ABSTRACT
Introduction: Over the past few decades, antimicrobial resistance (AMR) has skyrocketed globally among bacteria within the Family Enterobacteriaceae (i.e. Enterobacter spp, Klebsiella spp, Escherichia coli, Proteus spp, Serratia marcescens, Citrobacter spp, and others). Enterobacteriaceae are intestinal flora and are important pathogens in nosocomial and community settings. Enterobacteriaceae spread easily between humans and may acquire AMR via plasmids or other mobile resistance elements. The emergence and spread of multidrug resistant (MDR) clones have greatly limited therapeutic options. Some infections are untreatable with existing antimicrobials.
Areas covered: The authors discuss the escalation of CRE globally, the epidemiology and outcomes of CRE infections, the optimal therapy, and the potential role of several new antimicrobials to combat MDR organisms. An exhaustive search for literature related to Enterobacteriaceae was performed using PubMed, using the following key words: antimicrobial resistance; carbapenemases; Enterobacterales; Enterobacteriaceae; Klebsiella pneumoniae; Escherichia coli; global epidemiology; metallo-β-lactamases; multidrug resistance; New Delhi Metalloproteinase-1 (NDM-1); plasmids
Expert opinion: Innovation and development of new classes of antibacterial agents are critical to expand effective therapeutic options. The authors encourage the judicious use of antibiotics and aggressive infection-control measures are essential to minimize the spread of AMR.
1. Introduction
Over the past few decades, antimicrobial resistance (AMR) to a variety of antibiotics has escalated dramatically within the USA and globally [Citation1]. Clonal spread of resistant organisms between hospitals, geographic regions, and continents has fueled the explosive rise in AMR [Citation1]. The incidence of AMR is highest in intensive care units (ICUs) [Citation2], because of the liberal use of antibiotics, prolonged hospital stays, need for invasive devices, and co-morbidities. However, AMR is increasing in diverse healthcare and community settings as well [Citation3]. Factors that enhance spread of AMR include: crowding; lack of hygiene; overuse of antibiotics; increased worldwide travel [Citation4,Citation5]. AMR is associated with a striking increase in morbidity and mortality, prolonged hospitalizations, need of ICU care, time lost from work, and substantial economic costs [Citation6,Citation7]. Clones of multidrug resistant (MDR) bacteria have disseminated globally, limiting therapeutic options [Citation1,Citation4]. Judicious use of antibiotics and aggressive infection-control measures are essential to minimize spread of AMR [Citation5].
2. Family Enterobacteriaceae
Over the past few decades, AMR has skyrocketed globally among bacteria within the Family Enterobacteriaceae (i.e. Enterobacter spp, Klebsiella spp, Escherichia coli, Proteus spp, Serratia marcescens, Citrobacter spp, and others) [Citation8]. Enterobacteriaceae comprise inhabitants of intestinal flora and are important pathogens in nososomial and community settings [Citation9]. Enterobacteriaceae spread easily between humans (via hand carriage, contaminated food or water, or environmental sources), and may acquire AMR via plasmids, transposons, or other mobile resistance elements [Citation5,Citation9,Citation10]. Early studies in the 1980’s and 1990s cited production of extended-spectrum β-lactamases (ESBLs) by Enterobacteriaceae, creating resistance to cephalosporins (CEPHS) and β-lactam antibiotics [Citation9]. Subsequent studies from the mid-1990’s to the present have documented dramatic increases in carbapenem-resistant Enterobacteriaceae (CRE) (especially among K. pneumoniae) that in some cases are untreatable with existing antimicrobial agents [Citation1,Citation4]. In a comprehensive meta-analysis of nine publications before April 9, 2012, CRE was associated with significantly higher mortality compared to carbapenem (CP)-susceptible infections; in 7 publications, mortality attributed to CRE infections ranged from 27 to 44% [Citation11]. An analysis by the Centers for Disease Control and Prevention evaluated the economic burden in the USA attributed to CRE [Citation7]. Costs associated with CRE infections were substantial and higher than annual costs associated with many chronic and acute diseases [Citation7]. In 2017, Logan et al recognized that global dissemination of carbapenemase (CPase)-producing Enterobacteriaceae was ‘increasing at an alarming pace’ and efforts to curtail spread of these deadly pathogens were essential [Citation12]. In this review, we focus on CRE with an emphasis on CPase-producing strains. CPases in Enterobacteriaceae derive from different sources [e.g., Ambler class A (e.g., KPC); class B (IMP, VIM, NDM), and class D (OXA-48) genes] [Citation5,Citation10]. CPase-encoding genes are often located on plasmids or various mobile genetic elements (MGEs) like transposons and integrons, which contribute significantly to their spread. These genes are usually associated with other AMR genes such as other β-lactamases as well as genes conferring resistance to aminoglycosides (AG), fluoroquinolones (FQ), and other classes of antibiotics, leading to MDR phenotypes [Citation5,Citation9,Citation10]. In this review, we discuss in detail the escalation of CRE globally, review epidemiology and outcomes of CRE infections, and the potential role of several new antimicrobials.
3. Mechanisms of Antimicrobial Resistance (AMR)
The most common mechanism of AMR among Gram negative bacteria (GNB) is hydrolysis of the β-lactam ring by β-lactamases although additional mechanisms (e.g., alterations in porin channels, alteration in bacterial target sites, efflux pumps, combinations of mechanisms) may contribute to or amplify resistance [Citation1,Citation2,Citation9,Citation13]. The dramatic escalation of AMR among Enterobacteriaceae worldwide over the past three decades primarily reflects the emergence and widespread dissemination of novel CPases [Citation1,Citation5,Citation9,Citation11] and ESBLs [Citation2,Citation9] that may be spread rapidly via mobile genetic elements (e.g., plasmids, transposons). In this context, plasmids containing ESBLs were first identified in Germany in 1983 in K. pneumoniae and S. marcescens, and were transferable to other Enterobacteriaceae [Citation2]. A few epidemic ESBL clones (TEM or SHV mutants) were described in hospitals in France and Belgium in the late 1980’s and mid-1990’s [Citation14–19] and rapidly spread throughout Europe and globally [Citation2,Citation3,Citation9,Citation14,Citation20–27]. In the late 1990s, ESBLs were almost exclusively TEM or SHV variants, predominantly in K. pneumoniae [Citation14] . Subsequently, ESBLs not closely related to TEM or SHV (e.g., CTX-M types) arose [Citation24–26]. Clonal dissemination was noted within and between hospitals [Citation15] and long-term care facilities (LTCFs) [Citation19], associated with endemic and epidemic outbreaks. Although some ESBL-producing Enterobacteriaceae confer CP resistance [Citation9], CPs remain the preferred therapy for most strains of ESBL-producing Enterobacteriaceae [Citation2,Citation28]. A prospective multicenter trial of 85 blood stream infections (BSIs) due to ESBL-producing K. pneumoniae showed that treatment with a CP was independently associated with a lower 14-day mortality compared to other in vitro active antibiotics [Citation29].
