ABSTRACT
Introduction: The 4-aminoquinolines, chloroquine, and hydroxychloroquine have been used for over 70 years for malaria and rheumatological conditions, respectively. Their broad-spectrum antiviral activity, excellent safety profile, tolerability, low cost, and ready availability made them prime repurposing therapeutic candidates at the beginning of the COVID-19 pandemic.
Areas covered: Here, the authors discuss the history of hydroxychloroquine and chloroquine, the in vitro data which led to their widespread repurposing and adoption in COVID-19 and their complex pharmacokinetics. The evidence for the use of these drugs is assessed through in vivo animal experiments and the wealth of conflicting data and interpretations published during COVID-19, including the more informative results from randomized controlled trials (RCTs). The safety aspects of these drugs, in particular cardiotoxicity, are then reviewed.
Expert opinion: The evidence from clinical trials in COVID-19 supports the well-established safety record of the 4-aminoquinolines at currently recommended dosage. In hospitalized patients with severe COVID-19 RCTs show clearly that the 4-aminoquinolines are not beneficial. The only treatments with proven benefit at this stage of infection are immunomodulators (dexamethasone, IL-6 receptor antagonists). No antiviral drugs have proven life-saving in late-stage COVID-19.
1. Introduction
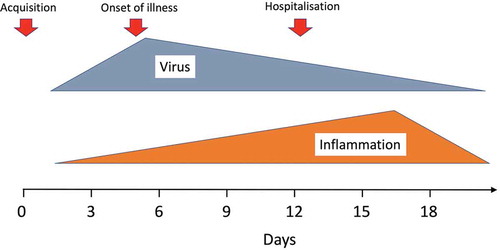
Chloroquine and hydroxychloroquine have been used for over 70 years in malaria and rheumatological conditions [Citation1]. Their broad-spectrum antiviral activity, excellent safety profile, tolerability, low cost, and ready availability made them prime repurposing therapeutic candidates in the COVID-19 pandemic. Unfortunately, excessive and contradictory (and in one case fabricated) claims for either efficacy or lack of efficacy, and for cardiotoxicity, compounded by intense politicization and mixed messages from regulatory authorities, have created substantial antipathy and compromised their rigorous evaluation in the prevention and early treatment of COVID-19.Footnote1 Another major source of confusion has been conflation of antiviral effects in prevention of COVID-19, or in early treatment (at the time of symptom onset and peak viral burdens), with later effects in more severely ill patients (in whom viral burdens are much lower and inflammatory processes predominate) [Citation2]. Large randomized controlled trials (RCTs) in hospitalized patients show clearly that high-dose hydroxychloroquine is not beneficial in late-stage illness [Citation3,Citation4]. In contrast, the signal from smaller RCTs in prevention and early disease is in the direction of clinically significant, albeit modest, benefit, but this is far from conclusive [Citation5–11]. Observational studies have provided a broad range of preventive and treatment efficacy estimates. There has been a plethora of case reports and uncontrolled observations rediscovering electrocardiograph QT prolongation but, with the exception of one study in which chloroquine was clearly overdosed [Citation12], and a potentially cardiotoxic interaction with azithromycin [Citation13], there is no convincing evidence for significant cardiotoxicity in the treatment of COVID-19 when these drugs are used alone. Completion of large RCTs, with sufficient power to identify smaller benefits, is needed to determine if 4-aminoquinolines do have significant efficacy in the prevention or in the early treatment of COVID-19, and thus if they still have any role in the COVID-19 pandemic. This is especially important while there exists no alternative. Much of the world’s population will remain unvaccinated over the next one or 2 years, and contingency measures are needed if vaccine escape mutants emerge and spread. Meanwhile, hydroxychloroquine continues to be recommended for COVID-19 prevention or treatment in several countries.
1.1. History
1.1.1. Chloroquine
Chloroquine [7-chloro-4-[[4-(diethylamino)-1-methylbutyl]amino] quinoline has been one of the most widely used medicines ever [Citation1]. It is a 4-aminoquinoline compound which was discovered in Germany in 1934 in a research program to develop new antimalarial drugs. Early clinical pharmacology assessments in the USA, as part of the war-time drug discovery effort, characterized the safety, tolerability, and efficacy of potential antimalarial drugs. Chloroquine was selected from a very long list of potential antimalarials as the most promising, although it was not evaluated fully until the end of the Second World War [Citation14,Citation15]. By the 1950s, its excellent efficacy, simple dose regimen, good tolerability, safety, and low cost resulted in chloroquine becoming the treatment of choice for all malaria throughout the world. Hundreds of metric tons (corresponding to nearly 100 million malaria treatment doses) were dispensed each year [Citation16]. Chloroquine was a key component of the global malaria eradication effort (1955–1967). Industrial production peaked in 2004 when, in the last quarter of the year, China alone reported production of over 400 tons.Footnote2 But by then, chloroquine resistance in P. falciparum was widespread across the tropics, and chloroquine use has declined as countries switched to the more effective artemisinin combination treatments (ACTs) for falciparum malaria. Overall, well over 5 billion treatments have been dispensed worldwide over the past 60 years. As it is very slowly eliminated, chloroquine can claim to be among the drugs to which humans have been most exposed. Chloroquine was used both in the prevention and in the treatment of malaria, and today it remains a first-line treatment for non-falciparum malaria, except in Indonesia and Papua New Guinea where there is high-level chloroquine resistance in P. vivax [Citation17]. Chloroquine was, and still is, used to prevent malaria in pregnancy although it is no longer effective in preventing falciparum malaria [Citation17–19]. In the 1950s, chloroquine was even added in large quantities to table salt in some regions to provide mass antimalarial prophylaxis. Chloroquine was widely used for antimalarial chemoprophylaxis across the tropics and, in many cases, was taken continuously for many years. Chloroquine was also found to be effective in the treatment of amoebic liver abscesses and to possess important anti-inflammatory properties which provided benefit in rheumatological conditions. The treatment of malaria required a short course treatment (25 mg base/kg total dose-up to 50 mg/kg) given over 2 or 3 days, whereas high total doses (10 mg base/kg daily for 2 days followed by 5 mg base/kg daily for 2–3 weeks) were used for the treatment of hepatic amoebiasis. Daily dosing (3–5 mg base/kg/day) was required in rheumatological conditions. Confusingly, because there are several different salts of chloroquine, dose regimens are prescribed in weights of base equivalent.
