ABSTRACT
IntroductionCalcitonin gene-related peptide (CGRP) is a vasodilatory neuropeptide involved in the pathophysiology of migraine, a highly disabling neurovascular disorder characterized by severe headache attacks. Rimegepant is a small-molecule CGRP receptor antagonist approved by the FDA for acute treatment of migraine and currently under investigation for migraine prophylaxis. Areas covered The authors summarize available data on safety and tolerability of rimegepant and provide insights on its use for acute migraine treatment. Expert opinion Rimegepant seems to be well tolerated and superior to placebo for two-hour pain freedom. Moreover, rimegepant does not induce vasoconstriction, and is therefore not contraindicated in patients with cardiovascular disease, nor does it seem to induce medication-overuse headache. However, the therapeutic gain of rimegepant is only small, and since CGRP is a vital rescue molecule during ischemia, blocking the CGRP pathway might be detrimental. Although current evidence on CGRP receptor blockade has shown no cardiovascular adverse events, clinicians should remain critical about the use of rimegepant, as well as other CGRP (receptor)-inhibiting drugs. Further research should focus on determining the consequences of long-term CGRP blockade, especially during ischemia or cardiovascular disease, the exact receptors antagonized by rimegepant, and potential effects of combining rimegepant with other antimigraine treatments.
KEYWORDS:
1. Introduction
Migraine is a debilitating neurovascular disorder, mainly characterized by severe unilateral headache attacks of pulsating quality. Other symptoms include nausea, vomiting, and phono- and photophobia [Citation1]. The headache phase of a migraine attack lasts up to 72 hours and can be preceded by an aura phase in which fully reversible visual, sensory, speech, motor, brainstem or retinal disturbances take place (). According to the Global Burden of Disease Study 2016, migraine is the second most disabling disorder worldwide, and the first cause of disability in people under the age of fifty [Citation2,Citation3]. In addition, migraine has a global age-standardized prevalence of around 14%, affecting twice as many women as men [Citation4].
The pathophysiology of migraine involves activation of the trigeminovascular system [Citation5]. Neuropeptides, including calcitonin gene-related peptide (CGRP), are released by perivascular branches of the trigeminal nerve, induce vasodilation, and activate nociceptors on the trigeminal nerve, which subsequently leads to pain perception. CGRP is a highly potent vasodilator and is thought to play an important role in the migraine pathophysiology. CGRP levels were found to be elevated during migraine headaches [Citation6,Citation7] and infusion of CGRP has been shown to induce a migraine-like headache in migraine patients with and without aura [Citation8,Citation9], implying a causative role of CGRP.
2. Overview of the market
Non-steroidal anti-inflammatory drugs (NSAIDs) and other analgesics are often used to treat migraine attacks, although they are not specifically developed to treat migraine. The current golden standard for the specific acute treatment of migraine is the triptans, wich are 5-HT1B/D receptor agonists. It was discovered that the triptans could reduce elevated CGRP levels during nitroglycerin-induced migraine attacks and spontaneous headaches, leading to pain relief [Citation7,Citation10]. Moreover, sumatriptan inhibits the increase in dermal blood flow after capsaicin application on the forehead, induced by CGRP release [Citation11]. Another novel drug that has been approved by the FDA for the treatment of migraine is the 5-HT1F receptor agonist lasmiditan. Lasmiditan was found to inhibit CGRP release in the trigeminovascular system of mice [Citation12] and Sprague–Dawley rats [Citation13], suggesting that, apart from its direct central effects, this novel anti-migraine drug potentially also functions through suppression of CGRP release. Over the last years, CGRP has become a major target for specific anti-migraine medication. Recently, two new classes of drugs specifically targeting CGRP or its receptor have been developed, i.e. monoclonal antibodies and the small-molecule CGRP receptor antagonists called gepants. Four monoclonal antibodies have been developed, of which one, erenumab, targets the CGRP receptor and the other three, eptinezumab, fremanezumab, and galcanezumab, target the CGRP peptide itself. All four have been approved for clinical use.