4. Carbapenemases (CPases) and international spread
The emergence of CRE has been associated with heightened morbidity and mortality in nosocomial and community settings [Citation11]. Carbapenemases (CPases) are a diverse group of enzymes detected in Enterobacterales, Pseudomonas aeruginosa, Acinetobacter baumannii and other GNB that confer resistance to CPs [Citation2,Citation30,Citation31]. CPases in Enterobacteriaceae may belong to the Ambler class A (KPC) [Citation30], class B (IMP [Citation25,Citation32],VIM [Citation30], New Delhi Metalloproteinase-1 (NDM-1) [Citation33], or class D (OXA-48) types [Citation1,Citation5] (). CPase-encoding genes are usually located on plasmids or other mobile genetic elements (MGEs) like transposons and integrons, which contribute significantly to their spread. The specific CPase genes vary depending upon geographic location, antibiotic use in the region, and clonal transmission. For example, NDM-1 producing strains are most common in India, the Middle East, and the Balkans [Citation34,Citation35] but are uncommon in the USA [Citation36]. It should be emphasized that some CRE isolates have multiple different CPase genes [Citation37]. The first metallo-β-lactamase (MBL), IMP-1, reported in S. marcescens in Japan in 1991 [Citation32], spread (via plasmids) to Ps. aeruginosa and Enterobacteriaceae [Citation2]. MBLs are usually produced in combination with a second or third β-lactamase enzyme [Citation13]. Importantly, MBL are not inhibited by β-lactamase inhibitors (BLI) [Citation2]. The first IMP-1 MBL-producing K. pneumoniae isolates were detected in Turkey in 2003 [Citation38]. Another MBL, NDM-1, was initially discovered in 2008 in K. pneumoniae from a Swedish patient previously hospitalized in New Delhi, India [Citation33], and rapidly spread worldwide [Citation39–41]. NDM-1 is located on plasmids, most of which are transferable between species [Citation41]. By 2010, NDM-1 had been detected in five continents; most early cases were linked to travel in India or Pakistan [Citation42]. Many strains were MDR [Citation43]. By 2012, NDM-1-producing A. baumannii isolates were reported from several European countries [Citation44,Citation45] and rapidly spread globally [Citation46–49]. Some NDM-1 isolates acquired additional genes conferring resistance to all CPs, CEPHS, and BLI [Citation34]. NDM-1-producing strains are most common in India, the Middle East, and the Balkans [Citation34,Citation35]. However, clonal spread in Europe and elsewhere has occurred. In Tuscany, Italy, a clone of NDM-producing Enterobacteriaceae [sequence type (ST)147] spread rapidly from November 2018 to October 2019; 90.9% were K. pneumoniae [Citation49]. Additionally, E. coli carrying NDM-5 on plasmids recently emerged in Pakistan (in chickens) and humans (in Canada) [Citation50]. NDM-1 remains rare in the USA. Among 13,929 isolates of Enterobacteriaceae from hospitals in the USA from 2016 to 2018, only 152 (1.1%) were CRE; only 4 isolates carried NDM-1 [Citation36]. Klebsiella pneumoniae carbapenemase (KPC) rapidly emerged as a cause of MDR-infections globally beginning in the mid-1990’s [Citation51]. KPC-2, the most common CPase in the USA, emerged in North Carolina in 1996 [Citation52], and spread within the USA [Citation53,Citation54], South America [Citation55], Europe [Citation30,Citation56], Israel [Citation57], South Africa [Citation58], Asia [Citation1,Citation59–61], and worldwide [Citation30]. CRE is predominantly due to KPC-2 on plasmids or other MGEs (e.g. insertion sequences, and phage-related sequences) [Citation62,Citation63]. In Brazil, KPC-2 is the most common CPase, carried on plasmids among K. pneumoniae belonging to different international lineages (e.g. sequence type (ST)258, ST101, and ST15) [Citation64]. Clonal spread in Brazil [Citation65,Citation66] and certain South American countries has been extensive [Citation64,Citation67]. Reyes et al reviewed publications (in English or Spanish) until 2019; data from seven South American countries noted that K. pneumoniae belonging to clonal complex 258 was the most common; the most prevalent CPase genes were KPC and NDM [Citation68]. A recent study in a hospital in Brazil reported 27 isolates of CP-resistant K. pneumoniae (CR-Kp); 24 (88.8%) produced KPC; 16 (59.2%) produced NDM; 13 (48.1%) expressed both genes [Citation65]. In Venezuela, 19 isolates of CRKP co-expressing KPC-2 and VIM-2 were reported in a pediatric hospital in 2014 [Citation69]. In 2015, 4 strains of CR-Kp co-expressing KPC and NDM were described in a Venezuelan hospital [Citation67]. In Europe, the first reports of outbreaks of infections due to KPC-2 were reported in Greece from 2007 to 08 [Citation70]. Subsequent spread through Europe has been sporadic, with high rates in endemic countries (primarily Greece [Citation71,Citation72] and Italy [Citation66,Citation73–76]) whereas CR-Kp has been uncommon in France and Scandinavian countries [Citation77]. In Italy, 60 consecutive clinical isolates of CRE in 2013–2014 revealed clonal spread of KPC-2 producing K. pneumoniae ST101 and ST1789 epidemic clones [Citation73]. Some strains also expressed VIM-1 or CTX-M genes [Citation73]. A national surveillance of BSI due to CRE (K. pneumoniae and E. coli) was initiated in Italy in 2013 [Citation74]. From 2014–2017, 7,632 CRE-BSI cases were reported from all 21 regions and autonomous provinces in Italy. The cases increased from 1,403 in 2014 to 2,208 in 2017. Most cases originated in hospitals (87.2%), mainly in ICUs (38.0%). Almost all CPE-BSI (98.1%) were due to K. pneumoniae carrying the KPC enzyme (95.2%) [Citation74]. In Tuscany, Italy, clonal spread of NDM-producing Enterobacteriaceae (n = 1,645) was documented from November 2018 to October 2019; 90.9% were K. pneumoniae, with a ST147/NDM dominant clone [Citation49].
Table 1. Carbapenemase classification and features
CPases of the oxacillinase type (e.g. OXA-48) were first detected in 2001 in Istanbul in K. pneumoniae [Citation78]. Within one decade, OXA-48-producing CRE were endemic in Turkey [Citation79], and widely disseminated to countries around the Mediterranean (Egypt, Algeria, Libya, Tunisia, Morocco) [Citation80] and western European [Citation1,Citation81] countries. OXA-48-like CPases are endemic in North Africa and the Middle East and are the most common CPases in Enterobacteriaceae in certain parts of the world [Citation25]. OXA-48, OXA-181, OXA-232, OXA-204, OXA-162, and OXA-244, in that order, are the most common enzymes derived from the OXA-48-like CPase group [Citation25]. By 2011, isolates of OXA-181-producing K. pneumoniae were described in New Zealand [Citation82], India [Citation83], and Singapore [Citation84]. OXA-181 CPases are endemic in India and certain sub-Saharan African countries [Citation25]. OXA-162 and OXA-244 (also derivatives of OXA-48) are present in Europe and globally [Citation25]. Certain high-risk clones among K. pneumoniae and E. coli (ST38 and ST410) have been associated with the global dispersion of OXA-48, OXA-181, OXA-232, and OXA-204 [Citation25]. In one hospital in Madrid, Spain, 178 isolates of CPase-producing Enterobacter spp. were recovered from 2005–2018, the most common CPases were VIM (73.5%) and OXA-48 (18.8%); only 11 (6.2%) were due to KPC-2 [Citation85]. In North America, OXA enzymes among Enterobacteriaceae have been uncommon [Citation54].
Until recently, CPs were the agents of choice to treat infections due to E. coli ST131, well known for its MDR profile. However, CP resistance emerged via CPase-encoding genes [Citation86]. Transmission of a KPC-2-producing plasmid to ST131 E. coli led to infections in multiple wards in a hospital [Citation86]. Eight of 30 patients with respiratory infections died within 28 days of the first isolation of ST131 E. coli. Diverse MDR elements were transferred and rearranged between these plasmids [Citation86]. Disturbingly, hypervirulence genes incorporated into CR-K. pneumoniae (CR-hvKp) have been increasingly reported [Citation87,Citation88]. These isolates produce CPases (e.g. OXA-48, NDM-1, ST11, ST15) but may also carry resistance genes (e.g., CTX, TEM, SHV-type) and virulence genes (e.g., rmpA), on plasmids. Clonal spread of these hypervirulent strains within hospitals has been described [Citation88]. The prevalence of specific genotypes of CRE may change over time in hospitals and geographic regions. Surveillance of CRE isolates in ICU patients with BSIs in a University Hospital in Greece demonstrated substantial variation over time [Citation89]. From 2015 to 2017, 110/125 (88%) of CRE BSI isolates produced KPC, followed by NDM-1 (n = 15, 12%); none produced VIM. In 2018, 22/45 (49%) of CRE isolates were due to KPC, followed by VIM (n = 17, 38%), both KPC and VIM (n = 5, 11%) and NDM-1 (n = 1, 2%) [Citation89]. The change in the type of CPase correlated with the introduction of ceftazidime/avibactam (CAZ-AVI) in January 2018 at that hospital (p = 0.014, OR 16.7) [Citation89].
5. International dissemination of antimicrobial resistant genetic elements
The prevalence of CRE has spread at an alarming rate globally [Citation1,Citation30] and multiple factors have been implicated in contributing to this spread (). Importation of CRE from refugees, medical tourism, travel, and cross-border transfer of patients has fueled spread of AMR [Citation1,Citation30,Citation90–92]. Travelers to areas with high AMR prevalence may be exposed to resistant bacteria and return to their home countries colonized. Differences in the prevalence of AMR between countries depend on multiple factors such as levels of antibiotic consumption (including inappropriate use), access to clean water, adequate sanitation, vaccines, and the availability and access to quality healthcare [Citation5,Citation90]. Acquisition of MDR genes occurs most commonly in hospitals and chronic care facilities, but community-acquired sources including wild and domestic animals, food products, and environmental contamination may drive resistance [Citation5,Citation10,Citation50,Citation93,Citation94]. Spread of MDR genes has also been linked to community and hospital effluent treatment systems [Citation95,Citation96]. Movement of humans, wild or domestic animals, livestock and food products is a key factor in spreading MDR in the environment [Citation93,Citation94,Citation97–99]. In a recent study of 88 seagull fecal samples from the Lisbon coastline in Portugal, CPase- and ESBL-producing Enterobacteriaceae were detected in 16 and 55% of samples, respectively [Citation97]. Isolation of MDR bacteria including CRE and ESBL-producing Enterobacteriaceae have been reported in islands in the South Western Indian Ocean [Citation100].