1.1.2. Hydroxychloroquine
Hydroxychloroquine, in which one ethyl group in the alkyl side chain is hydroxylated, was synthesized in 1946. Hydroxychloroquine was shown to have equivalent antimalarial activity and to be slightly less toxic in experimental animals [Citation20]. It was developed more for its use in rheumatological conditions [Citation21]. Initially, both chloroquine and hydroxychloroquine were used to treat rheumatoid arthritis, systemic lupus erythematosus and other rheumatological diseases, but in recent years, hydroxychloroquine has predominated. Hydroxychloroquine is generally considered to be slightly safer than chloroquine [Citation20] although the evidence for this is not strong. There is extensive experience with long-term use mainly in the 3–6 mg base/kg day range (corresponding to adult doses of 155 to 310 mg given as 200 or 400 mg of sulfate salt). Daily doses up to 620 mg base (800 mg salt) have been used.
2. Antiviral activity
2.1. Pharmacodynamics: mechanism of antiviral action
Both chloroquine and hydroxychloroquine have moderate to weak broad-spectrum antiviral activities [Citation22]. There are several possible mechanisms of antiviral action against the single-stranded RNA-enveloped virus SARS-CoV-2. These 4-aminoquinolines interfere with the terminal glycosylation of the angiotensin-converting enzyme 2 (ACE2) virus receptor on host cells [Citation23] and also the viral spike S protein which may alter the affinity of SARS-CoV-2 for its receptor, potentially inhibiting key steps in cell entry. These are basic drugs which accumulate within cytoplasmic acidic organelles, including lysosomes and endosomes, increasing their pH and inhibiting the activity of pH-dependent lysosomal/endosomal proteases [Citation23,Citation24]. This reduces the fusion process between the viral envelope and lysosomal or endosomal membrane. Thus, the 4-aminoquinolines may prevent viral entry by reducing receptor binding and membrane fusion. Another mechanism of cellular accumulation is by nonspecific binding to membrane phospholipids, although the role of this is uncertain. An in vitro experiment suggested that low-dose chloroquine only had a small effect in raising intravacuolar pH but acted on distal Golgi and pre-lysosomal compartments to prevent normal sorting of lysosomal enzymes, and thus induce mis-sorting of these enzymes in a pH-independent manner [Citation25]. In silico modeling suggests that the 4-aminoquinolines may inhibit the viral RNA dependent RNA polymerase [Citation26]. Thus, although the exact mechanism of antiviral activity of the 4-aminoquinolines is currently unclear, there is no reason to believe that antiviral activity against vaccine escape mutants would be compromised. In COVID-19 treatment, their multiple immunomodulatory actions may also be therapeutically relevant.
2.2. In-vitro antiviral activity
In vitro studies have been conducted on different cell lines. Most studies have used Vero cells although antiviral activity has been demonstrated in others, including respiratory cell lines. These studies have reported half maximal effective concentration (EC50) values for SARS-CoV-2 ranging from 0.72 to 17.31 μM [Citation27,Citation28]. There has been substantial variation between studies. EC50 values decrease with longer incubation times, suggesting that a longer incubation allows for greater drug accumulation and an augmented antiviral effect [Citation29]. These concentrations are some 10–1000 times higher than the levels required to inhibit the growth of malaria parasites [Citation30]. The antiviral activity of the 4-aminoquinoline drugs is greater when given earlier, at virus entry rather than following entry into the cells [Citation29]. Experiments done on the closely related SARS-CoV with chloroquine demonstrated a marked decline in activity if given later (90% inhibition at 1 hr and 15% inhibition if given 7 h after infection) [Citation31]. These experiments suggest activity early in viral replication and point to their most effective use early in the viral infection.
2.3. Transmembrane protease serine 2 and viral entry
Hoffmann et al. demonstrated decreased activity of chloroquine in a Vero cell line overexpressing a cellular protease, Transmembrane Protease, Serine 2 (TMPRSS2), and the human cancer cell line CALU-3 and concluded from this that ‘ … chloroquine and hydroxychloroquine will exert no antiviral activity in human lung tissue and will not be effective against COVID-19, in keeping with the results of recent clinical trials’ [Citation32]. This sweeping conclusion is unjustified for the following reasons. First, chloroquine clearly does exhibit antiviral activity against SARS-CoV-2 in human lung cell lines [Citation33], as it did in their experimental model, albeit at higher levels. Second, this conclusion presupposes that the mechanism of antiviral action in vitro or in vivo is understood and that chloroquine will not work in the presence of TMPRSS2. However, chloroquine does inhibit another human coronavirus which uses TMPRSS2 (HCoV-229E) in a human epithelial lung cell line (L-132) [Citation34] with activities in the low micromolar range. Third, it also presupposes that TMPRSS2 is the critical mechanism of viral entry in humans. This is unproven. The two in vivo mouse models cited as evidence for the role of TMPRSS2 suggest that this is not the only mechanism of viral entry, as the TMPRSS2-independent inhibitor has some antiviral effect [Citation35], TMPRSS2 knockout mice do get disease [Citation36] and, in another in vivo experiment in mice with SARS-CoV, chloroquine did show clinical benefit against another coronavirus which uses TMPRSS2 [Citation22,Citation37]. Furthermore, type I pneumocytes, the majority of type II pneumocytes and some blood vessel endothelial cells in human lungs all lack TMPRSS2 [Citation38–40]. TMPRSS2 cannot therefore be ‘the whole story.’ Even if it is accepted that TMPRSS2 does play an important role in human lung tissue, there is still no reason to consider definitively that one particular lung cancer cell line is a better model than others which also express TMPRSS2 (such as the A549 human respiratory cell line above [Citation41]) or indeed other cell lines which overexpress TMPRSS2, such as Caco-2, where an in vitro antiviral effect of chloroquine has been demonstrated [Citation42].
2.4. Animal studies
The 4-aminoquinolines have demonstrated in vivo antiviral activity against a range of viruses, including the human coronavirus, HCoV-OC43, in newborn mice pups, and HIV in humans [Citation43], but other studies have been negative, and no animal studies have reported in vivo activity against SARS-CoV-2 [Citation43]. Mice, Syrian hamsters, ferrets, and non-human primates have been used in therapeutic and vaccine animal assessments [Citation44–46]. A study using a Syrian hamsters and rhesus macaques infected with SARS-CoV-2 did not report any benefit from chloroquine administered either prophylactically or therapeutically in terms of clinical, virologic or pathology measures. However, the plasma concentrations achieved in the macaques were low; 1.2 to 10 ng/ml in prophylaxis and 8 to 98 ng/ml in treatment [Citation47]. Tissue levels were comparable. These levels are ~100 times lower than the EC50 of these drugs against SARS-CoV-2 in cell culture and were generally much lower than those readily achieved in humans and considered as therapeutic in rheumatological conditions (500–2000 ng/ml in whole blood-plasma concentrations are 3–10 times lower than that in whole blood). However, a later study using cynomolgus macaques, in which exposures were much higher (plasma concentrations ~200 ng/ml) again failed to demonstrate benefit. In five cynomolgus macaques infected by the intranasal and intratracheal route, pre-exposure prophylaxis with hydroxychloroquine also did not confer protection against infection with SARS-CoV-2. Laboratory studies do provide useful information, but direct predictions of in vivo activity extrapolated from free-drug concentrations and in vitro activity in cell culture are probably not justified.