The first gepants that were developed were olcegepant, telcagepant, MK3207, and BI44370TA. Olcegepant could effectively relieve acute migraine pain when administered intravenously [Citation14]. However, an alternative route of administration is preferred for the acute treatment of migraine, leading to discontinuation of olcegepant. The other gepants were developed for oral intake and have also been shown to be effective for treating migraine [Citation15–18]. Yet, their development was halted due to elevated aminotransferase levels, suggesting liver toxicity [Citation15,Citation19]. Subsequently, a second generation of gepants was developed, including atogepant, rimegepant and ubrogepant. These three compounds made it to clinical trials successfully without signs of liver toxicity. Both rimegepant and ubrogepant have already received FDA approval for the acute treatment of migraine [Citation20,Citation21], while atogepant, which was developed for migraine prophylaxis, is still in the last phase before approval. In addition, rimegepant is currently under investigation for the prophylactic treatment of migraine.
3. Introduction of the compound
Rimegepant, previously known as BMS-927711, is a small molecule that antagonizes the CGRP receptor. Biohaven Pharmaceutical Holding Company Ltd developed rimegepant for the acute treatment of migraine with and without aura. It is marketed as an orally disintegrating tablet (ODT) called NURTEC ODT. The recommended dose of NURTEC ODT is 75 mg, which should be taken maximally once in a 24-hour period. The safety of treatment of more than fifteen migraines in a 30-day period has yet to be established [Citation22].
4. Pharmacodynamics, pharmacokinetics, and metabolism
Rimegepant is a very potent antagonist of the CGRP receptor with a Ki of 27 pM, determined in SK-N-MC cells [Citation23], a human neuroblastoma cell line which expresses the CGRP receptor. Moreover, rimegepant could inhibit CGRP-stimulated cAMP production in these cells, with an IC50 of 0.14 nM. Oral administration of NURTEC ODT in a 75 mg tablet leads to a maximum concentration of rimegepant in serum around 1.5 hours after intake [Citation24], with significant pain relief after 60 minutes [Citation25], and an oral bioavailability of 64% [Citation22] (). Its efficacy lasts up to 48 hours after a single dose, and the drug has a relatively long half-life in healthy subjects, of around 10–12 hours [Citation26], giving it the potential to be used as migraine prophylaxis in addition to the acute treatment of migraine attacks. The volume of distribution of rimegepant is 120 L at steady state [Citation22]. Together with a high partition coefficient (Log P = 2.95) [Citation27], this large distribution volume suggests a relatively high lipid solubility of the compound. Besides, as rimegepant is approximately 96% plasma protein bound, only a very small biologically available free fraction remains, with a high lipid solubility. Rimegepant is mainly metabolized by CYP3A4, and partly by CYP2C9 as well. It is also a substrate of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) efflux transporters. Therefore, it is not recommended to take rimegepant simultaneously with strong CYP3A4 inhibitors (e.g. ketoconazole, imatinib, voriconazole), strong (e.g. carbamazepine, rifapentine) or moderate (e.g. dexamethasone, oxcarbazepine) CYP3A inducers, or inhibitors of P-gp (e.g. propranolol, progesterone, diltiazem) or BCRP [Citation22,Citation28]. Around 77% of rimegepant is eliminated in unchanged form and can be found in urine (51% as unchanged drug) and feces (42% as unchanged drug) [Citation22]. Sex, age, race or ethnicity, bodyweight or CYP2C9 genotype did not affect the pharmacokinetics of rimegepant with clinical significance according to the FDA prescribing information [Citation22], although, as will be discussed below, it might be hypothesized that bodyweight may affect the pharmacokinetic behavior of rimegepant in a population with a wider range of variation in bodyweight than that assessed by the FDA.
Table 1. Pharmacodynamic and pharmacokinetic parameters of NURTEC ODT (75 mg)
5. Clinical safety, efficacy, and tolerability
Before rimegepant was tested on migraine patients in clinical trials, the safety and tolerability of the compound were assessed in healthy subjects [Citation29]. A single dose up to 1500 mg rimegepant was well tolerated, and daily doses of rimegepant up to 600 mg showed no significant adverse effects for fourteen days. In this phase I study, the maximum tolerated dose was not reached, even though plasma levels were up to 50 times higher than those reached after the therapeutic 75 mg dose.