Global spread of AMR is amplified by antibiotic use. Importantly, global antibiotic consumption has increased substantially over the past two decades [Citation101]. Klein et al analyzed the trends and drivers of antibiotic consumption from 2000 to 2015 in 76 countries [Citation101]. Between 2000 and 2015, global antibiotic consumption increased 65%. The increase was driven by low- and middle-income countries (LMICs), where rising consumption correlated with gross domestic product per capita (GDPPC) growth (p = 0.004). In high-income countries (HICs), overall consumption increased modestly, and there was no correlation with GDPPC. However, of particular concern was the rapid increase in the use of ‘last-resort’ compounds such as glycylcyclines, oxazolidinones, CPs, and polymyxins [Citation101]. Antibiotic consumption in LMICs is rapidly converging to rates similar to HICs. Reducing global consumption is critical for reducing the threat of AMR. In Europe, outpatient antibiotic use was analyzed in 33 countries from 1997 to 2009. Total outpatient antibiotic use in 2009 varied by a factor of 3.8 between the countries with the highest defined daily dose (DDD) (38.6 in Greece) and lowest (10.2 in Romania) [Citation102]. Additionally, the use of specific classes of antibiotics varied among countries [Citation102]. Importantly, efforts are needed to curb inappropriate antibiotic use worldwide [Citation103]. High-income countries must ensure their use of antimicrobials is appropriate to reduce selection for AMR. Surveillance across all countries is needed to monitor and respond to this emerging threat [Citation90].
6. Risk factors for Carbapenem Resistant Enterobacteriaceae (CRE)
Rates of AMR (including CRE) are highest in ICUs, because of the liberal use of antibiotics, prolonged hospital stays, need for invasive devices, and co-morbidities [Citation2,Citation34,Citation104,Citation105] (). Endemic and epidemic spread of CRE has been described in hospitals [Citation15], LTCF [Citation17,Citation18,Citation106,Citation107] and less commonly in community settings [Citation2,Citation30]. Not surprisingly, the incidence of infections with CRE is much higher in patients with co-morbidities [Citation108], immunodeficiency, neutropenia [Citation109,Citation110], organ transplant recipients (OTR) [Citation109,Citation111,Citation112], diabetes mellitus [Citation34,Citation109,Citation113], recent hospitalization [Citation104,Citation105], ICU stay [Citation34], hematological malignancy [Citation109,Citation110,Citation114,Citation115], cardiac surgery [Citation66,Citation116,Citation117], rectal colonization [Citation109,Citation118], receipt of broad spectrum antimicrobials [Citation17,Citation104,Citation119], invasive procedures [Citation113], peripheral or central venous catheters (CVC) [Citation34,Citation74,Citation105,Citation112,Citation118,Citation120], indwelling urinary catheters [Citation17], and tracheostomy [Citation113]. Rectal colonization with CRE is a risk factor for subsequent infection with CRE [Citation113,Citation118]. In a cohort of 464 hospitalized patients colonized with CP-resistant K. pneumoniae (CR-Kp), clinical infections with CR-Kp developed in 42 (9.1%) [Citation113]. The following factors were associated with increased risk of CR-Kp infection: invasive procedure (OR 5.74, p = 0.02); diabetes mellitus (OR 4.36, p = 0.17); solid tumor (OR 3.4, p = 0.024); tracheostomy (OR 4.98, p = 0.042); urinary catheter insertion (OR 4.74, p = 0.04); antipseudomonal penicillin use (OR 2.31, p < 0.0001) [Citation113]. Tumberello retrospectively reviewed clinical samples of 657 hospitalized adults in whom KPC-Kp had been isolated from clinical samples and compared with two matched controls with no KPC-Kp-positive cultures during their hospitalizations [Citation121]. Independent predictors of KPC-Kp isolation included: recent admission to an ICU; indwelling urinary catheter, CVC, or surgical drain; recent surgery; neutropenia; ≥2 hospitalizations; hematological cancer, recent FQ and/or CP therapy. A Charlson index of ≥3, indwelling CVC, recent surgery, neutropenia, ≥2 recent hospitalizations, and recent FQ and/or CP use were independent risk factors for KPC-Kp infection [Citation121]. Models to predict KPC-Kp isolation or infection showed good prediction power, with areas under receiver operating curves of 0.82 and 0.82, respectively. Giannella et al developed a bacteremia risk score (GRS) for KPC-Kp colonized patients based on four independent variables [Citation122]. Colonization at multiple sites with KPC-Kp was the strongest predictor of BSIs. Cano et al validated the GRS score and showed very good prediction for developing not only BSI but also any type of KPC-Kp infection [Citation123]. In a tertiary care hospital in Israel, 66 patients with prior rectal carriage of CRE were assessed at their next hospital encounter [Citation124]. Persistent rectal carriage was documented in 23 patients; factors associated with persistent carriage included: prior FQ use (OR 4.27); admission from another hospital (OR 3.49); time interval ≤3 months since first (+) CRE culture (OR 3.59). In a subsequent matched case control study by these investigators, 132 newly identified CRE rectal carriers were followed; 44 developed clinical infections, 88 did not [Citation118]. The median time between screening and subsequent clinical infection was 11 days. Independent predictors of subsequent CRE clinical specimens included: admission to ICU; presence of CVC; receipt of antibiotics; diabetes mellitus [Citation118]. In an Italian teaching hospital, emergence and progressive spread of KPC-Kp was noted over a 4-year period in a cardiac surgical unit [Citation66]. Following implementation of aggressive infection control efforts and antibiotic stewardship, the outbreak was curtailed [Citation117]. A retrospective study of 22 hematological patients hospitalized between February 2012 and May 2013 in an Italian hospital identified 14 BSIs due to CR-Kp [Citation109]. CR-Kp BSIs developed mainly during neutropenia (86%) and in CR-Kp-carriers (79%). Ten of 14 patients with CR-Kp BSIs died (71%); all had acute myelogenous leukemia. Initial adequate antibiotic therapy was the only independent factor protective against death (p = 0.02). Infection control measures and weekly screening for CR-Kp were effective in reducing the spread of CR-Kp [Citation109]. These various studies underscore the importance of aggressive infection control efforts [Citation125] and the importance of antibiotic use and CVCs in increasing the risk for carriage.
7. Incidence of AMR among different geographic regions and countries
The incidence of AMR is highly variable among countries, and changes (increases or decreases) are unpredictable. In this section, we briefly review trends including types of genetic resistance elements within certain countries/regions and clinical outcomes, but recognize that dissemination of resistance genes can be explosive. A meta-analysis of studies published until December 2015 evaluated global mortality in cases with CR-Kp [Citation111]. Pooled mortality was 42.1% among 2,462 patients infected with CR-Kp versus 21.2% in those infected with CP-susceptible K. pneumoniae (CS-Kp). Mortality rates of CR-Kp infections were as follows: BSI (54.3%); urinary tract infections (UTIs) (13.5%); admission to an ICU (48.9%); solid organ transplant (SOT) (43.1%) [Citation111]. Mortality rates were 47.7% in patients infected with KPC-CPase compared to 46.7% in those infected with VIM-CPase. Mortality varied among continents (i.e., 33.2% in North America; 46.7% in South America; 50.1% in Europe; 44.8% in Asia). In summary, mortality rates were higher in cases infected with CR-Kp compared to CS-Kp, especially in association with BSI, ICU admission, or SOT recipients. In a global study of 15,202 adults treated in ICUs at 1150 centers in 88 countries, 24-h point prevalence of hospital acquired infection (HAI) and longitudinal follow-up was assessed [Citation126]. Overall, 54% had suspected or proven infection, including 1,760 (22%) with ICU-acquired infections. The proportion of patients with suspected or proven infection ranged from 43% in Australasia to 60% in Asia and the Middle East. In-hospital mortality rate was 30% in patients with suspected or proven infection; ICU-acquired infection was independently associated with increased mortality compared with community-acquired infection (CAI) (p = 0.003). Klebsiella resistant to β-lactam antibiotics, including third-generation cephalosporins and CPs, was independently associated with a higher risk of death compared to infections with other microorganisms (OR, 1.29 [95% CI, 1.02–1.63]; p = .03)
In the sections below, we review resistance trends in specific countries or regions, but due to space constraints will only cite a few examples.
7.1. USA
Surveillance of CRE from sterile sites or urine cultures was performed in seven metropolitan areas in the USA (Colorado, Georgia, Maryland, Minnesota, New Mexico, New York, and Oregon) from 2012 to 2013 [Citation105]. Among 599 CRE cases in 481 individuals, 520 (86.8%) were isolated from urine and 68 (11.4%) from blood. Of 188 isolates tested, 90 (47.9%) produced CPases. Overall annual CRE incidence rate per 100,000 population was 2.93. The CRE standardized incidence ratio was significantly higher than predicted for the sites in Georgia, Maryland and New York and significantly lower than predicted for the sites in Colorado, New Mexico and Oregon. Most cases occurred in individuals with prior hospitalizations (75.1%) or indwelling devices (72.8%); 55.9% of admitted cases were discharged to a LTCF. Mortality rate was 9.0%.