2.5. Pharmacokinetic properties and biotransformation
Chloroquine and hydroxychloroquine have unusual pharmacokinetic properties. They have very large apparent volumes of distribution and very slow terminal elimination (terminal half-life >1 month) [Citation48,Citation49]. Absorption is relatively rapid and reliable, but elimination is multiphasic with distribution rather than elimination determining blood concentration profiles [Citation50,Citation51]. The desethylated metabolites are also biologically active. There are no clinically significant pharmacokinetic interactions. These drugs concentrate markedly in platelets and leukocytes so whole blood rather than plasma or serum is the preferred matrix for pharmacokinetic studies [Citation1]. Chloroquine concentrates in cells [Citation48] but tissue concentrations at the site of viral infection are not well-characterized. The very large apparent volume of distribution necessitates giving a loading dose, but this must be titrated against distribution, so it is often split to avoid potential cardiovascular toxicity associated with transiently high concentrations in the distribution phase [Citation52,Citation53].
2.6. Observational studies
During the first months of the COVID-19 pandemic, when 4-aminoquinolines were promoted vigorously, a large number of observational studies were reported. Overall, the observational data are confusing, and provide no clear signal. Some studies suggested benefit (in some the reported protective benefit was remarkable – see below) whereas other studies suggested no benefit. This familiar problem in clinical research raises concerns over biases and whether confounders have been dealt with adequately. Reporting of toxicity (notably electrocardiograph effects) has been very variable with many observational studies repeating descriptions of the predictable concentration-dependent ECG QT prolongation and describing this as ‘cardiotoxicity’ (and also failing to distinguish QRS widening from JT prolongation – see section 3.5).
2.7. Prevention
The largest data sets describing pre-exposure prophylactic efficacy (PrEP) against COVID-19 infection come from observational studies of those already taking hydroxychloroquine for rheumatological conditions. A database analysis in Portugal of 26,815 SARS-CoV-2 positive patients and 333,489 negative patients showed a nearly 50% (OR 0.51 (0.37–0.70)) reduction in COVID-19 cases in those taking HCQ, after adjusting for age, sex, and chronic treatments with corticosteroids and other immunosuppressants [Citation54]. An even larger beneficial effect size was seen (OR 0.09 [95% CI 0.01–0.94]) in a Chinese study, where 616 patients with rheumatological disease taking hydroxychloroquine were compared to those taking other disease-modifying antirheumatic drugs although the numbers actually exposed to COVID-19 were low [Citation55]. Another case series of 1641 patients with rheumatological autoimmune diseases from Italy suggested a higher prevalence of COVID-19 in those with autoimmune systemic diseases and in those not taking conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), of which hydroxychloroquine is a major representative [Citation56]. A retrospective cohort study of veterans in the US Veterans Health Administration clinical database showed a non-statistically significant reduction in SARS-CoV-2 infections (OR 0.79, 95% CI 0.52–1.20), but the mortality was significantly lower in those taking hydroxychloroquine (OR 0.70, 95% CI 0.55–0.89) [Citation57]. In contrast to these larger ‘positive’ reports, and other smaller reports of benefit, there have been several other observational studies which have not shown benefit in patients receiving hydroxychloroquine for rheumatological conditions, including another database analysis of 159 patients taking hydroxychloroquine compared with historical controls [Citation58]. A publication reporting a large retrospective observational study using the OpenSAFELY platform, which comprises 40% of health records in the UK, and 30,569 patients with rheumatological conditions who had received ≥2 prescriptions of hydroxychloroquine in the previous 6 months found no difference in the COVID-19 mortality of hydroxychloroquine users [Citation59]. These studies suffer variably from uncertain drug exposure, confounding by indication and underlying disease severity, as patients with milder rheumatological illness are normally started on drugs such as hydroxychloroquine and graduate to other medications with more severe illness. In addition, those with the rheumatological conditions requiring hydroxychloroquine treatment may be more susceptible to COVID-19 [Citation56]. Finally, although the databases were often very large, the numbers of patients receiving hydroxychloroquine that met the endpoints were often small.
An alternative assessment of the efficacy of HCQ in pre-exposure prophylaxis comes from observational data from those taking the drugs specifically to prevent COVID-19. In one study, 106 health-care workers who were high-risk contacts of COVID-19 positive cases and had been tested for SARS-CoV-2 by RT-PCR were included. There was an 80.7% reduction in SARS-CoV-2 in the hydroxychloroquine group. Our interpretation of all the available evidence and the widely divergent estimates of benefit is that large benefits are unlikely, but it is not possible to state with certainty whether or not chloroquine or hydroxychloroquine provide modest but significant prophylactic efficacy against COVID-19. There remains substantial uncertainty. Definitive statements will require evidence from large well-conducted randomized control trials if these can be completed.
2.8. Treatment studies
Over 100 studies describing the use of 4-aminoquinolines in the treatment of COVID-19 have now been published. The vast majority were observational. Early reports of benefit in observational studies largely came from China, although these were small and often in the form of a press-release rather than a formal publication.
An open-label non-randomized trial published at the end of March 2020 as a pre-print (and still not peer-reviewed), in which 26 patients hospitalized with COVID-19 received hydroxychloroquine, and 6 azithromycin, was very influential. At day 6, fewer in the treated compared to the control arm were PCR positive. This led to widespread advocacy and the adoption of hydroxychloroquine into treatment guidelines across the globe, in many places accompanied by azithromycin. Many concerns were voiced, but the study had a substantial impact. For only the second time in its history, the US FDA used its emergency authority to permit use of a medication (hydroxychloroquine) for an unapproved indication (COVID-19). ‘Off-label’ and self-medication use skyrocketed, inadvertent fatal self-poisoning was reported, while people who really needed these drugs for rheumatological conditions suddenly found them hard to find. In the US alone, by May 22, the US Strategic National Stockpile had dispensed approximately 2.4 million 7-day hydroxychloroquine treatment courses to state and local health authorities. Numerous observational studies were published. The momentum stopped abruptly on 22 May, 2020 when an observational study was published in The Lancet. It purported to describe data from nearly 100,000 patients who had received hydroxychloroquine or chloroquine for COVID-19 treatment. The study claimed these drugs increased the risk of ventricular arrhythmias and death. This hit news headlines and was very widely publicized. Opinion quickly swung strongly against the drugs. Within hours, several regulatory authorities withdrew authorizations for use of these drugs or for clinical investigation. However, it very soon transpired that the data were suspect and could not be verified, and the paper was ultimately retracted. But the damage was lasting, the regulatory bans were slow to lift, and clinical trials across the world found recruitment difficult thereafter.