Next, Marcus et al. performed a phase IIb dose-ranging study [Citation30]. Eight hundred eighty-five migraine patients received 10 mg, 25 mg, 75 mg, 150 mg, 300 mg or 600 mg rimegepant, 100 mg sumatriptan as an active comparator, or a placebo. The number of patients that reached pain freedom two hours after intake was significantly higher in the 75 mg, 150 mg, and 300 mg dose groups of rimegepant (31.4%, 32.9%, and 29.7%, respectively) and the sumatriptan group (35%) compared to placebo (15.3%). No treatment-related serious adverse events (SAEs) occurred and no patients discontinued due to adverse events (AEs). However, dose-dependent nausea was observed in the rimegepant groups, with up to 8% of patients experiencing nausea in the 600 mg dose group.
Subsequent phase III trials continued with the 75 mg dose of rimegepant. Three pivotal phase III trials for the acute treatment of migraine have been completed, while a phase III study in Chinese and Korean patients is still ongoing. Two of these clinical trials (NCT03235479 and NCT03237845) [Citation31,Citation32] used rimegepant as a regular oral tablet while the third (NCT03461757) used an orally disintegrating tablet (ODT) with an improved absorption rate, allowing intake without liquids [Citation25]. In all three studies, rimegepant was superior to placebo at two hours post-dose for pain freedom and freedom from the most bothersome symptom. A meta-analysis on the pivotal phase IIb and phase III studies showed that especially photophobia and phonophobia improved when using rimegepant [Citation33]. Nausea could be relieved as well, albeit to a lesser extent, which is remarkable as nausea is also a side-effect of rimegepant. Pain freedom and pain relief did not differ much between 24 hours and 48 hours after intake of rimegepant, which could be due to the slow metabolism of rimegepant [Citation33]. Moreover, more patients experienced sustained pain relief compared to sustained pain freedom, suggesting that rimegepant might be more effective for pain relief. No SAEs occurred that were related to the treatment, and no hepatotoxicity was described. Nausea and urinary tract infections were the most common AEs in these clinical trials.
The safety of rimegepant was assessed in a phase II/III open-label study (NCT03266588) [Citation34], in which participants were allowed to take a 75 mg dose when needed, with a maximum of one tablet per day for 52 weeks. Another group of participants in this study took 75 mg rimegepant every other day for 12 weeks, supplemented by an extra dose of rimegepant on nonscheduled dosing days when necessary to treat an attack. The most common AEs were upper respiratory tract infection (8.8%), nasopharyngitis (6.8%), and sinusitis (5.1%). The majority of AEs were considered unrelated to the treatment. No deaths were reported during the study period, and no SAEs were considered related to rimegepant by the investigators. Hepatotoxicity was not observed with long-term and high-frequency dosing of rimegepant. Based on the efficacy and safety data of the clinical trials, rimegepant (NURTEC ODT) received approval from the U.S. Food and Drug Administration (FDA) for the acute treatment of migraine in February 2020 [Citation20].
Doses of NURTEC ODT need no adjustments in patients with mild, moderate, or severe renal impairment or in patients with mild or moderate hepatic impairment. However, the use of NURTEC ODT should be avoided in patients with severe hepatic impairment, as plasma concentrations of rimegepant were significantly higher in these patients in clinical trials [Citation22]. Moreover, hypersensitivity reactions have been reported in clinical trials, including dyspnea and rash. If hypersensitivity occurs, NURTEC ODT should be discontinued immediately [Citation22].
A subset of patients in the open-label study (NCT03266588) used a monoclonal antibody against CGRP or its receptor for migraine prevention next to rimegepant for the acute treatment of migraine attacks [Citation35,Citation36]. Two patients who received a monthly dose of erenumab could successfully treat all their attacks with a 75 mg dose of rimegepant, while they reported no related AEs [Citation35]. The safety of the combined treatment of migraine with a monoclonal antibody and rimegepant was assessed in 13 patients [Citation36]. The patients received a stable dose of erenumab, fremanezumab or galcanezumab for over two months before starting the study period in which they could use rimegepant to treat their migraine attacks. In three patients, an AE occurred that was potentially related to the treatment. The investigators evaluated these AEs, of which a viral gastroenteritis was considered to be unlikely related to the treatment in a patient using galcanezumab, while a first-degree atrioventricular block and dizziness were considered to be possibly related to treatment in patients on erenumab. No SAEs were observed, and AEs did not lead to discontinuation of the study drug. Overall, rimegepant was found to be well tolerated in patients using concomitant preventive treatment with a monoclonal antibody targeting CGRP or its receptor, although this should be studied in more extensive and heterogeneous patient populations.