7.2. Canada
In Canada, participating acute care facilities submitted CRE isolates in 2014 to the National Microbiology Laboratory [Citation92]. Among 261 CRE isolates from 58 hospitals, the most common CPase genes were KPC-3 (64.8%) and NDM-1 (17.6%). Patients who had a history of medical attention during international travel accounted for 21% of cases. In Alberta, Canada, 12 patients with infections due to CRE during 2012–2013 were linked to recent international travel [Citation91]. All had contact with health care systems abroad. In British Columbia, Canada from 2008 to March 2014, surveillance detected 177 isolates of CPase-producing bacteria [Citation127]. CP-resistance among K. pneumonia was plasmid-mediated but lacked clonality, whereas NDM-producing E. cloacae isolates exhibited clonality. Surveillance of clinical isolates from 70 Canadian hospitals between January 1, 2014 and December 31, 2015 noted that CPase-producing Enterobacteriaceae infection rates remained low and stable whereas colonization increased by 375% (p = 0.014) [Citation128].
7.3. Europe
The French National Reference Center evaluated 140 CPase-producing E. coli isolates in 2012 and 2013; 74% produced OXA-48-like CPase; 21% produced NDM CPase [Citation129]. A wide diversity of isolates were found [50 different sequence types (ST)]. The most prevalent clones were ST38-producing OXA-48, a clone linked to Turkey and North African countries [Citation129]. In 2012, the European Center for Disease Prevention and Control (ECDC) launched the ‘European survey of CPase-producing Enterobacteriaceae (EuSCAPE)’ in 38 countries [Citation130]. Since then, the prevalence of CRE has worsened, in particular with the rapid spread of OXA-48 and NDM-1-producing Enterobacteriaceae. In 2015, 13 of 38 countries reported inter-regional spread of or an endemic situation for CRE, compared with 6 of 38 in 2013 [Citation130]. In 2018, all 37 participating countries reported CPE cases [Citation131]. However, the incidence of CRE in Europe is very heterogeneous. CRE is endemic in Greece [Citation72,Citation89] and Italy [Citation66,Citation117,Citation132], but is rare in France and northern European countries (e.g., Finland, Norway). In November 2018, an outbreak of NDM-1 CRE emerged in Tuscany, Italy; by October 2019, 1,643 patients with CRE isolates (infections or colonization) mediated by NDM-1 (ST147 dominant clone) had been identified; 1,495 (90.9%) of isolates were K. pneumoniae [Citation49]. Aggressive surveillance, contact precautions, and cohorting were successful in curtailing the outbreak. Interestingly, in 2020, 2 cases of K. pneumoniae infections with NDM-9, ST147 genotype were detected in a Tuscany hospital; genomic and phylogeneic analysis demonstrated a relationship of these isolates to the earlier 2018–2019 clone; these new isolates had also acquired additional resistance genes reflecting prior exposure to colistin, tigecycline, and fosfomicin [Citation133]. In France, a multihospital program from 2010 to 2015 comprising 21,000 hospital beds implemented efforts to reduce infections due to CRE and glycopeptide-resistant Enterococcus faecium [Citation125]. Outbreaks of clinical infections were significantly reduced if contact precautions were instituted (OR 0.31) and even more when dedicated nursing staff were provided within 2 days of hospitalization of index cases (OR 0.09) [Citation125]. In 2018, 1,704 isolates of extensively Drug Resistant (eXDR) CRE were detected by the National Reference Center for Antimicrobial Resistance (NRC) in France from January 1–31, 2018 [Citation134]. Most (81%) of isolates reflected rectal colonization (only 3% of isolates were BSI). In Poland, a retrospective study in a tertiary hospital between 2011 and 2018 et al reported 741 hospital-acquired infections (HAI) among 3708 patients hospitalized in the ICU [Citation120]. Sites of infections included ventilator-associated pneumonia (VAP) (54.1%), UTI (32.4%); central line-associated (CLA)-BSI (13.2%). K. pneumoniae-ESBL (+) was implicated in 11.6% of VAP and 13.2% of CLA-BSI. The incidence of Enterobacteriaceae (+) ESBL among all HAIs increased from 11.8% in 2011 to 15.4% in 2018 [Citation120]. The observed period was marked by an increase in the consumption of CPs: 197.7 vs. 235.9 defined daily dose/1000 patients-days.
7.4. Asia
In China, 1,801 isolates of CRE were obtained from 65 hospitals from 2012 to 2016; the most common strains included K. pneumoniae (n = 1,201), E. coli (n = 282), and E. cloacae (n = 179) [Citation135]. CPase genes were detected in 91% of K. pneumoniae, 80% of E. coli, and 72% of E. cloacae isolates. KPC predominated among K. pneumoniae (77%); NDM predominated in E. coli (75%) and E. cloacae (53%). Sequence types ST11 and ST67 predominated among 100 K. pneumoniae and 47 E. coli isolates. The proportion of KPC and NDM enzymes in CRE increased from 2012–2016 from 54% to 59% and from 12 to 28%, respectively [Citation135]. Two clusters of infections due to CPR-Kp involving 31 patients occurred in a Chinese hospital between October 2017 to August 2019 [Citation136]. Resistance genes were KPC-2 and/or NDM-1 in 20 (64.5%). Aggressive infection control measures were successful in controlling the outbreak. In Singapore, a prospective study of 249 adult inpatients with positive cultures for Enterobacteriaceae found that 161 (71.3%) had CRE [Citation114]. In one multivariate analysis, prior exposure to CPs (OR 3.23) and hematological malignancies (OR 2.83) were associated with non-CPase-producing CRE. Among the CPase producers, 50.2% were KPC, 31.6% NDM, and 13.7% OXA (+). Whole gene sequencing identified five transmission clusters involving 13 patients. In India from November 2018 to May 2019, 350 patients from nine hospitals were colonized or infected with NDM-1-producing Enterobacteriaceae [Citation34]. Among 40 cases with BSI, 30-day mortality was 42.5%. Mortality was lower (30.8%) if patients received appropriate antibiotics compared to 64.3% mortality if the organism was resistant to the antibiotics administered. Not surprisingly, prior CP use (OR 8.4) and the presence of a CVC (OR 4.8) predicted acquisition of NDM-1.
7.5. Africa
The extent of CPE in Africa has not been well studied [Citation137]. In one study, surveillance in 31 of 47 (66%) countries identified low rates of CP resistance among E. coli (> 5% in only one country); for Klebsiella spp., rates of CP resistance exceeded 5% in only 2 nations (13%) [Citation137]. In a study of 292 clinical isolates of Enterobacteriaceae from two hospitals in Nigeria between January and June 2019, 129 (44.2%) were resistant to 3rd-generation cephalosporins but only 19 (6.5%) were CRE [Citation138]. Only 7 of 19 (36.8%) CRE isolates produced CPases; genotypes included NDM-5 (n = 5) and OXA-181 (n = 2), respectively. Other CPase genes, including VIM, KPC and IMP, were not detected. However, 53% of isolates in this study were non-fermenters (Ps. aeruginosa or Acinetobacter spp). Manenzhe et al performed a comprehensive review of 83 studies of CPase-producing GNB in Africa as of February 28, 2014 [Citation139]. Although the prevalence of CR-GNB was not well defined, rates in hospital settings ranged from 2.4 to 67.7% in North Africa and 9–60% in sub-Sahara Africa [Citation139]. In South Africa, 1,193 isolates of suspected CREs from 46 laboratories from 2012–2015 were assessed by polymerase chain reaction reaction (PCR), the gold standard [Citation140]. CPase-producing genes were confirmed in 812 (68%); the three most common genes were NDM, OXA-48, and VIM. Most CRE were Klebsiella spp. (71%) [Citation140].
8. Treatment of Infections due to CRE
8.1. Carbapenems (alone or in combination) are the mainstay of therapy for CRE
CRE have emerged as a major public health challenge globally [Citation141]. Importantly, mortality rates are much higher in patients with CPR compared to CPS strains; further, mortality is higher in KPC-producing K. pneumoniae compared to other genotypes. A recent study in China reported 285 inpatients with BSI due to K. pneumoniae; 46 (16.1%) were CPR [Citation142]. Independent risk factors for CPR included recent ICU stay, malignancy, immunosuppression, and exposure to broad-spectrum antibiotics, FQs, or antifungal agents. Mortality rates at 28 days were 14.6% for CPS strains compared to 50.0% among CPR strains. Italian investigators reported 278 cases of BSIs among 13 hematology units from January 2010 to June 2014 [Citation115]. Mortality rates at 21 days were 84/161 (52.2%) for CPR cases compared to 17/117 (14.5%) with CPS strains. Septic shock, inadequate antimicrobial therapy, and CPR were independently associated with mortality. As has been discussed, AMR among Enterobacteriaceae is mediated via multiple genotypes, KPC, VIM; NDM-1, IMA, OXA and others. Some resistance mechanisms reflect the production of CPases whereas a variety of chromosomal and genetic mobile elements may cause resistance among non-CPase producing bacteria. Regardless of mechanism(s), the escalation of AMR has been associated with significant increases in morbidity and mortality, and drug choices may be limited. A meta-analysis of 37 studies comprising 5,125 patients with KPC-Kp infections from 2005 through 2017 cited overall mortality of 41.0%, with the highest mortality rates observed in oncology patients (56.0%) and in Brazil (51.3%) [Citation141].