A retrospective study of hydroxychloroquine ± azithromycin use in treatment of hospitalized veterans in the US did not identify a significant reduction in mortality or the need for mechanical ventilation with hydroxychloroquine ± azithromycin and there was a signal for increased mortality [Citation60] although other studies finding no benefit did not find this worrying mortality signal [Citation61]. Others showed quite dramatic benefits in those receiving hydroxychloroquine [Citation62], but incomplete correction for biases plagues the observational study results and their conclusions.
3. Randomized controlled trials
3.1. Prevention
In contrast to the wealth of observational data, there have been few RCTs reported. Two pre-exposure prophylaxis trials and three post-exposure prophylaxis trials have now been published in peer-reviewed journals [Citation5–8,Citation11]. Post-exposure prophylaxis (PEP), which may be regarded as a hybrid of prevention and early treatment, would be expected a priori to provide less benefit than pre-exposure prophylaxis. These published studies were relatively small and were powered therefore only to demonstrate large benefits (a minimum of 50% reduction in cases). None were able to reject the null hypothesis. However, the majority did demonstrate non-significant reductions in cases of the order of 15% [Citation63]. So, although the available data from the prevention studies are currently indicative of small benefit, the results are far from conclusive. Dose is also a consideration. Rajasingham et al. in their pre-exposure prophylaxis study used low doses (once weekly and twice weekly dosing, which are closer to those used in malaria chemoprophylaxis) which meant that the levels of hydroxychloroquine were significantly lower than those achieved in the treatment of rheumatological conditions with once daily dosing. This would have reduced the likelihood of a significant antiviral effect [Citation11] although there was a non-statistically significant trend to increased benefit at the higher dose.
3.2. Treatment
In contrast to the prevention trials, the two large COVID-19 platform treatment RCTs in hospitalized patients have produced clear and actionable results. The RECOVERY Trial recruited large numbers of participants from many NHS hospitals in the UK in the first wave of the pandemic. This was an open-label RCT in which hospitalized patients with COVID-19 were randomized to one of the several treatments, including hydroxychloroquine, or to standard of care. The World Health Organization’s SOLIDARITY trial was similar in design and was conducted in many sites globally (405 hospitals in 30 countries). It was slower in producing results and assessed some different interventions. Both trials evaluated the same high-dose hydroxychloroquine regimen designed to provide drug exposures which were as high as safely possible [Citation1]. In the RECOVERY trial, 1561 patients were randomized to receive hydroxychloroquine versus 3155 in the standard of care arm [Citation3]. The mortality was 27% in the HCQ arm vs 25% in the standard of care (RR = 1.09 [95% CI 0.97–1.23]). A similar lack of efficacy was demonstrated in the SOLIDARITY trial; mortalities were 11% (104/947) in patients randomized to hydroxychloroquine and 9.3% (84/906) in patients receiving standard of care (RR = 1.19 [0.89–1.59]) [Citation4].
These definitive negative results should stop the use of hydroxychloroquine in COVID-19 illness requiring hospitalisation. Importantly, despite the large doses used, and the very high incidence of myocardial involvement in severe COVID-19 illness [Citation64], there was no excess of cardiac arrhythmias in hydroxychloroquine recipients. This provides powerful counterevidence to the numerous published opinion pieces, uncontrolled studies, and case-reports that claimed harm from QT prolongation. There is substantial clinical and epidemiological evidence for the safety of these drugs at currently recommended doses, supported by cardiovascular safety margins derived from self-poisoning toxicokinetics [Citation65]. Many observers also confounded the risks associated with the use of the drugs alone (which appears to be safe) versus in combination with azithromycin, where there is evidence for a cardiotoxic (arrhythmogenic) interaction [Citation13].
The results of the RECOVERY and SOLIDARITY trials provide an important insight into the pathogenesis of COVID-19. Anti-inflammatory drugs, dexamethasone and IL-6 receptor antagonists, have proved life-saving in late-stage illness, whereas antivirals (including remdesivir which had been shown to shorten the duration of hospitalization) were not [Citation4,Citation66,Citation67]. This suggests that clinical deterioration in late-stage illness results primarily from inflammation, and not active viral replication. Indeed, corticosteroids often worsen viral infections in the phase of active replication. In the RECOVERY trial, there was a non-significant trend toward a worse outcome in those not receiving respiratory support at baseline [Citation66]. Viral burdens are highest much earlier, at the time of symptom onset [Citation68], and then decrease, whereas deterioration resulting in hospitalization often occurs after a week, with hospitalization for several weeks [Citation2]. This supports the general paradigm of viral replication early, and inflammatory sequelae and deterioration late. This paradigm suggests that any benefits from antiviral drugs in late-stage illness are likely to be modest, at best. By contrast, antivirals would have a much better chance of preventing or reducing disease severity if given earlier, when viral burdens and replication are greater. The results of the RECOVERY and SOLIDARITY trials have been misunderstood by many and taken as conclusive evidence that the tested antiviral drugs will not work at any stage of illness, such as the WHO’s living guideline on drugs for COVID-19 [Citation69], whereas current knowledge suggests different pathophysiological processes at different phases of the COVID-19 infection [Citation70]. Although there is little definitive RCT evidence describing the use of HCQ in early disease treatment, the two published RCTs by Skipper et al. [Citation9] and Mitjà et al. [Citation10]. demonstrated a non-significant trend toward benefit when given early, with a relative risk of hospitalization of 0.75 [0.32; 1.77] [Citation10]. This is in keeping with the trend toward benefit seen when these drugs are given even earlier in prevention (PrEP and PEP). Thus, the question of whether chloroquine or hydroxychloroquine provide benefit in the treatment of early COVID-19 still remains open.Footnote3
Figure 1. Meta-analysis of published hydroxychloroquine prevention studies showing a non-significant overall approximate 15% reduction in COVID-19 [Citation5–8,Citation11]
![Figure 1. Meta-analysis of published hydroxychloroquine prevention studies showing a non-significant overall approximate 15% reduction in COVID-19 [Citation5–8,Citation11]](/cms/asset/57f3cc17-fa19-428c-ab09-7abaa5357500/ieop_a_1898589_f0001_b.gif)
3.3. Safety and tolerability
Chloroquine and hydroxychloroquine are dangerous in overdose [Citation65] but, at the correct dosages, they are generally well tolerated and safe. The evidence base for this safety assessment has been established over decades of real-world use and after billions of doses have been given. Gastrointestinal disturbance and nausea are the most commonly reported side effects, but these are usually mild. This was reconfirmed in the RCT results evaluating their use for COVID-19 [Citation71,Citation72]. Taken in high doses for years, cumulative toxicity to the retina and to the heart is well documented, but this is not relevant to short-term use [Citation1]. Joint analysis of the outpatient RCTs has not confirmed the safety concerns raised early in the pandemic by numerous commentators; instead, they have reinforced the generally good safety profile of the drugs given over the shorter term. Nevertheless, the retracted Lancet publication, which reported harm [Citation73], together with the exaggeration of toxicity concerns, overgeneralization of lack of efficacy, politicization, excessive regulatory responses, and intense media interest, all continues to negatively influence public perceptions of these drugs.