In addition to the clinical trials on rimegepant for the acute treatment of migraine, another phase II/III trial assessed the efficacy of rimegepant for migraine prevention (NCT03732638) [Citation37]. Participants received a 75 mg dose of NURTEC ODT every other day for three months. The primary outcome was a reduction in mean monthly migraine days, measured in week nine until twelve. Rimegepant showed a statistically larger reduction in monthly migraine days (4.3 days) than placebo (3.5 days). Safety analyses showed that a similar number of AEs were reported by the participants who received rimegepant compared to placebo. Of the treatment group, seven participants discontinued the study due to AEs, compared to four patients in the placebo group. A supplemental New Drug Application for rimegepant (NURTEC ODT) for the preventive treatment of migraine was submitted to the FDA and accepted for review in October 2020. If accepted, NURTEC ODT will become the first CGRP targeting drug for both the acute and preventive treatment of migraine.
6. Expert opinion
In general, rimegepant seemed to be well tolerated in the clinical trials, with the most commonly reported side effect being nausea, and rimegepant intake is not associated with any SAEs. However, CGRP is a highly potent vasodilator and serves as a rescue molecule during cardiac or cerebral ischemia [Citation38]. As migraine is an underestimated risk factor for cardiovascular disease, including stroke [Citation39], blocking CGRP in these patients could be detrimental in the longer term [Citation40]. In mice, administration of both olcegepant and rimegepant resulted in an increase of infarct volumes and mortality after middle cerebral artery occlusion, suggesting that medication that blocks the CGRP pathway could aggravate cerebral ischemia in mice [Citation41]. It is still uncertain how these findings translate to the clinical setting. However, it was shown that both antagonists are much more potent in human blood vessels compared to mice vessels, which could suggest a considerable effect of these compounds on ischemia in humans. Until now, the large clinical trials using monoclonal antibodies targeting CGRP or its receptor have not shown a significant increase in cardiovascular AEs compared to the placebo groups, suggesting that long-term CGRP blockade is relatively safe [Citation42–45]. However, preclinical data state that CGRP receptor blockade leads to a worsening in ischemic outcome [Citation41], not an increased risk of ischemic events. Current clinical trials are not designed to detect this worsening of ischemia. Therefore, future research should focus on the assessment of the impact of CGRP (receptor) blockade on ischemic outcome in both males and females with and without comorbid cardiovascular disease.
Rimegepant was developed to target the canonical CGRP receptor, which is composed of receptor activity-modifying protein 1 (RAMP1), calcitonin like receptor (CLR) and receptor component protein (RCP). However, other receptors in the CGRP receptor family are closely related. The amylin 1 (AMY1) receptor differs from the CGRP receptor by having a calcitonin receptor (CTR) subunit instead of CLR. The adrenomedullin 1 and 2 receptors differ by having a different RAMP unit (RAMP2 and RAMP3, respectively, instead of RAMP1 for the CGRP receptor). Recently, rimegepant was found to be able to target the AMY1 receptor as well, albeit with a lower potency [Citation46]. The role of the AMY1 receptor in migraine needs to be investigated further and the effects of blocking this receptor should be identified.
At present, it is difficult to estimate the advantage of rimegepant over other antimigraine medication, as direct comparator trials are necessary to elucidate this issue. However, when considering the therapeutic gain of medications that are used to treat migraine attacks versus placebo, the effect of the new gepants seems to be relatively small [Citation47]. Indeed, statistically significant results do not directly imply that these results are clinically significant or relevant. Ubrogepant and rimegepant show a therapeutic gain of 5–10% for two-hour pain freedom in the pivotal phase III trials [Citation25,Citation31,Citation32], while for triptans (e.g. sumatriptan, eletripan, and rizatriptan) the therapeutic gain is between 16% and 32% [Citation47]. Medication that is not specifically developed for the treatment of migraine, such as aspirin and diclofenac, also show a higher therapeutic gain (13% and 11%, respectively) [Citation48,Citation49] compared to the gepants that are currently available. However, a direct comparison might not be feasible as trials investigating rimegepant might include patients with more persistent migraine. Interestingly, telcagepant, an older gepant whose development was halted due to liver toxicity, showed a much higher therapeutic gain of around 17% [Citation50].
