1. Introduction
In all living creatures, a predetermined period of endogenously driven motor inactivity occurs during the 24-h day [Citation1,Citation2]. Mostly, this period coincides with the time creatures could be in danger; for example, species that use their eyes to detect enemies are in danger during the dark night. Another evolutionary fact is that during this time of inactivity, creatures respond only to individually relevant, learned events. In humans, for instance, a mother is more likely to awaken at the slightest whisper from her new-born child than to a loud noise, whereas the father sleeping next to her may react in the opposite way. Moreover, events in the environment during that period will not be remembered. This behavioral state of endogenously driven motor inactivity, selective ability to respond to one’s environment and low memory is called sleep.
During sleep, the body’s organs are highly active, fulfilling many functions in a predetermined and coordinated manner [Citation3–5]. Functions might include restoration of the body like, for instance, healing wounds from a scuffle with lions during the hunt earlier in the day; strengthening the immune system after being exposed to virus-laden aerosols; consolidating memories to have working memory storage for new information at the cabinet meeting the next day; encoding motor sequences to be retrieved the next day for a piano recital; and coordinating metabolism to supply organs with the substances needed for all of these processes within sleep.
Unfortunate and unique in clinical medicine, the person affected by disturbed sleep cannot report specifically on the changes related to this disturbance for the simple reason that the person is sleeping. The person may report problems initiating and maintaining sleep or experience non-restorative sleep or daytime dysfunction. But these complaints are side effects and unspecific symptoms comparable to fever or pain. The underlying disturbances during sleep remain out of the patient’s sight.
2. Sleep in the elderly
There is still no universally accepted definition of sleep. In 1968, a group of sleep researchers operationalized sequences of bio-signals to determine stages of sleep [Citation6]. Although the sleep period differs in length from individual to individual, sleep stages in healthy subjects occur in a stereotypic pattern. The complexity in particular of the neural mechanisms behind these coordinated processes cannot be overestimated [Citation3,Citation4]. Changes in any of these processes during sleep result in specific disturbances and functional deficits that can lead to impaired well-being, performance and health [Citation5,Citation7–9].
A common belief is that humans need less sleep as they age. No evidence for this exists, however. Indeed, it is more likely that age-related disorders (co)affecting the nervous system, as well as psychotropic medications, spoil the quality of sleep, leading to an increased need for sleep time in order to fulfill its functions. For reasons only partly understood, some sleep parameters change with healthy aging [Citation10,Citation11]: during midlife, between the ages of 35 and 50 years, deep slow wave sleep (SWS) is replaced by lighter NREM (non-rapid eye movement) sleep, whereas in late life, after the age of 70, REM sleep declines and sleep becomes more fragmented. These changes are paralleled by hormonal variations. Less SWS sleep coincides with reduced levels of growth hormone, whereas less REM sleep coincides with increased cortisol levels in the evening. Experimental evidence indicates that a homeostatic sleep drive is responsible for midlife changes in SWS and changes in growth hormone production, whereas REM and cortisol changes are due to flattening of the circadian drive to sleep and wakefulness. This flattening of endogenously generated 24-h variation is also thought to result in instability of REM sleep and sleep fragmentation in later life [Citation11,Citation12].
Epidemiological studies show that elderly individuals report problems initiating and maintaining sleep more often than their younger counterparts but, at the same time, are to report the feeling of not being restored by sleep less frequent [Citation13]. Possible reasons could be the presence of fewer structured daytime activities, as well as sleeping during the day (such as taking naps after lunch or in the early evening) because of reduced circadian drive to sleep during the night and wakefulness during the day.
Insomnia in the elderly should not be neglected. It has been known for some time that insomnia is a predictor of any number of psychiatric disorders [Citation8]. Moreover, elderly individuals with insomnia often have impaired functional status, as well as substantially higher health-care costs and burden of disease [Citation9]. Another crucial aspect is the growing acceptance that disturbed sleep is a causal factor in the development of the major groups of neurodegenerative disorders, including Alzheimer’s and Parkinson’s diseases [Citation14]. The underlying mechanisms, such as the clearing of metabolic waste (e.g. amyloid and tau- and synuclein-protein) from the brain during sleep, are becoming clearer [Citation4,Citation15,Citation16]. Fragmented sleep in otherwise healthy elderly people predicts later neurocognitive impairment [Citation17].