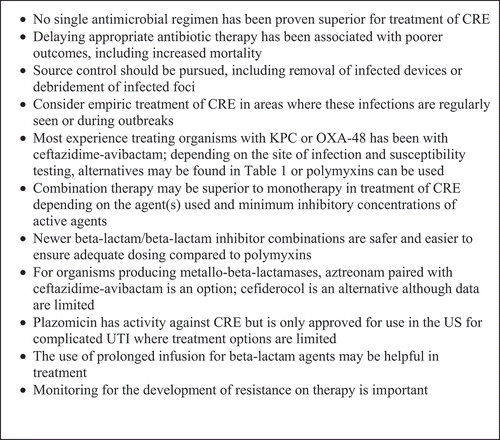
Carbapenems remain the empirical agents of choice for most infections due to Enterobacteriaceae (including some CPase-producing isolates) [Citation71,Citation105,Citation143]. In a non-randomized cohort of 331 patients with ESBL-producing Enterobacteriaceae seen during the period 2007–2014, 14-day mortality was compared between patients who initially received empirical treatment with either piperacillin/tazobactam (PTZ) (n = 103) or a CP (n = 110) [Citation144]. Patients were excluded if they were not treated with a CP after (+) ESBL status was detected [Citation144]. The adjusted risk of death was 1.92 times higher for patients receiving empiric PTZ compared with empiric CP therapy (95% confidence interval, 1.07–3.45) [Citation144]. These findings should not be extended to newer BLI combinations, nor can they be extrapolated to CPase-producing Enterobacteriaceae. Although data are limited, high dose CP (typically meropenem) [Citation132,Citation143,Citation145] and optimization of PK/PD [Citation146] may improve outcomes. In various studies, CP has been combined with a second or even third agent [e.g. polymyxins (PMX), tigecycline (TGC), fosfomycin (FOS), AGs, ceftazidime/avibactam (CAZ-AVI)] but results have been mixed, and the optimal agents have not been established. Several non-randomized observational studies suggest that outcomes with CRE infections are superior with combination antimicrobial therapy compared to monotherapy [Citation71,Citation147,Citation148]. A multicenter retrospective study in three Italian teaching hospitals examined 125 cases of BSIs caused by KPC-producing K. pneumoniae (KPC-Kp) [Citation147]. Overall 30-day mortality rate was 41.6%. Mortality rate was significantly higher among patients treated with monotherapy compared to combination therapy (54.3% vs 34.1%, p = .02). Factors independently associated with 30-day mortality included septic shock at BSI onset (OR, 7.17; p = 0.008); inadequate initial antimicrobial therapy (OR: 4.17, p = 0.003); high APACHE III scores (OR: p < .001). Therapy with a combination of TGC, colistin, and meropenem (MERO) was associated with lower mortality compared to monotherapy (OR 0.11; p = .01) [Citation147]. Similarly, in a series of 205 cases of CR-Kp BSI from two hospitals in Athens, Greece, combination therapy was associated with reduced mortality compared to monotherapy [Citation71]. In that cohort, 163 (79.5%) cases were infected with KPC or KPC plus VIM. 103 patients received combination therapy (two or more active drugs); 72 received monotherapy; 12 received no active drug. The remaining 18 patients died within 48 h of onset of bacteremia. All-cause 28-day mortality was 40%. Mortality rate was significantly higher in those treated with monotherapy (44.4%) compared to combination therapy (27.2%; p = 0.018). Combinations containing a CP were associated with the lowest mortality rate (19.3%). Combination therapy was strongly associated with survival [hazard ratio (HR) of death for monotherapy versus combination, 2.08; 95% CI, 1.23 to 3.51; p = 0.006], mostly due to the effectiveness of CP-containing regimens [Citation71]. A retrospective study from two medical centers in the USA identified 41 cases with BSI due to KPC-Kp; all infections were either HA (78%) or HCA 22%) [Citation148]. Overall 28-day mortality rate was 39.0% (16/41); mortality was lower in the combination therapy group (13.3%) compared to 57.8% in the monotherapy group (p = 0.01). The most commonly used combinations were CP + PMX +TGC. The mortality in this combination therapy group was 12.5% (1/8). Despite in vitro susceptibility, monotherapy with PMX or TGC was associated with increased mortality (8/12) 66.7%). In the multivariate analysis, definitive therapy with a combination regimen was independently associated with survival (OR, 0.07, p = 0.02). Giannella et al. reported 595 adults inpatients in 3 hospitals in Genoa, Italy with CR-Kp BSIs; 127 (21.5%) died within 14 days of onset [Citation132]. Factors associated with increased mortality included Charlson comorbidity index (HR 1.31, p < 0.001); septic shock at BSI onset (HR 3.14, p < 0.001), colistin-resistant isolates (HR 1.02. p = 0.003). Protective factors included admission to a surgical ward (HR 0.44, p = 0.005) and high dose CP use (HR 0.69, p = 0.05). Russo et al retrospectively analyzed patients admitted to the ICU with septic shock from November 2010 to December 2015 with either KPC-Kp infections (n = 128) or MDR A. baumannii (MDR-AB (n = 92); 30-day mortality with KPC-Kp was 44.5% compared to 84.8% with MDR-AB [Citation149]. For patients with KPC-Kp infections, the use of ≥ 2 in vitro-active antibiotics as empirical or definitive therapy was associated with higher 30-day survival, while isolation of PMX-resistant strains was linked to mortality [Citation149]. In a children’s hospital in Spain, 38 cases of CRE were reported from 2005 to 2018; 76.3% were HA; 23.7% were HCA [Citation112]. All children had at least one comorbidity; 52.6% were OTRs. Sources of infection included: intestinal tract (26.3%) and CVC (21.1%). VIM CPase was demonstrated in 92.1%. Crude mortality at 30-days was 7/38 (18.4%); attributable mortality was 10.5%. Two independent factors predicted mortality: ICU admission and inadequate empirical antimicrobial therapy (p < 0.05). Lodise et al, using a large USA hospital data base, identified all admissions between July 2011 and September 2014 with serious Enterobacteriaceae infections [Citation150]. Infections were classified as CRE or CP-susceptible (CSE). Receipt of antimicrobials with activity against all index pathogens on the index date or ≤2 days thereafter was deemed as ‘timely’; all other instances were ‘delayed.’ Associations between CRE status and delayed appropriate therapy on outcomes were estimated using inverse probability weighting and multivariate regression models. A total of 50,069 patients met selection criteria; 514 patients (1.0%) had CRE. Overall, 67.5% of CSE patients (vs 44.6%, CRE) received timely appropriate therapy (p < .01). Irrespective of CRE status, patients who received delayed appropriate therapy had longer duration of antibiotic therapy and length of stay (LOS), higher costs, lower likelihood of discharge to home, and greater likelihood of the composite mortality outcome (p < .01) [Citation150]. The above studies do not establish optimal therapy for CRE infections, but strongly support early treatment of effective antibiotic therapy prior to identifying the susceptibility pattern of the organism. A panel of 11 experts from the Critically Ill Patients Study Group of the European Society of Clinical Microbiology and Infectious Disease (ESCMID) recommended combination therapy for severe CRE infections; they acknowledged that appropriate therapy for non-critically ill patients with non-severe infections required randomized clinical trials to assess benefit/risks [Citation143]. For severe CRE infections, we initiate therapy with a CP plus a second agent while awaiting results of susceptibility tests. General principles regarding the treatment of CRE infections are summarized in .
8.1.1. Use of Dual Carbapenems to treat CRE
Dual beta lactam therapy has been tried in anecdotal cases in patients with CRE infections [Citation151–154] and in vitro and murine models [Citation155], but randomized trials are lacking and its value is unproven.
8.2. Nebulized antibiotics as adjunctive therapy for VAP
In a randomized placebo-controlled trial (RPCT) in patients with VAP due to KPC-Kp treated with IV antimicrobials, adjunctive treatment with nebulized amikacin + fosfomycin showed no clinical benefit, but was associated with reduced selection pressure and greater eradication of pathogens compared to the placebo arm [Citation156]. In 2017, the European Society of Clinical Microbiology and Infectious Disease recommended avoiding nebulized antibiotics in VAP due to weak level of evidence of efficacy and potential adverse effects [Citation157].