Although the therapeutic gain seems smaller, rimegepant has the advantage over triptans that it does not induce vasoconstriction. Therefore, in contrast to triptans, rimegepant is not contraindicated in patients with cardiovascular disease comorbidity [Citation51]. Moreover, based on preclinical data, latent sensitization or cutaneous allodynia are not induced by gepants, suggesting an absence of the risk of medication overuse headache (MOH) for this class of drugs [Citation52,Citation53]. Opposite results were found for triptans or the novel class of anti-migraine medication targeting the 5-HT1F receptor called ditans, which induce cutaneous allodynia [Citation52–54]. The preclinical data are in agreement with preliminary clinical results, which show no evidence of MOH development after exposure to gepants [Citation55].
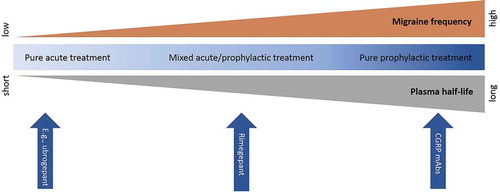
From a safety point of view, temporarily blocking the CGRP pathway with a small-molecule CGRP receptor antagonist such as rimegepant for the acute treatment of migraine might be favorable over long-term blockade, as observed after administration of monoclonal antibodies or a gepant used for migraine prophylaxis. Indeed, this would greatly diminish the chances of an ischemic event occurring during the time of CGRP receptor blockade, although no conclusions can be drawn yet concerning the potential safety of repeated short-term blockade. Following this line of reasoning, small-molecule CGRP receptor antagonists with a half-life that is even shorter than that of rimegepant might be preferred, such as ubrogepant (half-lives are, respectively, around 10–12 hours for rimegepant and 5–7 hours for ubrogepant). Indeed, this might decrease the period during which the CGRP receptor is blocked, thereby potentially decreasing the chance of concurring ischemia and the occurrence of potential AEs due to CGRP receptor blockade. On the other hand, a shorter half-life might result in a less sustained efficacy compared to compounds with longer half-lives. Thus, the choice for a drug with certain pharmacokinetics should be based on the patient characteristics and preferences, including cardiovascular risk profile and migraine frequency ().
Figure 1. Rimegepant as a continuum for mixed acute and prophylactic treatment in migraine. Rimegepant is situated between pure acute treatments (e.g. ubrogepant) and pure prophylactic treatments (e.g. monoclonal antibodies (mAbs)) in migraine, based on its plasma half-life. This allows clinicians to choose the optimal treatment based on the migraine frequency
Further studies on potential disadvantages of blocking the CGRP receptor and a direct comparison of different gepants should be conducted to determine clinical effectiveness of rimegepant. Moreover, a more heterogeneous population should be studied in order to be able to draw conclusions on the safety and efficacy of rimegepant in specific subpopulations. In the current randomized controlled trials, most of the subjects were white females with an average age of around 40 years and a body mass index (BMI) around 31 [Citation33]. Therefore, it is difficult to estimate the efficacy and safety in males, patients of an older age or with different races or ethnicities. Moreover, an average BMI of 31 suggests a high prevalence of obesity in these groups, which could affect the pharmacokinetics of rimegepant, especially since rimegepant has a relatively high lipid solubility. More research is warranted on the potentially altered pharmacokinetics. Indeed, this might have implications for the safety and efficacy of rimegepant and possibly for the dosing in migraine patients with a lower BMI [Citation56], despite statements of the FDA prescribing information mentioning an absence of clinically significant differences in the pharmacokinetics [Citation22].
7. Conclusions
Considering the modest therapeutic gain of rimegepant and the possible (long-term) risks of blocking the CGRP pathway, clinicians should remain critical about the use of rimegepant for migraine treatment. However, considering the fact that rimegepant does not induce vasoconstriction, it can be prescribed in patients with comorbid cardiovascular disease. Also, rimegepant does not seem to induce medication overuse headache. These are arguments in favor of the clinical use of rimegepant. Moreover, the relatively long half-life of rimegepant opens up new avenues in the migraine field, forming a continuum between the acute and prophylactic treatment of migraine attacks (see ) [Citation56]. Therefore, rimegepant might be ideal in patients with intermediate migraine frequency. In conclusion, rimegepant could be helpful for the acute treatment of migraine symptoms in a select, but yet to be more specifically defined, group of migraine sufferers.