To conclude, having specific and diverse treatment options for sleep disturbances in the elderly would probably be one of the best ways to achieve profound improvements in their health.
3. Nonpharmacological therapies
Operationalized in ICD-10 non-organic sleep-related complaints were basically considered symptoms of psychiatric disorders. As a consequence, psychotherapeutic approaches such as cognitive behavioral therapy (CBT) are the mainstay of treatment [Citation18]. Nonpharmacological therapies for insomnia are helpful and easy to assess. Sleep hygiene can be improved by following recommendations available from reputable and evidence-based sources on the internet. A very effective and simple therapy for strengthening the circadian timing system is to be exposed to high amounts of natural daylight prior to midday and to avoid bright, especially blue light in the evening [Citation19]. CBT is the treatment of choice to reduce the symptoms of psychophysiological insomnia, a sleep disorder that co-occurs in most sleep disorders: many patients with sleep disturbances experience daytime dysfunction, and most of these patients develop a vicious cycle of arousal, right before bedtime, characterized by a fear of poor sleep. Such hyperarousal hinders the initiation of sleep and leads to sleep fragmentation, impaired sleep maintenance, and daytime dysfunction [Citation18]. In regions or countries with poor access to CBT, online programs can offer solutions. In patients suffering from psychophysiologic insomnia, pharmacological hypnotic treatment is sometimes even contraindicated.
4. Pharmacological treatment
In the treatment of insomnia, one has to consider that indicating diagnostic features such as problems with initiation or maintaining sleep are unspecific symptoms – like fever or pain – of a huge variety of yet to define disorders (DSM-5). These unspecific symptoms necessitate complex differential diagnosis of underlying pathologies and specific treatment. Diagnostic characteristics of insomnia disorder in DSM-5 include more than 30 disturbances with different pathophysiologies. Polysomnography is a highly precise diagnostic tool to monitor a variety of diverse bio-signals over several hours without the masking influence of e.g. attention, concentration, or motivation [Citation6]. Polysomnography thus provides the basis for specific, diverse treatments such as in apnea, periodic limb movement disorder, or narcolepsy. Recent reports on the interplay of the sleep-waking brain give evidence that insomnia-like sleep disturbances are causally related to e.g. anxiety [Citation20] and neurocognitive disorders [Citation14–16] as it is long hypothesized for affective illnesses [Citation8,Citation21]. In case true, specific sleep-related treatments have yet to be determined with the side effect of improving symptoms such as anxiousness, depressed mood, and cognitive impairment. Perhaps, the most important aspect in ‘pharmacological’ treatment of insomnia symptoms is the avoidance of polypharmacy. Even substances that are not known to cross blood–brain barrier or have been used in the same dosage for years may eventually induce insomnia in the aging brain.
For symptomatic treatment of unspecific symptoms of insomnia, various substances are in general use. Alcohol is used by a large number of patients, for example. Tolerance develops within a few days, leading to the risk of abuse, as well as vegetative withdrawal symptoms with early morning awakening [Citation22]. Many sedating psychotropic drugs, such as antipsychotics or antidepressants, are accompanied by impairment the next day [Citation18]. Indeed, most antidepressants spoil or suppress REM sleep. One-night suppression of REM sleep by anticholinergic amitriptyline has been shown to impair procedural learning [Citation23], and the long-term use of anticholinergic antidepressants is associated with an increased risk of developing dementia. Benzodiazepines should be avoided in general because of a substantial risk of developing tolerance and long-lasting withdrawal symptoms [Citation24].
