7 points is diagnostic for pouchitis [Citation5]. Second, while the PDAI allows diagnosis of pouchitis, it does not distinguish idiopathic pouchitis from secondary causes of pouchitis or other causes of diarrhea such as celiac disease. Hence, this approach should be coupled with a fecal sample tested for C. difficile toxin, a review of NSAID intake and celiac serology. Importantly, special attention should be made to the onset of pouch symptoms in relation to stoma closure. Those whose symptoms started immediately after stoma closure should be suspected of and investigated for a pouch leak or sinus. Third, and particularly in patients with antibiotic refractory pouchitis, Crohn’s-like disease of the pouch (CLDP) needs to be ruled out. In the absence of defining Crohn’s-like features, such as complex peri-pouch fistulas or proximal small bowel strictures, the distinction may be difficult. This is particularly true since granulomas are only seen in 12–13% of CLDP cases. A diagnosis of CLDP is suggested by the presence of deep ulcerations in the pouch body combined with risk factors for CD such as a preoperative diagnosis of indeterminate colitis, active smoking, and positive anti-Saccharomyces cerevisiae antibodies (ASCA).
Once the diagnosis of idiopathic acute pouchitis is confirmed, if a patient has failed one 2-week course of antibiotics such as metronidazole (15–20 mg/kg/day), they should be treated with a 2-week course of another antibiotic such as ciprofloxacin (500 mg twice daily) [Citation6]. Ciprofloxacin appears to be more effective than metronidazole and with fewer adverse effects [Citation6]. Tinidazole (500 mg twice daily) can be used as an alternative in those failing ciprofloxacin and is considered one of the most potent antibiotics for acute pouchitis. Rifaximin 500 mg twice daily is also effective, but due to its cost and low side effect profile, it is best reserved for chronic antibiotic-dependent pouchitis (CADP) requiring ongoing antibiotics [Citation7]. Patients failing the above, should be offered longer courses of two antibiotics combined [Citation8]. Combination therapy of ciprofloxacin and metronidazole for 4 weeks achieved clinical remission in 82% of patients in one study [Citation9], and combination of ciprofloxacin and tinidazole achieved clinical remission in 88% of patients in another study [Citation10]. Those intolerant to metronidazole or tinidazole can be treated with a 2–4 week course of ciprofloxacin and rifaximin, which achieved clinical response or remission in 87% of patients in an open-label study [Citation11].
Those who have failed combination antibiotics, and in whom underlying secondary causes of pouchitis or an alternate diagnosis have been excluded, are considered to have chronic antibiotic refractory pouchitis (CARP). Accurate subtyping of antibiotic-refractory pouchitis is essential for a targeted approach as different sub-types appear to be mediated by different pathogenic processes and have different treatment response rates. Antibiotic-refractory pouchitis should be divided into four subtypes, IgG 4-associated pouchitis, Primary sclerosing cholangitis (PSC)-associated pouchitis, pouchitis associated with prepouch ileitis and autoimmune-CARP. IgG4-associated pouchitis seems to be a predominately immune-mediated process [Citation12]. Similarly, pouchitis associated with prepouch ileitis, particularly when the underlying colonic disease was indeterminate colitis, is a predominately immune-mediated process. Therefore, early recognition of these two subtypes helps minimize antibiotic use and direct treatment toward the mucosal immune component early on. PSC-associated pouchitis also has a high failure rate to antibiotics commonly used for pouchitis. Furthermore, there are limited data on the efficacy of therapies targeting the immune component such as 5-amino salicylate (5ASA), budesonide and biologics[Citation8]. Vancomycin, however, has been reported to successfully achieve and maintain remission in PSC-associated pouchitis at the Cleveland Clinic, with a similar experience in our center[Citation13]. When CARP is not associated with PSC, IgG4 or prepouch ileitis, it is subtyped auto-immune CARP. While 5ASA can be tried, most patients need induction with budesonide and maintenance with an immunosuppressant or a biologic. Preliminary data suggest better clinical response rates to vedolizumab and ustekinumab than immunosuppressants and anti-TNFs. In one study, the 6 months clinical and endoscopic response to vedolizumab in 29 CARP patients was 52% and 58%, respectively [Citation14]. Similarly, in a recent retrospective study of 24 patients with CARP who received ustekinumab, there was a 50% clinical and endoscopic response rate[Citation8]. Conversely, in a metanalysis that included 313 patients treated with infliximab (n = 194) or adalimumab (n = 119) for CARP and Crohn’s-like disease of the pouch (CLDP), clinical remission rates at 8 weeks appeared to be lower in CARP patients compared with CLDP group (0.10 (95% CI 0.00–0.35) vs 0.64 (95% CI 0.5–0.66), p = 0.06) [Citation15].