Table 2. Drug summary box for rimegepant
Declaration of interest
A. MaassenVanDenBrink has received research grants, served as a consultant and has received speaker’s fees from Allergan, AbbVie, Amgen, Novartis, Eli Lilly and Company and Teva Pharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
One referee is an investigator for and has received research support from Biohaven and AbbVie. Another referee has served as a consultant and speaker for Eli Lilly and Company, Novartis, Teva Pharmaceuticals, and Grunenthal. Peer reviewers in this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders. Jan. Cephalalgia. 2018;38(1):1–211. PubMed PMID: 29368949. 3rd edition.
- Steiner TJ, Stovner LJ, Vos T, et al. Migraine is first cause of disability in under 50s: will health politicians now take notice? J Headache Pain. 2018;19(1):17. Feb 21; PubMed PMID: 29468450; PubMed Central PMCID: PMCPMC5821623.
- GBD. Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2016;390(10100):1211–1259. 2017 Sep 16; PubMed PMID: 28919117; PubMed Central PMCID: PMCPMC5605509
- Collaborators GH, Nichols E, Steiner TJ. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018 Nov;17(11):954–976. PubMed PMID: 30353868.
- Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain. 2013;(Suppl 1): Dec;154 . 10.1016/j.pain.2013.07.021. PubMed PMID: 24347803; PubMed Central PMCID: PMCPMC3858400.
- Goadsby PJ, Edvinsson L, Ekman R, Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990 Aug;28(2):183–187. 10.1002/ana.410280213. PubMed PMID: 1699472.
- Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993; Jan;33(1):48–56. PubMed PMID: 8388188.
- Lassen LH, Haderslev PA, Jacobsen VB, et al. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54–61. Feb; PubMed PMID: 11993614.
- Hansen JM, Hauge AW, Olesen J, et al. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30(10):1179–1186. Oct; PubMed PMID: 20855363.
- Juhasz G, Zsombok T, Jakab B et al., Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia 2005 Mar;25(3):179–183. PubMed PMID: 15689192.
- Ibrahimi K, Danser A, Terwindt GM et al., A human trigeminovascular biomarker for antimigraine drugs: a randomised, double-blind, placebo-controlled, crossover trial with sumatriptan. Cephalalgia 2017 Jan;37(1):94–98. PubMed PMID: 26951335.
- Labastida-Ramírez A, Rubio Beltrán E, Garrelds IM, et al. Lasmiditan inhibits CGRP release in the mouse trigeminovascular system. Cephalalgia 2017;37:362–363.
- Labastida-Ramírez A, Rubio-Beltrán E, Haanes KA, et al. Lasmiditan inhibits dural CGRP release form the rat trigeminovascular system. MTIS; London 2018.
- Olesen J, Diener HC, Husstedt IW. et al., Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004; Mar 11;350(11):1104–1110. PubMed PMID: 15014183.
- Diener HC, Barbanti P, Dahlof C et al., BI 44370 TA, an oral CGRP antagonist for the treatment of acute migraine attacks: results from a phase II study. Cephalalgia Apr;31(5):573–584. PubMed PMID: 21172952. 2011
- Hewitt DJ, Aurora SK, Dodick DW et al., Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia Apr;31(6):712–722. PubMed PMID: 21383045. 2011
- Ho TW, Mannix LK, Fan X. et al., Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology 2008; Apr 15; 70(16):1304–1312. PubMed PMID: 17914062.
- Connor KM, Shapiro RE, Diener HC. et al., Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology 2009; Sep 22;73(12):970–977. PubMed PMID: 19770473.
- Ho TW, Connor KM, Zhang Y. et al., Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology 2014 Sep 9; 83(11):958–966. PubMed PMID: 25107879.