3.4. Cardiovascular toxicity
Cardiovascular toxicity is the principal immediate concern with high doses of 4-aminoquinolines [Citation1]. Parenteral chloroquine causes hypotension if administered too rapidly or when a large dose (5 mg base/kg or more) is given by intramuscular or subcutaneous injection [Citation53]. Chloroquine and hydroxychloroquine (and the structurally related 4-aminoquinoline amodiaquine and also the bisquinoline piperaquine) block several different cation channels [Citation74–76]. In a standard laboratory model (voltage-clamped cat ventricular myocytes), chloroquine blocked both inward and outward membrane currents. The relative potencies were: inward rectifying potassium current (IK1) > rapid delayed rectifying potassium current (IKr) > sodium current (INa) > L-type calcium current (ICa-L). This prolongs the cardiac action potential duration, enhances automaticity, and reduces the maximum diastolic potential. Chloroquine blocks the rapid component (IKr) but not the slow component (IKs) of the delayed rectifying potassium current. These different electrophysiological effects explain the electrocardiograph changes, notably, prolongation of the QRS (ventricular depolarization) and JT intervals (repolaristion). The blockade of INa and ICa-L reduces the early afterpotentials that trigger ventricular arrhythmias. Chloroquine and hydroxychloroquine also block the hyperpolarization-activated funny current (If) which plays an important role in the sinoatrial node pacemaker and so may cause bradycardia [Citation77].
3.5. QT prolongation concerns
Blockade of the IKr (hERG) channel, which delays ventricular repolarization (measured as prolongation of the ECG QT or, more specifically, the JT interval), has been the primary focus of concern in 2020 [Citation53]. Prolongation of the QT-interval is a risk factor for polymorphic ventricular tachycardia (TdP: torsade de pointes) although there is extensive debate about the risk relationship and the potential ameliorating effects of multichannel blockade. While chloroquine and hydroxychloroquine consistently prolong the electrocardiograph J to T-peak interval and are potentially ‘torsadogenic,’ how much they increase the risk of TdP is unclear. Assessments of QT prolongation commonly omit measurement of QRS prolongation (QT = QRS + JT), which is also a consistent effect of 4-aminoquinolines, and reflects slowing of intraventricular conduction. As a result, the degree of JT prolongation (relevant to the TdP risk) is overestimated [Citation53]. Nevertheless, the rediscovery of the effects of the 4-aminoquinolines on ventricular repolarization has generated a large number of publications citing the potential (but not observed) risk of ventricular arrhythmia. The important point is that this inferred risk has not been confirmed in extensive clinical trials both before COVID-19 and again in the high-quality randomized controlled trials conducted during COVID-19 (see later). With the exception of one trial in which chloroquine was clearly overdosed [Citation12], the randomized trials (which are the best source of evidence) do not show an excess of arrhythmias [Citation3,Citation72]. Severe COVID-19 itself is an important cause of myocardial dysfunction and arrhythmia. Many of the recent articles and warnings around the well-known QT prolongation associated with these drugs simply extrapolate from QT prolongation to risk without considering the paucity of reports in relation to the enormous usage of these drugs (over 5 billion malaria treatments given and approximately 1 million receiving chronic treatment). Overall, despite the extensive use of these drugs, there are few case reports of presumed iatrogenic TdP [Citation1].
The WHO pharmacovigilance database (VigiBase) contains reports of 83 episodes of TdP or other forms of ventricular tachycardia, which occurred in patients taking hydroxychloroquine over a 52-year period, of which seven were fatal. This experience does not distinguish acute from chronic use, and most pertains to conditions with an increased risk of cardiac disease. This should be viewed in the context of approximately one million people (mainly older adults) using hydroxychloroquine continuously worldwide (based on manufacturing outputs). In a recent retrospective observational review of 956,374 rheumatoid arthritis patients starting treatment with hydroxychloroquine, the risk of arrhythmia in the first 30 days of treatment (calibrated hazard ratio (CalHR) 0.89 (95% confidence interval 0.77 to 1.04)) was lower compared with sulphasalazine recipients (n = 310; 350) [Citation13]. As sulphasalazine has no known cardiac effects, this suggests that hydroxychloroquine was acting as an antiarrhythmic.
TdP may occur in chloroquine or hydroxychloroquine overdose, but other arrhythmias usually predominate. There is no evidence for a significant risk of TdP in acute treatment with the doses that have been used in malaria or rheumatological conditions. Sudden unexplained death has not been associated with antimalarial use of oral chloroquine previously despite administration of literally billions of malaria prophylaxis and treatment courses and wide variation in dosing. Recent prospective studies providing data from 200,000 patients treated with the related bisquinoline antimalarial compound piperaquine (which has similar hERG blocking properties to chloroquine) found no increased risk of TdP after standard treatment [Citation78]. Thus, the concerns that chloroquine or hydroxychloroquine alone given in currently recommended doses over the short term are likely to provoke TdP, which have seriously hindered studies in COVID-19, are largely unfounded. In contrast, chloroquine clearly does have anti-arrhythmic properties which are under-recognized [Citation13,Citation79]. In laboratory studies, chloroquine and hydroxychloroquine have also been shown to reduce myocardial ischemia reperfusion injury [Citation80]. Confusion and concern over cardiotoxicity have arisen by extrapolating from the undoubted cumulative long-term risks of myocardial damage to short-term exposures, assuming that QT prolongation per se equates to a high risk of ventricular arrhythmias and, in COVID-19 treatments, underestimating the very significant contribution of azithromycin to combined cardiovascular toxicity [Citation13].