Source Control:
Particularly in post-surgical patients, removal of implanted or tunneled devices, drainage or debridement of infected foci or abscess may be critical to improve outcome. Several studies in ICU patients with CRE have demonstrated improved survival when source control has been achieved [Citation158–160]
9. Aminoglycosides have limited role to treat CRE
9.1. Plazomicin (next-generation AG)
Activity of aminoglycosides (AGs) against CRE is variable; however, many CREs contain AG resistance determinants on conjugative plasmids [Citation2]. Resistance to AGs may develop via enzymatic inactivation or 16S ribosomal RNA methylation [Citation161]. Isolates with this latter mechanism, expressed on transposons, although rare, often co-express ESBLs or MBL [Citation161]. We do not recommend AGs as monotherapy for ESBL infections; the role of combination therapy with AG and CP has not been established. Plazomicin is a next-generation AG that was synthetically derived from sisomicin [Citation162]. Plazomicin is active against both Gram-negative and Gram-positive bacterial pathogens, including isolates harboring all clinically relevant AG-modifying enzymes. Plazomicin, has in vitro activity against several MDR GNB, including CRE [Citation163–167]. In a surveillance of 4,680 clinical isolates from six European and adjacent countries during 2014–2015, plazomicin inhibited 95.8% of Enterobacteriaceae including CRE and was more active than other AGs against isolates carrying KPC, OXA-48, or CPase (-) isolates [Citation166]. Additionally, among 728 isolates carrying AG-modifying enzymes, 99.0% were inhibited by plazomicin at ≤2 µg/ml [Citation166]. Recently, plazomicin was reported to be active versus E. coli (including 100% of ESBL-producing and CPR isolates) and other Enterobacteriaceae tested, including gentamicin-resistant isolates [Citation168]. Plazomicin is approved by the FDA to treat c-UTI [Citation163–165] and will likely have a future role to treat serious CRE infections at other sites as part of combination therapy. Emergence of resistance mediated by 16S ribosomal RNA methylation genes among CRE has been described [Citation169].
10. Polymyxin B and polymyxin E (Colistin)
Polymyxins [e.g. polymyxin B and polymyxin E (colistimethate sodium)] are cationic detergents that disrupt bacterial cytoplasmic membranes, causing leakage of cytoplasmic contents [Citation170]. Colistimethate sodium can be administered by intravenous (IV) or inhaled routes [Citation170]. Polymyxins (PMX) have been used primarily as salvage therapy for MDR-GNB including CRE [Citation146] but are associated with significant neurotoxicity (principally paresthesias) and nephrotoxicity (20–50%) [Citation170]. These complications are less common with current formulations and regimens [Citation170]. International Guidelines were recently published regarding the use of PMX [Citation170]. Polymyxin B is preferred, as this has superior pharmacokinetics (PK) [Citation170] and less nephrotoxicity [Citation170]. Optimal dosing and monitoring is difficult; therapeutic dose management is advised [Citation170]. Dosing according to PK and pharmacodynamic (PD) principles and the use of combination regimens may maximize efficacy of antibiotics for MDR-strains and may minimize emergence of resistance [Citation170]. PMX-CP combinations are synergistic in vitro against CR-GNB [Citation171], and some studies suggest improved outcomes in CR-GNB but data are mixed. de Oliveira et al retrospectively reviewed 118 cases of CRE infections in 3 Brazilian hospitals from October 2009 to June 2013; 78 had BSI [Citation172]. K. pneumoniae was implicated in 108 cases (92%). Fifty-seven patients (48%) received monotherapy; 61 (52%) received combination therapy. Overall 30-day mortality was 45%. The following factors were associated with increased mortality: renal failure at end of therapy, older age, and use of PMX. UTIs and monotherapy with CP were protective. Given the retrospective nature of this study, no clear conclusions can be drawn although efficacy of PMX was suboptimal. A randomized, open label controlled trial in 6 hospitals in Israel, Greece, and Italy included adults with BSI, VAP, HAP, or urosepsis caused by CPR-GNB [Citation173]. Between October 1, 2013 and December 31, 2016, 406 patients were randomly assigned to IV colistin or colistin plus MERO. Treatment success was defined by clinical and microbiological parameters. The primary outcome was treatment failure at 14 days post randomization. Most (87%) patients had pneumonia or BSI; 77% of infections were caused by A. baumannii. Combination therapy was not superior to monotherapy in severe A. baumannii infections. However, the trial was underpowered to specifically address other bacteria such as CRE [Citation173]. In one review of 15 studies comprising 55 patients with CRE, PMX monotherapy was associated with clinical successes in only 14%; response rates were higher (73%) when used as combination therapy [Citation51]. Secondary analysis of that RCT evaluated whether the initial empirical antibiotic therapy (EAT) influenced outcomes [Citation174]. Initial EAT covered the responsible organisms in 209 patients (51.5%), mostly colistin (n = 200). Mortality was 96 of 209 (45.9%) when EAT covered the organisms compared to 84 of 197 (42.6%) when EAT did not cover the organisms (p = 0.50). Hence, covering EAT was not associated with improved survival; rather, there was a weak association with mortality (OR = 1.37). Since 77 percent of infections were caused by A. baumannii, these data may not be representative of CRE. Two publications cited the superiority of combination therapy over monotherapy for BSI due to CPase-producing K. pneumoniae [Citation147,Citation148]. In the largest published series (125 patients with BSI due to KPC-Kp), 30-day mortality with colistin monotherapy was 54% compared to 34% with combination therapy with TGC, colistin and MERO (OR 0.11, p = 0.01) [Citation147]. Emergence of plasmid-mediated colistin resistance against mcr-1 genes and its variants have been described among Enterobacteriaceae in humans, animals, and environment (farms, communities), and pose a threat to public health and the livestock industry [Citation98,Citation175] .
11. Tigecycline (TGC) and Eravacycline
Tigecycline (TGC), the first of a new class of glycylcyclines, is active in vitro against >95% of ESBL-producing and CPase-producing E. coli or K. pneumoniae [Citation176]. However, clinical studies of TGC for documented CRE infections are limited. TGC has limited urinary tract penetration [Citation51], and is not reliable for UTIs. For BSI, peak serum concentrations of TGC do not exceed 1 µg/ml, a concentration similar to the MIC of many ESBL-producing and/or CPase-producing organisms [Citation177]. Thus, efficacy of TGC for BSI cannot be assured, particularly when MICs exceed 1 µg/ml [Citation177]. Further, in a multicenter RCT trial of 945 patients with HAP randomized to monotherapy with either TGC or imipenem (IMI), cure rates were 67.9% with TGC and 78.2% with IMI [Citation178]. Overall mortality rates were similar (14.2% with TGC; 12.2% with IMI); however, among patients with VAP, mortality was significantly higher with TGC compared to IMI [Citation178]. Additionally, Cheng et al reported 176 adults with BSI due to XDR A. baumannii (defined as nonsusceptible to all drug classes except for colistin and TGC) at 3 hospitals in Taiwan from 2010 to 2013 [Citation179]. In that prospective study, 55 patients received combination therapy [colistin + TGC (n = 29) or colistin + CP (n = 26)] [Citation179]. Crude 14-day and in-hospital mortality rates were higher among patients receiving colistin +TGC with TGC MIC > 2 µg/ml compared to patients receiving colistin + CP (p = 0.105). Breakthrough XDR A. baumannii bacteremia under steady state concentrations of combination therapy for colistin-TGC group was 18% and for colistin-CP group was 0% (HR, 6.93, p = 0.009) [Citation179]. Peleg et al cautioned that TGC resistance may emerge on therapy and MDR infections may persist despite maintenance of in vitro susceptibility [Citation177]. Based upon these features, TGC has a very limited role as monotherapy for MDR GNB infections, and should only be considered as a part of combination therapy.
Eravacycline, a novel synthetic fluorocycline structurally similar to TGC, has excellent activity against resistant GNB and Gram-positive cocci [Citation180,Citation181] and is ~4 times more potent than TGC against GNB, including MDR strains [Citation180,Citation182]. Eravacycline has excellent in vitro activity against Enterobacteriaceae including ESBL and CPase-producing strains (KPC-2 and NDM-1), and those exhibiting colistin resistance (mcr-1), with MICs 2–4-fold lower than TGC [Citation183]. In a worldwide surveillance study of 36 countries from 2013–2017 (> 13,000 clinical isolates of Enterobacteriaceae), eravacycline was 4 times more potent than TGC [Citation184]. Currently, eravacycline is FDA-approved only for treating c-IAI . Pooled data from two phase 3 clinical trials (IGNITE 1 and IGNITE 4) found that eravacycline was non-inferior to ertapenem or meropenem as treatment for c-IAI including patients with bacteremia [Citation185].