- Biohaven’s NURTEC™ ODT (rimegepant) receives FDA approval for the acute treatment of migraine in adults [Internet]. [cited 2020 Nov 27]. Available from: https://www.prnewswire.com/news-releases/biohavens-nurtec-odt-rimegepant-receives-fda-approval-for-the-acute-treatment-of-migraine-in-adults-301013021.html: PR Newswire; 2020
- Allergan receives U.S. FDA approval for UBRELVY™ for the acute treatment of migraine with or without aura in adults [Internet]. Available from: https://www.prnewswire.com/news-releases/allergan-receives-us-fda-approval-for-ubrelvy-for-the-acute-treatment-of-migraine-with-or-without-aura-in-adults-300979082.html: PR Newswire; 2019
- Biohaven Pharmaceutical Company Holding Ltd. Rimegepant (Nurtec™ ODT): US prescribing information: FDA; 2020 [cited 2020]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212728s000lbl.pdf
- Luo G, Chen L, Conway CM. et al., Discovery of (5S,6S,9R)-5-amino-6-(2,3-difluorophenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyri din-9-yl 4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxylate (BMS-927711): an oral calcitonin gene-related peptide (CGRP) antagonist in clinical trials for treating migraine. J Med Chem. 2012 Dec 13;55(23):10644–10651. PubMed PMID: 23153230.
- Croop R, Ivans A, Stock D, et al. A phase 1 study to evaluate the bioequivalence of oral tablet and orally diossolving tablet formulations of rimegepant, a small molecule CGRP receptor antagonist. 17th Biennial Migraine Trust International Symposium; London, UK 2018.
- Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019 Aug 31;394(10200):737–745. PubMed PMID: 31311674.
- Tong G, Savant I, Jariwala N, et al. Phase I single and multiple dose study to evaluate the safety, tolerability, and pharmacokinetics of BMS-927711 in healthy subjects. J Headache Pain. 2013;14(Suppl 1):P118.
- Drugbank. Rimegepant (Accession Number DB12457) 2020 [updated 02-11-2020; cited 2020 27-11-2020]. Available from: https://go.drugbank.com/drugs/DB12457
- The Medical Letter on Drugs and Therapeutics. Inhibitors and inducers of CYP enzymes and P-glycoprotein [updated10–09–2020;14–12–2020].
- Conway CM, Dubowchik GM, Croop R, et al. Phase 1 and 2 safety, tolerability and pharmacokinetics of single and multiple dose rimegepant as compared to the predicted clinically efficacious dose range. American Headache Society 61st Annual Scientific Meeting; Philadelphia, PA: Biohaven Pharmaceuticals; 2019.
- Marcus R, Goadsby PJ, Dodick D, et al. BMS-927711 for the acute treatment of migraine: a double-blind, randomized, placebo controlled, dose-ranging trial. Cephalalgia. 2014 Feb;34(2):114–125. PubMed PMID: 23965396.
- Lipton RB, Croop R, Stock EG, et al. Rimegepant, an Oral Calcitonin Gene–Related Peptide Receptor Antagonist, for Migraine. N Engl J Med. 2019 Jul 11;381(2):142–149. PubMed PMID: 31291516.
- Lipton RB, Conway CM, Stock EG, et al. Efficacy, safety, and tolerability of rimegepant 75 mg, an oral CGRP receptor antagonist, for the acute treatment of migraine: results from a phase 3, double-blind, randomized, placebo-controlled trial, study 301. American Headache Society 60th Annual Scientific Meeting; San Fransisco CA: Biohaven Pharmaceuticals; 2018.
- Gao B, Yang Y, Wang Z, et al. Efficacy and safety of rimegepant for the acute treatment of migraine: evidence from randomized controlled trials. Front Pharmacol. 2020;10:1577. PubMed PMID: 32038251.
- Croop R, Berman G, Kudrow D, et al. Long-term safety of rimegepant 75 mg for the acute treatment of migraine (study 201). American Academy of Neurology 2020 Anual Meeting; Virtual Poster 2020.
- Mullin K, Kudrow D, Croop R. et al., Potential for treatment benefit of small molecule CGRP receptor antagonist plus monoclonal antibody in migraine therapy. Neurology 2020 May 19;94(20):e2121–e2125. PubMed PMID: 31932515.
- Berman G, Croop R, Kudrow D. et al., Safety of rimegepant, an oral cgrp receptor antagonist, plus CGRP monoclonal antibodies for migraine. Headache 2020; Aug 16;60(8):1734–1742. PubMed PMID: 32799325.
- Croop R, Lipton RB, Kudrow D. et al., Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet 2021 Jan 2; 397(10268):51–60. PubMed PMID: 33338437.
- MaassenVanDenBrink A, Meijer J, Villalón CM, et al. Wiping out CGRP: potential cardiovascular risks. Trends Pharmacol Sci. 2016 Sep;37(9):779–788. PubMed PMID: 27338837.