3.6. Disease–toxicity interactions
Malaria and malarial fever have independent effects on the QT interval and heart rate although the heart is relatively spared even in severe malaria. There is increasing evidence for myocarditis and arrhythmias in COVID-19 [Citation64,Citation81]. It is unclear whether the mechanism for the cardiotoxic hydroxychloroquine–azithromycin interaction is explained only by iatrogenic TdP or whether there is a febrile illness interaction.
3.7. Information from ongoing trials
In the RECOVERY and SOLIDARITY platform trials in hospitalized patients, there was a non-significant excess of deaths in high-dose hydroxychloroquine recipients although there was no excess of ventricular arrhythmias [Citation3,Citation4]. This reemphasizes that high-dose hydroxychloroquine or chloroquine should not be used to treat severe COVID-19 infection. The data from outpatient RCTs, using lower doses, are very reassuring for the safety of these drugs [Citation71,Citation72]. In contrast to observational data, RCTs do control adequately for confounders of the disease and its treatment. Of the 2795 participants recruited into three RCTs evaluating hydroxychloroquine in the outpatient setting, 1633 received HCQ and the pooled study concluded that ‘Randomized clinical trials, in cohorts of healthy outpatients, can safely investigate whether hydroxychloroquine is efficacious for COVID-19’[Citation72]. Together these data suggest, unsurprisingly, that the safety and tolerability profile of hydroxychloroquine in COVID-19 is similar to that in rheumatological conditions.
4. Expert opinion
The repurposed 4-aminoquinolines, chloroquine, and hydroxychloroquine have been used extensively in the COVID-19 pandemic. We now know that high-dose hydroxychloroquine is not life-saving in late disease, nor indeed is any antiviral to date, whereas dexamethasone significantly reduces mortality. Antivirals are likely to have their best chance of providing benefit given either as prevention or early in the course of COVID-19 illness when viral replication is greatest and before the late-stage inflammatory sequelae predominate. The results of the two, large COVID-19 RCTs in hospitalized patients (RECOVERY and SOLIDARITY) have been misunderstood by many and taken as conclusive evidence that the tested antiviral drugs will not work at any stage of illness, whereas current knowledge suggests different pathophysiological processes at different phases of the infection [Citation2].
Despite their in vitro antiviral activity, it is unclear whether the 4-aminoquinolines have any useful preventive or curative efficacy in early COVID-19 (early treatment or prevention). This has not yet been assessed adequately in large RCTs. The reported RCTs in prevention and early treatment were powered only to show large benefits and had relatively few end-points. When combined they do not provide a conclusive assessment of efficacy although they point in the direction of a modest clinical benefit. Statements that these drugs ‘do not work’ in prevention or early treatment are not justified by the available data, but they have defined the narrative around the 4-aminoquinolines in the COVID-19 pandemic. The observational data have produced conflicting results in all stages of disease, and the available RCT evidence leaves substantial uncertainty. Meanwhile, many countries continue to recommend these drugs actively. Well-designed, large randomized controlled trials evaluating chloroquine and hydroxychloroquine in the prevention and early treatment of COVID-19 need to be completed to answer these important questions adequately [Citation82].
The excellent safety profile of the 4-aminoquinolines at currently used doses over the short term has been established through extensive clinical use over 70 years. Despite adverse commentaries, and an influential and alarmist study which appears to have been fabricated, this good safety profile has generally been reinforced by the results of the COVID-19 hydroxychloroquine randomized controlled trials. Unfortunately, politicization, overgeneralization, polarized opinion, and negative media coverage have all made it very difficult to continue the 4-aminoquinoline randomized trials needed to assess the benefits and risks objectively, and to inform policies and practices. Licensed vaccines with good efficacy have arrived and are being administered, but it may be years before there is adequate coverage to create herd immunity, and the appearance of vaccine escape mutants may threaten this progress. Therapeutics are still needed, but one of the most discussed topics in the entire pandemic ‘can hydroxychloroquine prevent COVID-19 illness?’ may remain unanswered.
Article highlights
The 70-year-old antimalarial and antirheumatic drugs, chloroquine and hydroxychloroquine, have broad-spectrum antiviral activity, including against SARS-CoV-2 in vitro.
These drugs were rapidly repurposed and widely used early in the COVID-19 pandemic before there was convincing evidence of benefit.
Hydroxychloroquine has clearly been shown to be ineffective in the treatment of severe hospitalized COVID-19 infections, but chloroquine and hydroxychloroquine have not been assessed adequately in prevention or early treatment.
Evidence from randomized controlled trials confirms the good safety profile of these drugs at currently recommended doses.
Larger, high-quality RCTs are needed to determine if the 4-aminoquinolines have any role in prevention or early treatment in COVID-19.
This box summarizes key points contained in the article.
Declaration of interest
Both authors are co-principal investigators of the hydroxychloroquine/chloroquine prevention of coronavirus disease study (COPCOV). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
Notes
2. CEIC. China CN production; 2020. https://www.ceicdata.com/en/china/pharmaceutical-production-ytdantiparasitics-vitamins-and-minerals/cn-production-ytd-hydroxychloroquine.
References
- White NJ, Watson JA, Hoglund RM, et al. COVID-19 prevention and treatment: a critical analysis of chloroquine and hydroxychloroquine clinical pharmacology. PLoS Med. 2020 Sep; 17(9):e1003252.
- White N, Strub-Wourgraft N, Faiz A, et al. COVID-19 therapeutic reviews and guidelines should not pool evidence from uncomplicated illness in outpatients and severely ill hospitalized patients. Lancet. 2021; 396(10267):1976–1977. In Press.
- Recovery, Horby P, Mafham M, et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020 Nov 19;383(21).
- Solidarity, Pan H, Peto R, et al. Repurposed antiviral drugs for Covid-19 – interim who solidarity trial results. N Engl J Med. 2021 Feb 11; 384(6):497–511.
- Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020 Jun; 383(6):3.
- Mitjà O, Corbacho-Monné M, Ubals M, et al. A cluster-randomized trial of hydroxychloroquine for prevention of Covid-19. N Engl J Med. 2020 Feb 4;384:417-427
- Abella BS, Jolkovsky EL, Biney BT, et al. Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers: a randomized clinical trial. JAMA Intern Med. 2020 Sep 30;181(2):195–202.