12. Fosfomycin (antibiotic with a novel mechanism of action)
Fosfomycin is a bactericidal agent that inhibits cell wall synthesis using a mechanism of action distinct from β-lactams or other antimicrobial agents [Citation186]. Fosfomycin has a broad-spectrum and is often active against MDR Enterobacteriaceae (including ESBL- and CP-resistant strains) [Citation187,Citation188]. Fosfomycin has been used as part of combination therapy in hospitalized patients who have not responded to or cannot tolerate first and potentially second-line antimicrobials [Citation186,Citation189]. In a recent prospective multicenter study in 20 ICUs in Germany and Austria, 209 patients with serious bacterial infections (194 were in ICUs) were treated with fosfomycin, almost exclusively (99%) in combination with other antibiotics [Citation189]. Among evaluable patients, clinical response was favorable in 81.3% (148/182) of cases, including 84.8% (39/46) favorable responses among patients with MDR pathogens. Fosfomycin was effective therapy for UTIs caused by ESBL-producing E. coli [Citation190]. Resistance to fosfomycin is rare [Citation191,Citation192], but fosfomycin-resistance genes (fosA3) have been documented among ESBL-producing E. coli and K. pneumoniae isolates; all co-harbored blaCTX-M resistance genes [Citation193]. Clinical and microbiological resistance to fosfomycin among patients with CPase-producing K. pneumoniae has been reported [Citation194]. Combinations of 3-drugs [MERO, PMX, and rifampin (RIF)] have been evaluated in a hollow fiber infection model in the laboratory [Citation195]. A single-dose of PMX, combined with prolonged infusion MERO 8 gm every 8 hours, and RIF 600 mg every 24 hours was associated with bacterial counts below the quantitative limit within 24 hours and remained undetectable throughout the 10-day experiment. These results are interesting but data in humans are lacking.
13. Newer β-lactam/β-lactamase inhibitors for CRE
Newer β-lactam/β-lactamase inhibitor (BLBLI) combinations have been used to treating ESBL-producing GNB [Citation196]. However, these agents are usually not active against MBLs (including NDM-1).
13.1. Ceftazidime/avibactam (CAZ-AVI)
The combination of CAZ-AVI, the first in the class of diazabicyclooctanes, improves the in vitro activity of ceftazidime against some Enterobacteriaceae, including KPC and OXA-48 producers [Citation196–202]. However, activity against MBLs (including NDM-1) is poor. In a cohort of 37 patients with CRE infections treated with CAZ-AVI, clinical success and survival rates at 30 days were 59% (22/37) and 76% (28/37), respectively. In 23% (5 of 22) clinical successes, CRE infections recurred within 90 days. Microbiologic failure rate was 27%. CAZ-AVI resistance was detected in 3 of 10 microbiological failures [Citation198]. In a compassionate use trial of severe GNB-resistant infections in Italy, 138 patients with KPC-KP were treated with CAZ-AVI after failing initial therapy (median 7 days) with other antibiotics [Citation203]. CAZ-AVI was administered with at least one other active antibiotic in 100 (78.9%) cases. Thirty-day mortality after infection onset was 34.1% (47/138). Among 104 patients with bacteremic KPC-KP infections, 30-day mortality was significantly lower with CAZ-AVI compared to drugs other than CAZ-AVI (36.5% vs 55.8%, respectively) (p = 0.005). Multivariate analysis of the 208 cases with KPC-KP bacteremia identified septic shock, neutropenia, Charlson comorbidity index and need for MV as independent risk factors for mortality whereas CAZ-AVI was the sole independent predictor of survival [Citation203]. Although data are limited, in vitro studies and murine models [Citation204,Citation205] and one case report [Citation206] cited success with CAZ-AVI plus aztreonam for MBL-producing Enterobacteriaceae.
13.2. Imipenem-relebactam (IMI-REL)
Imipenem-relebactam (IMI-REL) is a novel CP-BLI combination. Relebactam (REL) is a non-β-lactam, bicyclic diazabicyclooctane BLI that is structurally related to avibactam [Citation207]. REL displays activity against Ambler class A (including ESBLs), KPCs and class C β-lactamases (AmpC), but does not inhibit NDM or OXA-48-producing strains [Citation207]. Surveillance of 22 European countries from 2015–2017 of clinical isolates of non-Proteus Enterobacteriaceae (NPE) from intraabdominal infections (IAIs) (n = 10,465) and UTIs (n = 7,446) cited susceptibility rates to IMI-REL of 98.4% and 98.5%, respectively [Citation208]. Recently Karlowsky et al. reported that 99.5% of NPE collected from patients with IAIs or UTIs were susceptible to IMI-REL; further, 77.9% of IMI-non-susceptible strains, 96.3% of KPC-producing K. pneumoniae, and 98.7% of MDR isolates were S to IMI-REL [Citation209]. Motsch et al. recently published the results of a randomized double blind clinical trial (RESTORE-IMI 1) in patients with HA-VAP, complicated-IAI (c-IAI), or c-UTI caused by imipenem non-susceptible pathogens [Citation210]. Patients were treated with either IMI-REL (n = 31) or colistin plus IMI/cilastatin (n = 16). Favorable clinical responses at 28 days were achieved in 71% of patients receiving IMI-REL and in 40% receiving colistin plus IMI/cilastatin; 28-day mortality rates were 10 and 30%, respectively. IMI-REL was safer than colistin plus IMI/cilastatin with treatment-emergent nephrotoxicity (10% and 56%, p = .002, respectively). These authors concluded that IMI-REL is an efficacious and well-tolerated therapeutic option for CP-nonsusceptible infections.
13.3. Meropenem/vaborbactam (MEV)
Vaborbactam is a non-β-lactam, cyclic, boronic acid-based, BLI that displays activity against Ambler class A (including ESBLs, KPCs, and class C β-lactamases (AmpC) but lacks activity against MBLs or class D OXA. Vaborbactam significantly (2 to >1024-fold MIC reduction) improves the activity of meropenem against most species of Enterobacteriaceae depending on the presence or absence of specific β-lactamase enzymes [Citation207]. In recent studies, MEV displayed excellent in vitro activity against resistant GNB, particularly KPC-producing Kp (>99% susceptibility; MIC50 typically ≤0.06 µg/ml) but activity against OXA-48 and MBLs was limited [Citation211–214]. Currently, MEV is indicated for treating c-UTIs [Citation211]. The efficacy and safety of MEV was assessed in two phase III randomized trials, Targeting Antibiotic Non-susceptible Gram Negative Organisms (TANGO-I and TANGO-II) of serious CRE infections [Citation215]. In TANGO-II (a phase 3 multi center open label trial conducted from 2014 to 2017), MEV was compared to best available therapy (BAT) for serious infections (BSI, c-UTI, HAP or VAP, c-IAI) due to CRE [Citation216]. Eligible patients were randomized 2:1 to MEV (2 g over 3 h, q8h for 7–14 days) or BAT (mono/combination therapy with PMX, CPs, AG, TGC; or CAZ/AVI alone). Patients with suspected or confirmed CRE infection were randomized, and 47 with confirmed CRE infection formed the primary analysis population. Efficacy endpoints included clinical cure, day-28 all-cause mortality, microbiologic cure, and overall success (clinical cure + microbiologic eradication). Clinical cure rates were 65.6% (21/32) with MEV vs 33.3% (5/15) with BAT [p = 0.03)] at end of treatment and 59.4% (19/32) and 26.7% (4/15) [p = 0.02] at test of cure. All-cause mortality at 28 days was 15.6% (5/32) with MEV vs 33.3% (5/15) with BAT, respectively. Treatment-related adverse events (AEs) and renal related AEs were 24.0% and 4.0% with MEV compared to 44.4% and 24.0% for BAT. In summary, for the treatment of CRE infections, monotherapy with MEV was associated with increased clinical cure, decreased mortality, and reduced nephrotoxicity compared with BAT [Citation216]. A multicenter, retrospective study of 131 adults with CRE infections who received MEV or CAZ-AVI for ≥ 72 hours from 2015 to 2018 was performed [Citation217]. Twenty-six patients received MEV; 105 received CAZ-AVI; 40% had bacteremia. The primary endpoint was clinical success; secondary endpoints included 30- and 90-day mortality, adverse events (AE), 90-day CRE infection recurrence, and development of resistance in patients with recurrent infection. No significant difference in clinical success was observed between groups (62% versus 69%; p = 0.49). Patients in the CAZ-AVI arm received combination therapy more often than patients receiving MEV (61% versus 15%; p < 0.01). Mortality rates at 30- and 90-days, and rates of AE were similar between groups. In patients with recurrent infection, resistance developed in three patients who received CAZ-AVI monotherapy and in no patient in the MEV arm [Citation217]. Importantly, MEV may be effective even against CRE carrying mutations in KPC genes that substantially reduce the potency of CAZ-AVI but have little effect on MEV [Citation218,Citation219]. In a recent cohort of 40 patients treated with MEV for serious GNB infections (80.0% were CRE), clinical success was achieved in 70.0% [Citation220]. Mortality and recurrence at 30 days were 7.5% and 12.5%, respectively [Citation220,Citation221] . MEV was effective as salvage therapy in a transplant patient with K. pneumoniae BSI and intraabdominal abscess resistant to CAZ/AVI [Citation218]. Resistance to MEV has been described among CRE and requires ongoing monitoring [Citation222].