Today, a good choice for symptomatic treatment is benzodiazepine-receptor agonists (Z-drugs). Unfortunately – and in the opinion of this expert, erroneously – this group of hypnotics is not generally recommended for long-term use because they are thought, like benzodiazepines, to lead to tolerance, somatic dependency, and next-day residual effects which – in the opinion of this expert – is not true. The electrophysiological profiles of benzodiazepines and Z-drugs are clearly different (). After long-term use, benzodiazepines are associated with the absence of SWS, a reduction in REM sleep, and a fingerprint-like high occurrence of sleep spindles in all sleep stages, including REM sleep [Citation24]. In sharp contrast, Z-drugs are associated with a healthy-like sleep pattern (). The only RCT with zolpidem in a long-term treatment over 8 months found no tolerance as measured using objective polysomnography [Citation25]. Discontinuing Z-drugs leads to the reoccurrence of insomnia symptoms – not surprising in symptomatic treatment – but not symptoms of somatic withdrawal. Moreover, next-day impairment after zolpidem has been shown for healthy subjects only. In contrast, zolpidem in patients with insomnia resulted in improved next-day driving performance compared to placebo and benzodiazepines [Citation26].
Figure 1. Hypnogram of three individuals: upper panel: healthy: 7.5 hours sleep with regular NonREM-REM cycles and sigma spindles predominantly in NREM sleep 2; medium panel: patient with insomnia using daily zolpidem (5 -10 mg) for 18 years; note more or less ‘healthy’ hypnogram; lower panel: patient with insomnia using daily diazepam (5 -10 mg) or brotizolam (0.25 mg) for 15 years; note absence of stage 3 (deep sleep, SWS), marked reduction in REM sleep and frequent sigma spindles during all sleep stages. W = stage awake, R = stage REM, 1/2 = stage NonREM 1/2 (light sleep), 3 = stage NonREM 3 (deep or slow-wave sleep), Sigma = sigma spindle frequency.
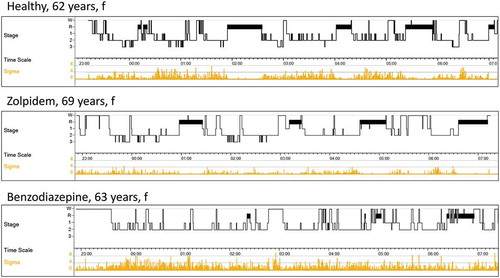
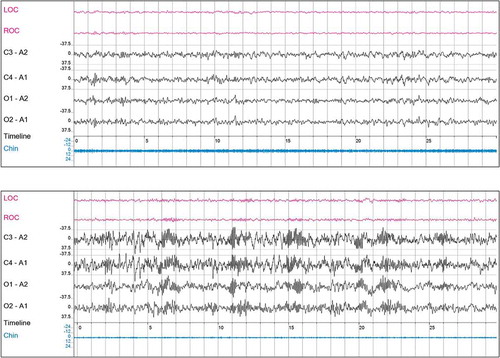
Figure 2. 30 second epochs of NonREM 2; upper panel: patient with insomnia using daily zolpidem (5–10 mg) for 18 years; note more or less ‘healthy’ EEG; low voltage, mixed frequency; alpha/theta range, with diffuse activity in the sigma range, though without pronounced spindles; lower panel: patient with insomnia using daily diazepam (5–10 mg) or brotizolam (0.25 mg) for 15 years; note additional ‘finger-print like’ excessive occurrence of spindle activity; often still to be detected months after benzodiazepine withdrawal
For specific treatment of sleep disturbances, two (groups of) substances are available today: the chronobiotic melatonin and dual orexin-receptor antagonists (DORA). A chronobiotic is defined as a substance capable of shifting phase of the circadian timing system and reentraining circadian rhythms that have been dissociated in the short term or desynchronized in the long term [Citation27]. The most widely recognized chronobiotic is melatonin. Unfortunately, melatonin is sold around the globe as a hypnotic to be administered ‘before bedtime’ or ‘after a meal.’ Because people’s bedtimes change, for example after watching a football match or attending a party, melatonin intake in this manner induces a jetlag-like condition, resulting in desynchronization and a worsening of target symptoms. In contrast, when administered ‘always at the same clock-time,’ melatonin stabilizes the circadian timing system and improves the coordination of processes resulting in positive effects such as a reduction in REM sleep fragmentation and an improvement of daytime tiredness [Citation14]. Interestingly – for being named a hypnotic – many patients being treated with melatonin report a combination of shortened sleep but improved daytime functioning.