- Barnabas RV, Brown ER, Bershteyn A, et al. Hydroxychloroquine as postexposure prophylaxis to prevent severe acute respiratory syndrome Coronavirus 2 infection: a randomized trial. Ann Intern Med. 2020 Dec 8; DOI:10.7326/M20-6519
- Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020 Jul 16; 173(8):623–631.
- Mitja O, Corbacho-Monne M, Ubals M, et al. Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomized-controlled trial. Clin Infect Dis. 2020 Jul 16;ciaa1009.
- Rajasingham R, Bangdiwala AS, Nicol MR, et al. Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial. Clin Infect Dis. 2020 Oct 17;ciaa1571.
- Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Network Open. 2020 Apr 1; 3(4):e208857.
- Lane JCE, Weaver J, Kostka K, et al. Risk of hydroxychloroquine alone and in combination with azithromycin in the treatment of rheumatoid arthritis: a multinational, retrospective study. Lancet Rheumatol. 2020 Nov; 2(11):e698-e711.
- Coatney GR. Pitfalls in a discovery: the chronicle of chloroquine . Am J Trop Med Hyg. 1963 Mar;12(2):121–128.
- Berliner RW, Earle DP Jr., Taggart JV, et al. Studies on the chemotherapy of the human malarias; of the human malarias the physiological disposition, antimalarial activity, and toxicity of several derivatives of 4-aminoquinoline. J Clin Invest. 1948 May; 27(3 Pt1):98–107.
- Advances in malaria chemotherapy. Report of a WHO Scientific group. World Health Organ Tech Rep Ser. 1984;711:1–218.
- WHO. Guidelines for the treatment of malaria. Geneva 2015.
- Villegas L, McGready R, Htway M, et al. Chloroquine prophylaxis against vivax malaria in pregnancy: a randomized, double-blind, placebo-controlled trial. Trop Med Int Health. 2007 Feb; 12(2):209–218.
- McGready R, Lee SJ, Wiladphaingern J, et al. Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy: a population-based study. Lancet Infect Dis. 2012 May; 12(5):388–396.
- McChesney EW. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med. 1983 Jul 18; 75(1A):11–18.
- Rynes RI. Hydroxychloroquine treatment of rheumatoid arthritis. Am J Med. 1988 Oct 14; 85(4A):18–22.
- Keyaerts E, Li S, Vijgen L, et al. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob Agents Chemother. 2009 Aug; 53(8):3416–3421.
- Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005 Aug 22; 2(1):69.
- Daniel WA, Bickel MH, Honegger UE. The contribution of lysosomal trapping in the uptake of desipramine and chloroquine by different tissues. Toxicol Pharmacol. 1995 Dec;77(6):402–406.
- Cardelli JA, Richardson J, Miears D. Role of acidic intracellular compartments in the biosynthesis of Dictyostelium lysosomal enzymes. The weak bases ammonium chloride and chloroquine differentially affect proteolytic processing and sorting. J Biol Chem. 1989 Feb 25;264(6):3454–3463.
- Doharey PK, Singh V, Gedda MR, et al. In silico study indicates antimalarials as direct inhibitors of SARS-CoV-2-RNA dependent RNA polymerase. J Biomol Struct Dyn. 2021 Jan;21:1–18.
- Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020 Mar 9; 71(15):732–739.
- Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020 Mar 18; 6(1):16.
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 Feb 4;30:269–271.
- Skinner TS, Manning LS, Johnston WA, et al. In vitro stage-specific sensitivity of Plasmodium falciparum to quinine and artemisinin drugs. Int J Parasitol. 1996 May;26(5):519–525.
- Keyaerts E, Vijgen L, Maes P, et al. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004 Oct 8; 323(1):264–268.
- Hoffmann M, Mosbauer K, Hofmann-Winkler H, et al. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020 Jul 22; 585(7826):588–590.
- Xie X, Muruato AE, Zhang X, et al. A nanoluciferase SARS-CoV-2 for rapid neutralization testing and screening of anti-infective drugs for COVID-19. Nat Commun. 2020 Oct 15; 11(1):5214.
- Kono M, Tatsumi K, Imai AM, et al. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antiviral Res. 2008 Feb;77(2):150–152.
- Zhou Y, Vedantham P, Lu K, et al. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. 2015; 116:76–84.
- Iwata-Yoshikawa N, Okamura T, Shimizu Y, et al. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after Coronavirus infection. J Virol. 2019 Mar 15; 93(6):6.
- Shirato K, Kawase M, Matsuyama S. Wild-type human coronaviruses prefer cell-surface TMPRSS2 to endosomal cathepsins for cell entry. Virology. 2018 Apr;517:9–15.
- Bertram S, Heurich A, Lavender H, et al. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One. 2012 Apr 30; 7(4):e35876.
- Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020 May 28; 181(5):1016–35 e19.
- Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020 Aug 1; 396(10247):320–332.
- Ma D, Chen CB, Jhanji V, et al. Expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in human primary conjunctival and pterygium cell lines and in mouse cornea. Eye (Lond). 2020 Jul; 34(7):1212–1219.
- Clementi N, Criscuolo E, Diotti RA, et al. Combined prophylactic and therapeutic use maximizes hydroxychloroquine Anti-SARS-CoV-2 Effects in vitro. Front Microbiol. 2020 Jul 10; 11:1704.
- Sperber K, Louie M, Kraus T, et al. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin Ther. 1995 Jul-Aug; 17(4):622–636.
- Park SJ, Yu KM, Kim YI, et al. Antiviral efficacies of FDA-approved drugs against SARS-CoV-2 Infection in Ferrets. mBio. 2020 May 22; 11(3):3.
- Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020 Oct 15; 383(16):1544–1555.
- Munoz-Fontela C, Dowling WE, Funnell SGP, et al. Animal models for COVID-19. Nature. 2020 Oct; 586(7830):509–515.
- Rosenke K, Jarvis MA, Feldmann F, et al. Hydroxychloroquine proves ineffective in hamsters and macaques infected with SARS-CoV-2. bioRxiv. 2020 Jun 11;10.145144.
- McChesney EW, Banks WF Jr., Fabian RJ. Tissue distribution of chloroquine, hydroxychloroquine, and desethylchloroquine in the rat. Toxicol Appl Pharmacol. 1967 May;10(3):501–513.
- McChesney EW, Fasco MJ, Banks WF Jr. The metabolism of chloroquine in man during and after repeated oral dosage. J Pharmacol Exp Ther. 1967 Nov;158(2):323–331.