- Øie LR, Kurth T, Gulati S et al., Migraine and risk of stroke. J Neurol Neurosurg Psychiatry 2020 Jun;91(6):593–604. PubMed PMID: 32217787.
- Favoni V, Giani L, Al-Hassany L et al., CGRP and migraine from a cardiovascular point of view: what do we expect from blocking CGRP? J Headache Pain 2019 Mar 12;20(1):27. PubMed PMID: 30866804.
- Mulder IA, Li M, De Vries T, et al. Anti-migraine calcitonin gene-related peptide receptor antagonists worsen cerebral ischemic outcome in mice. Ann Neurol. 2020 Oct;88(4):771–784. PubMed PMID: 32583883.
- Kudrow D, Pascual J, Winner PK. et al., Vascular safety of erenumab for migraine prevention. Neurology 2020 Feb 4;94(5):e497–e510. PubMed PMID: 31852816.
- Silberstein SD, McAllister P, Ning X et al., Safety and tolerability of fremanezumab for the prevention of migraine: a pooled analysis of phases 2b and 3 clinical trials. Headache Jun;59(6):880–890. PubMed PMID: 30977520. 2019
- Oakes TM, Kovacs R, Rosen N et al., Evaluation of cardiovascular outcomes in adult patients with episodic or chronic migraine treated with galcanezumab: data from three phase 3, randomized, double-blind, placebo-controlled EVOLVE-1, EVOLVE-2, and REGAIN Studies. Headache 2020 Jan;60(1):110–123. PubMed PMID: 31721185.
- Ashina M, Goadsby PJ, Reuter U, et al. Long-term efficacy and safety of erenumab in migraine prevention: results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur J Neurol. 2021. Jan 5. PubMed PMID: 33400330. 10.1111/ene.14715
- Pan KS, Siow A, Hay DL, et al. Antagonism of CGRP signaling by rimegepant at two receptors. Front Pharmacol. 2020;11:1240. PubMed PMID: 32973499.
- Tfelt-Hansen P, Loder LE. The emperor’s new gepants: are the effects of the new oral cgrp antagonists clinically meaningful? Headache. 2019 Jan; 59(1):113–117. PubMed PMID: 30451300.
- Kirthi V, Derry S, Moore RA Aspirin with or without an antiemetic for acute migraine headaches in adults Cochrane Database Syst Rev 2013 20134 CD008041 Apr 30; PubMed PMID: 23633350.
- Derry S, Rabbie R, Moore RA Diclofenac with or without an antiemetic for acute migraine headaches in adults Cochrane Database Syst Rev 2013 20134 CD008783 Apr 30 PubMed PMID: 23633360.
- Tfelt-Hansen P, Do TP Is oral telcagepant a relatively slowly acting drug? A mini-review of 4 RCTs. International Headache Conference: Cephalalgia; 2017. p. 81–82.
- Conway CM, Croop R, Dubowchik GM, et al. Cardiovascular safety of rimegepant 75 mg in 3 randomized clinical trials and systematic evaluations from in vitro, ex vivo, and in vivo nonclinical assays. American Headache Society 2019 Annual Scientific Meeting; Philadelphia, PA, 2019.
- Navratilova E, Behravesh S, Oyarzo J et al., Ubrogepant does not induce latent sensitization in a preclinical model of medication overuse headache. Cephalalgia Aug;40(9):892–902. PubMed PMID: 32615788. 2020
- Saengjaroentham C, Strother LC, Dripps I. et al., Differential medication overuse risk of novel anti-migraine therapeutics. Brain 2020; Sep 1;143(9):2681–2688. PubMed PMID: 32810212.
- Rau JC, Navratilova E, Oyarzo J et al., Evaluation of LY573144 (lasmiditan) in a preclinical model of medication overuse headache. Cephalalgia Aug;40(9):903–912. PubMed PMID: 32580575. 2020
- Holland PR, Saengjaroentham C, Sureda-Gibert P. et al., Medication overuse headache: divergent effects of new acute antimigraine drugs. Cephalalgia. 2020; Aug 40(9): 889–891 PubMed PMID: 32615789
- Al-Hassany L, Van Den Brink AM. Targeting CGRP in migraine: a matter of choice and dose. Lancet Neurol. Sep;19(9):712–713. PubMed PMID: 32822621. 2020