2. Expert opinion
This proposed treatment approach is based on observational studies and expert opinion. The management of refractory pouchitis remains challenging due in part to the paucity of randomized clinical trials to guide treatment decisions. Furthermore, the few available prospective studies often group together patients with potentially different phenotypes and subtypes when analyzing response rates. If pathogenesis is indeed different according to phenotype and subtype, then response to different strategies may also be different. Third, observational and interventional studies are often limited to assessing serum and fecal inflammatory markers and fail to include pathogenically relevant biomarkers such as microbial metabolic products (e.g. butyrate) and intestinal permeability. Investigating such markers would help our understanding of the pathogenesis of pouchitis, and could propose new treatment targets for assessment.
The two aspects expected to be central to pouchitis research in the near future include expanding our knowledge of disease pathogenesis and developing therapeutic strategies targeting the predominate process mediating pouchitis in individual patients (microbial vs immune). The former will require an increased understanding of the pathogenicity of individual components of the microbiome in IPAA patients, including assessing the response of various pathogenically relevant biomarkers to various therapeutic interventions. Success of the latter will depend on improved phenotyping and subtyping of pouchitis prior to individualizing therapeutic recommendations.
In conclusion, patients with confirmed antibiotic refractory pouchitis may benefit from a targeted approach that is based on accurate phenotyping and subtyping (). Well-designed prospective studies that incorporate accurate phenotyping and subtyping and assess pathologically relevant biomarkers of pouchitis are needed to help guide future treatment decisions.
Declaration of interest
MP Sparrow has received educational grants or research support from Ferring Pharmaceuticals, AOP Orphan Pharmaceuticals and Gilead Sciences. He has also received speaker’s fees from Janssen Pharmaceuticals, AbbVie, Ferring Pharmaceuticals, Takeda, Pfizer and Shire and has served on advisory boards for Janssen Pharmaceuticals, Takeda, Pfizer, Celgene, AbbVie, Merck Sharp and Dohme and Emerge Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One referee declares that they have served as consultant for AbbVie, Gilead Sciences, Pfizer, Takeda, and Target RWE although none are directly relevant to the material of this review. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Fazio VW, Kiran RP, Remzi FH, et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg. 2013;257:679–685.
- Quinn KP, Lightner AL, Pendegraft RS, et al. Pouchitis is a common complication in patients with familial adenomatous polyposis following ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2016;14:1296–1301.
- Shen B. Pouchitis: what every gastroenterologist needs to know. Clin Gastroenterol Hepatol. 2013;11:1538–1549.
- Shen B, Shermock KM, Fazio VW, et al. A cost-effectiveness analysis of diagnostic strategies for symptomatic patients with ileal pouch-anal anastomosis. Am J Gastroenterol. 2003;98:2460–2467.
- Sandborn WJ, Tremaine WJ, Batts KP, et al. Pouchitis after ileal pouch-anal anastomosis: a pouchitis disease activity index. Mayo Clin Proc. 1994;69:409–415.
- Shen B, Achkar JP, Lashner BA, et al. A randomized clinical trial of ciprofloxacin and metronidazole to treat acute pouchitis. Inflamm Bowel Dis. 2001;7:301–305.
- Isaacs KL, Sandler RS, Abreu M, et al. Rifaximin for the treatment of active pouchitis: a randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis. 2007;13:1250–1255.
- Quinn KP, Raffals LE. An update on the medical management of inflammatory pouch complications. Am J Gastroenterol. 2020;115:1439–1450.
- Mimura T, Rizzello F, Helwig U, et al. Four-week open-label trial of metronidazole and ciprofloxacin for the treatment of recurrent or refractory pouchitis. Aliment Pharmacol Ther. 2002;16:909–917.
- Shen B, Fazio VW, Remzi FH, et al. Combined ciprofloxacin and tinidazole therapy in the treatment of chronic refractory pouchitis. Dis Colon Rectum. 2007;50:498–508.
- Abdelrazeq AS, Kelly SM, Lund JN, et al. Rifaximin-ciprofloxacin combination therapy is effective in chronic active refractory pouchitis. Colorectal Dis. 2005;7:182–186.
- Shen B, Bennett AE, Navaneethan U. IgG4-associated pouchitis. Inflamm Bowel Dis. 2011;17:1247–1248.
- Ardalan ZS, Sparrow MP. A personalized approach to managing patients with an ileal pouch-anal anastomosis. Front Med (Lausanne). 2020;6:337.
- Gregory M, Weaver KN, Hoversten P, et al. Efficacy of vedolizumab for refractory pouchitis of the ileo-anal pouch: results from a multicenter US cohort. Inflamm Bowel Dis. 2019;25:1569–1576.
- Huguet M, Pereira B, Goutte M, et al. Systematic review with meta-analysis: anti-TNF therapy in refractory pouchitis and Crohn’s disease-like complications of the pouch after ileal pouch-anal anastomosis following colectomy for ulcerative colitis. Inflamm Bowel Dis. 2018;24:261–268.