- Gustafsson LL, Walker O, Alvan G, et al. Disposition of chloroquine in man after single intravenous and oral doses. Br J Clin Pharmacol. 1983 Apr; 15(4):471–479.
- Frisk-Holmberg M, Bergqvist Y, Termond E, et al. The single dose kinetics of chloroquine and its major metabolite desethylchloroquine in healthy subjects. Eur J Clin Pharmacol. 1984;26(4):521–530.
- Looareesuwan S, White NJ, Chanthavanich P, et al. Cardiovascular toxicity and distribution kinetics of intravenous chloroquine. Br J Clin Pharmacol. 1986 Jul; 22(1):31–36.
- White NJ, Miller KD, Churchill FC, et al. Chloroquine treatment of severe malaria in children. Pharmacokinetics, toxicity, and new dosage recommendations. N Engl J Med. 1988 Dec 8; 319(23):1493–1500.
- Ferreira A, Oliveira ESA, Bettencourt P. Chronic treatment with hydroxychloroquine and SARS-CoV-2 infection. J Med Virol. 2020 Jul 9; 93(2):755–759.
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020 Sep; 2(9):e557–e64.
- Ferri C, Giuggioli D, Raimondo V, et al. COVID-19 and rheumatic autoimmune systemic diseases: report of a large Italian patients series. Clin Rheumatol. 2020 Nov; 39(11):3195–3204.
- Gentry CA, Humphrey MB, Thind SK, et al. Long-term hydroxychloroquine use in patients with rheumatic conditions and development of SARS-CoV-2 infection: a retrospective cohort study. Lancet Rheumatol. 2020 Nov;2(11):e689–e97.
- Singer ME, Kaelber DC, Antonelli MJ. Hydroxychloroquine ineffective for COVID-19 prophylaxis in lupus and rheumatoid arthritis. Ann Rheum Dis. 2020 Aug 5; annrheumdis-2020-218500. DOI:10.1136/annrheumdis-2020-218500
- Rentsch CT, DeVito NJ, MacKenna B, et al. Effect of pre-exposure use of hydroxychloroquine on COVID-19 mortality: a population-based cohort study in patients with rheumatoid arthritis or systemic lupus erythematosus using the OpenSAFELY platform. Lancet Rheumatol. 2021 Jan; 3(1):e19–e27.
- Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. Med (N Y). 2020 Jun; 1(1):5.
- Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020 Jun 18; 382(25):2411–2418.
- Arshad S, Kilgore P, Chaudhry ZS, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020 Aug; 97:396–403.
- García-Albéniz X, Del Amo J, Polo R, et al. Systematic review and meta-analysis of randomized trials of hydroxychloroquine for the prevention of COVID-19. medRxiv. 2021 Jan 9; 2020092920203869
- Coromilas EJ, Kochav S, Goldenthal I, et al. Worldwide survey of COVID-19 associated arrhythmias. Circ Arrhythm Electrophysiol. 2021 Feb 7; DOI:10.1161/CIRCEP.120.009458
- Watson JA, Tarning J, Hoglund RM, et al. Concentration-dependent mortality of chloroquine in overdose. Elife. 2020 Jul 8; 9:9.
- Recovery, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 Feb 25;384(8):693-704.
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 – final report. N Engl J Med. 2020 Nov 5; 383(19):1813–1826.
- He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020 May; 26(5):672–675.
- Update to living WHO guideline on drugs for covid-19. BMJ. 2020 Dec 17; 371:m4779.
- Park JJH, Decloedt EH, Rayner CR, et al. Clinical trials of disease stages in COVID 19: complicated and often misinterpreted. Lancet Glob Health. 2020 Aug 20; 8(10):e1249-e1250.
- Grau-Pujol B, Camprubi D, Marti-Soler H et al. Pre-exposure prophylaxis with hydroxychloroquine for COVID-19: initial results of a double-blind, placebo-controlled randomized clinical trial. PREPRINT (Version 1) available at Research Square Access date: Nov 20 [+https://doiorg/1021203/rs3rs-72132/v1+] 2020.
- Lofgren SM, Nicol MR, Bangdiwala AS, et al. Safety of hydroxychloroquine among outpatient clinical trial participants for COVID-19. Open Forum Infect Dis. 2020 Nov; 7(11):ofaa500.
- Mehra MR, Desai SS, Ruschitzka F, et al. RETRACTED: hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020. Retraction in: Lancet. 2020 Jun 5.
- Sanchez-Chapula JA, Salinas-Stefanon E, Torres-Jacome J, et al. Blockade of currents by the antimalarial drug chloroquine in feline ventricular myocytes. J Pharmacol Exp Ther. 2001 Apr; 297(1):437–445.
- Sanson C, Schombert B, Filoche-Romme B, et al. Electrophysiological and pharmacological characterization of human inwardly rectifying Kir2.1 channels on an automated patch-clamp platform. Assay Drug Dev Technol. 2019 Apr;17(3):89–99.
- Rodriguez-Menchaca AA, Navarro-Polanco RA, Ferrer-Villada T, et al. The molecular basis of chloroquine block of the inward rectifier Kir2.1 channel. Proc Natl Acad Sci U S A. 2008 Jan 29; 105(4):1364–1368.
- Capel RA, Herring N, Kalla M, et al. Hydroxychloroquine reduces heart rate by modulating the hyperpolarization-activated current If: novel electrophysiological insights and therapeutic potential. Heart Rhythm. 2015 Oct; 12(10):2186–2194.
- Chan XHS, Win YN, Mawer LJ, et al. Risk of sudden unexplained death after use of dihydroartemisinin-piperaquine for malaria: a systematic review and Bayesian meta-analysis. Lancet Infect Dis. 2018 Aug;18(8):913–923.
- Harris L, Downar E, Shaikh NA, et al. Antiarrhythmic potential of chloroquine: new use for an old drug. Can J Cardiol. 1988 Sep;4(6):295–300.
- Bourke L, McCormick J, Taylor V, et al. Hydroxychloroquine protects against cardiac ischaemia/reperfusion injury in vivo via enhancement of ERK1/2 phosphorylation. PLoS One. 2015; 10(12):e0143771.
- Yancy CW, Fonarow GC. Coronavirus disease 2019 (COVID-19) and the heart – is heart failure the next chapter? JAMA Cardiology. 2020 Jul 27; 5(11):1216.
- Collins R, Bowman L, Landray M, et al. The magic of randomization versus the myth of real-world evidence. N Engl J Med. 2020 Feb 13; 382(7):674–678.