The orexin system has been implicated in the regulation of functions such as feeding behavior, locomotion, physical activity, and arousal from sleep [Citation3]. The levels in cerebrospinal fluid are highest at the end of the wake-period and lowest at the end of sleep. The orexin system stimulates the target neurons in the wake system – leading to the release of several chemicals (dopamine, serotonin, histamine, acetylcholine, norepinephrine) that promote wakefulness [Citation3]. DORAs have shown various effects, including the promotion of natural sleep [Citation28,Citation29] and clearance of waste from the brain [Citation30]. Moreover, DORAs – different to benzodiazepines, Z-drugs, and sedating antidepressants – do not impair physical and cognitive functions after e.g. forced awakening from night-sleep [Citation31,Citation32]. Orexin receptor antagonists therefore will very likely be first-choice agents in the treatment of insomnia with a special emphasis on the prevention of neurodegenerative disorders. Ongoing studies appear to confirm the absence of tolerance development over 12 months.
5. Expert opinion
In 2019, the World Health Organization (WHO) passed ICD-11, including Chapter 7 on Sleep-Wake Disorders. The functions of sleep include a large spectrum of essential tasks like learning, memory consolidation, organization of metabolic processes, or integration of the immune system. With the operationalization of more than 100 different disorders, the WHO acknowledged the complexity and bidirectional influences of the two behavioral states, sleep and wake. Sleep medicine is clearly underrepresented in general patient care, academic teaching, and research.
The behavioral state of sleep is highly complex, depends on processes that are coordinated in different ways at different times of the day, and involves more or less every part of the nervous system fulfilling a variety of important functions. Naturally, disturbances in any of the systems involved at any time during the sleep-period require specific treatment. For disease modification, a large number of specific agents are required.
To date, hypnotics have been defined as drugs that shorten the time to sleep onset and increase total sleep time. I argue that the main target for a hypnotic, however, should be to improve daytime performance and general health. While this might entail lengthening the sleep period in some patients, the focus more generally should be on improving the quality of sleep, which is highly complex. Today, this mode of action to promote specific aspects of natural sleep is achieved only by the chronobiotic melatonin and orexin receptor antagonists. Melatonin with its highly time-dependent dosing improves coordination of circadian processes underlying the 24-h sleep–wake propensity. DORAs reinforce low orexin naturally occurring during sleep, which appears to promote various sleep-specific functions, resulting in improved daytime well-being and clearance of brain wastes such as amyloid, tau-, and synuclein-protein during sleep.
To conclude:
1. The prevalence of insomnia symptoms in the elderly is high. The consequences for patients’ general health and the overall burden of disease are substantial. Improving and expanding current treatments for insomnia in this age group is essential.
2. Difficulty to initiate or maintain sleep are unspecific symptoms like fever and pain, which necessitate complex differential diagnosis of underlying pathologies and a multitude of specific treatments.
3. In contrast to the symptomatic hypnotics available today, a specific hypnotic could be defined as ‘a substance capable of promoting waketime functioning by means of its influence on sleep-related mechanisms (). Waketime functioning includes wellbeing, performance and health.’ The first substances available in this category of treatment are the chronobiotic melatonin and dual orexin receptor antagonists (DORAs).
Table 1. Hypnotics in chronic insomnia
4. The recommendation in current guidelines to limit the use of hypnotics to 4 weeks is not appropriate. Most sleep disturbances are chronic and not self-limiting. A restriction to short-term treatment leaves most of these patients without cure or even symptomatic relief. The target of treatment with specific hypnotics should be to modify or even cure the disease. If the aim of treatment is symptomatic only, long-term or even lifelong use needs to be accepted.
5. Z-drugs differ substantially from benzodiazepines. There is little evidence that Z-drugs lead to the development of tolerance and withdrawal symptoms. Z-drugs are a safe option for long-term symptomatic treatment of insomnia.
6. So-called non-organic sleep disturbances in ICD-10 are considered symptoms of psychiatric disorders. However, in most psychiatric disorders, the causal pathophysiology or specific biomarkers have yet to be determined. Sometimes it is useful to change perspective. Perhaps, it is the other way around: disturbances of sleep may result in psychopathologies during wakefulness such as depressed mood, loss of interest and drive, anxiety, cognitive impairment, and more.
The time is right to develop a differential pharmacology of sleep.
Declaration of Interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
One reviewer is a consultant for Eisai Inc, the manufacturer of a dual orexin receptor antagonist. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosc & Biobehav Rev. 1984;8(3):269–300.
- Liu D, Li W, Ma C, et al. A common hub for sleep and motor control in the substantia nigra. Science. 2020;367(6476):440–445. .
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263.
- Fultz NE, Bonmassar G, Setsompop K, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019;366(6465):628–631. .
- Niethard N, Born J. Back to baseline: sleep recalibrates synapses. Nat Neurosc. 2019;22:149–153.
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington: US Government Printing Office; 1968.
- Hudson AN, Van Dongen HPA, Honn KA. Sleep deprivation, vigilant attention, and brain function: a review. Neuropsychopharmacology. 2020;45(1):21–30.
- Hertenstein E, Feige B, Gmeiner T, et al. Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev. 2019;43:96–105.
- Schousboe JT,Kats AM, Stone KT, et al. Multidimensional sleep health and subsequent health-care costs and utilization in older women. Sleep. 2020;43
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284(7):861–868.
- Dijk DJ, Duffy JF, et al. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516(2):611–627.
- Kunz D, Mahlberg R, Müller C, et al. Melatonin in patients with reduced REM sleep duration: two randomized controlled trials. J Clin Endocrinol Metab. 2004;89(1):128–134.
- Ohayon MM. Prevalence and correlates of nonrestorative sleep complaints. Arch Intern Med. 2005;165(1):35–41.
- Postuma RB, Iranzo A, Hu, M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. BRAIN. 2019;0:1–16.
- Holth JK, Fritschi SK, Wang C, et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019;363(6429):880–884.
- Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370(6512):50–56.
- Lim AS, Kowgier YLM, Buchman AS, et al. Sleep Fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36(7):1027–1032.
- Riemann D, Baglione C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700.
- Münch M, Nowozin C, Regente J, et al. Light as a countermeasure to light at the wrong time: effects on cognition, sleepiness, sleep, and circadian phase. Neuropsychobiology. 2016;74(4):207–218.
- Ben Simon E, Rossi A, Harvey AG, et al. Overanxious and underslept. Nature Hum Behav. 2020;4(1):100–110.
- Reynolds III CF, Kupfer DJ. Sleep research in affective illness: state of the art circa 1987. Sleep. 1987;10(3):199–215.
- Roehrs T, Roth T. Insomnia as a path to alcoholism: tolerance development and dose escalation. Sleep. 2018;41(8):41.
- Goerke M, Cohrs S, Rodenbeck A, et al. Differential effect of an anticholinergic antidepressant on sleep-dependent memory consolidation. Sleep. 2014;37(5):977–985.
- Bastien CH, LeBlanc M, Carrier J, et al. Sleep EEG power spectra, insomnia, and chronic use of benzodiazepines. Sleep. 2003;26(3):313–317.
- Randall S, Roehrs TA, Roth T. Efficacy of eight months of nightly zolpidem: a prospective placebo-controlled study. Sleep. 2012;35(11):1551–1557.
- Staner L, Ertlé S, Boeijinga P, et al. Next-day residual effects of hypnotics in DSM-IV primary insomnia: a driving simulator study with simultaneous electroencephalogram monitoring. Psychopharmacol. 2005;181(4):790–798.
- Kunz D. Editorial: circadian rhythms are everywhere: except in neurodegenerative disorders. Curr Alz Res. 2017;14(10):1018–1021.
- Hoever P, Dorffner G, Beneš H, et al. Orexin receptor antagonism, a new sleep-enabling paradigm: a proof-of-concept clinical trial. Clin Pharmacol Ther. 2012;91(6):975–985.
- Zammit G, Dauvilliers Y, Pain S, et al. Daridorexant, a new dual orexin receptor antagonist, in elderly subjects with insomnia disorder. Neurology. 2020;94(21):e2222–e32.
- Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007.
- Seol J, Fujii Y, Park I, et al. Distinct effects of orexin receptor antagonist and GABAA agonist on sleep and physical/cognitive functions after forced awakening. PNAS. 2019;116(48):24353–24358.
- Neylan TC, Richards A, Metzler TJ, et al. Acute cognitive effects of the hypocretin receptor antagonist almorexant relative to zolpidem and placebo: a randomized clinical trial. Sleep. 2020;43(10):1–11.