13.4. Ceftolozane-tazobactam
Ceftolozane-tazobactam is a potent β-lactam/BLI combination approved for the treatment of c-IAI, c-UTIs, HAP and VAP [Citation223]. Ceftolozane-tazobactam is active versus ESBL-producing and AmpC-derepressed Enterobacteriaceae, but KPC, NDM and OXA-48 producers are typically resistant [Citation200,Citation223].
14. Cefiderocol (a novel siderophore cephalosporin)
Cefiderocol is a siderophore cephalosporin with unique ability to enter the bacterial periplasmic space that has enhanced stability against β-lactamases including ESBLs and CPases; its activity appears equal to or superior to CAZ-AVI against CPase-producing Enterobacteriaceae [Citation224]. Cefiderocol has potent activity against GNB including MDR Enterobacteriaceae and non-fermenters [Citation225–227]. Cefiderocol is active against β-lactamases including ESBLs (CTX, SHV, TEM) as well as KPC, NDM, IMP, VIM, OXA-24, OXA-48 type [Citation225]. Cefiderocol is FDA-approved to treat c-UTIs [Citation225] but data for other serious nosocomial infections are limited. In a non-randomized study, 10 critically ill patients with either BSI or VAP caused by CPR-GNB were treated with cefiderocol. All strains had MIC < 2 µg/ml [Citation228]. Clinical success and survival rates at 30 days were 70% and 90%, respectively; two exhibited microbiological failure. Two phase III clinical trials are in progress to evaluate its efficacy as in the treatment of adults with HAP, VAP, or HCAP caused by GNB [Citation224]. In a prospective, randomized, open label phase III trial of 152 patients with serious infections (HAP, BSI, sepsis, c-UTIs) caused by CPR-pathogens, 101 (85%) received monotherapy with cefiderocol and 49 received best available therapy (BAT) [Citation229]. Most common pathogens included A. baumannii (n = 54, 46%), K. pneumoniae (n = 39, 33%) and Ps. aeruginosa (n = 22, 19%). Among patients with HAP, clinical cure was achieved in 20/40 (50%) in the cefiderocol group compared to 10/19 (53%) in BAT group. For patients with BSI or sepsis, clinical cure was achieved in 10/23 (43%) with cefiderocol and 6/14 (43%) in the BAT group. For patients with c-UTI, microbiologic eradication was achieved in 9/17 (53%) in the cefiderocol group and 1/5 (20%) in BAT group. At the end of the study, 34/101 (34%) receiving cefiderocol and 9/49 (18%) patients receiving BAT died; one death (in BAT group) was considered to be related to the study drug. Although there were more deaths in the cefiderocol group, these deaths occurred primarily in patients with A. baumannii infections. Overall, this study suggests that cefiderocol is likely comparable to BAT in patients with severe, CRE infections and limited treatment options [Citation229]. Recently, Wunderink et al [Citation230] completed a randomized, double-blind, parallel-group, phase 3, non-inferiority trial in 76 centers in 17 countries in Asia, Europe, and the USA. Adult patients with HAP, VAP, or HCAP- due to GNB, were randomized to 3-h intravenous infusions of either cefiderocol 2 g or meropenem 2 g every 8 h for 7–14 days. All-cause mortality at day 14 was 12.4% with cefiderocol (18/145) and 11.6% with meropenem (17/146). The investigators concluded that cefiderocol was non-inferior to meropenem in terms of all-cause mortality in patients with GNB nosocomial pneumonia, with similar tolerability.
15. Conclusion
Antimicrobial resistance among Enterobacteriaceae is a major global threat. Despite increasing AMR, the development of new antibiotics has dwindled. From the 1980s to the early 2000s, approval of new antibiotics declined by 90% and few new novel classes have been discovered [Citation6]. Scientific, regulatory, and economic hurdles have made antibiotic development less attractive compared with more lucrative therapeutic areas [Citation6]. Some of the novel compounds in the antibiotic pipeline display increased activity, but preexisting resistance both within and across antibacterial classes limits the activity of many of the new agents against drug-resistant organisms.
16. Expert Opinion
Bacteria within the Family Enterobacteriaceae (i.e. Enterobacter spp, Klebsiella spp, Escherichia coli, Proteus spp, Serratia marcescens, Citrobacter spp, and others) are important pathogens in nosocomial and community settings. Over the past few decades, antimicrobial resistance (AMR) has skyrocketed globally among Enterobacteriaceae. AMR may develop via plasmids, transposons, or other resistance elements. Mutations conferring resistance typically increase over time; the rate of increase is amplified by selection pressure from antibiotic use. Factors that enhance spread of AMR include: overuse of antibiotics; crowding; lack of hygiene; tourism; refugees and international travel. Clonal spread of resistance among hospitals, geographic regions, and continents has fueled the explosive rise in resistance globally. Within the past 3 decades, several novel carbapenemase (CPase)-encoding genes have been described in Enterobacteriaceae the specific CPase genes vary depending upon geographic location, antibiotic use in the region, and clonal transmission. These CP-resistance genes are usually associated with other AMR genes such as other β-lactamases, as well as genes conferring resistance to aminoglycosides, fluoroquinolones, and other classes of antibiotics. The emergence and spread of multidrug resistant (MDR) clones has greatly limited therapeutic options. In some cases, infections due to MDR Enterobacteriaceae are untreatable with existing antimicrobial agents.
Optimal therapy for serious infections due to Enterobacteriaceae remains controversial, as prospective, randomized therapeutic trials are lacking. CPs remain the mainstay of therapy (alone or in combination), particularly for ESBL-producing Enterobacteriaceae. However, some CPases (e.g. KPC and NDM-1) may result in high grade resistance to CPs. In this context, optimizing pharmacodynamics and prolonging infusion time of CPs and/or using combinations of antibiotics may have value. Tigecycline and polymyxins demonstrate good in vitro activity versus CRE, but neither agent should be used as monotherapy due to low serum levels with tigecycline and resistance development with polymyxins. Combinations of CPs with second or even third agents (e.g. tigecycline, polymyxins, doxycycline, rifampin, fosfomycin, AGs, FQs) has been tried but efficacy appears limited, and toxicities may be increased with certain antibiotic combinations. Newer agents such as imipenem-relebactam, meropenem/vaborbactam, ceftazidime/avibactam, cefiderocol (a siderophore cephalosporin), and plazomicin (a novel aminoglycoside) are promising and will be discussed. Unfortunately, despite increasing AMR, the development of new antibiotics has dwindled. From the 1980’s to the early 2000’s, approval of new antibiotics declined by 90% and few novel classes have been discovered. Scientific, regulatory, and economic hurdles have made antibiotic development less attractive compared with more lucrative therapeutic therapeutic areas. Some of the novel compounds in the antibiotic pipeline display increased activity, but preexisting resistance both within and across antibacterial classes limits to activity of many of the new agents against extensively drug-resistant (XDR) and pan-drug-resistant (PDR) bacteria. Innovation and development of new classes of antibacterial agents is critical to expand effective therapeutic options. In addition, judicious use of antibiotics and aggressive infection-control measures are essential to minimize the spread of AMR.
Abbreviations
aminoglycosides (AG); antimicrobial resistance (AMR); β-lactamase inhibitors (BLI); blood stream infections (BSIs); carbapenem (CP); carbapenemase (CPase); carbapenem-resistant (CR); carbapenem-resistant Enterobacterales (CRE);β-lactam inhibitor (BLI); CP-resistant K. pneumoniae (CRKP); CP-susceptible K. pneumoniae (CSKP); ceftazidime/avibactam (CAZ-AVI); cephalosporins (CEPHS); central line-associated (CLA); central venous catheter (CVC); community-acquired infection (CAI); complicated intra-abdominal infection (c-IAI); complicated urinary tract infection (c-UTI); defined daily dose (DDD); extended-spectrum β-lactamases (ESBLs); extensively Drug Resistant (eXDR); fluoroquinolones (FQ); Gram negative bacteria (GNB); gross domestic product per capita (GDPPC); high-income countries (HICs); hospital acquired infection (HAI); imipenem-relebactam (IMI-REL); intensive care units (ICUs); Klebsiella pneumoniae carbapenemases (KPCs); long-term care facilities (LTCFs); low- and middle-income countries (LMICs); mechanical ventilation; metallo β-lactamase (MBL); minimum inhibitory concentration (MIC); mobile genetic elements (MGEs); multidrug resistant (MDR); New Delhi Metalloproteinase-1 (NDM-1); non-Proteus Enterobacterales (NPE); organ transplant recipients (OTR); piperacillin/tazobactam (PTZ); solid organ transplant (SOT); tigecycline (TGC); ventilator-associated pneumonia (VAP)
Article highlights
Over the past 3 decades, antimicrobial resistance (AMR) among bacteria within the family Enterobacteriaceae (within the order Enterobacterales) which include Enterobacter spp, Klebsiella spp, Escherichia coli, Proteus spp, Serratia marcescens, Citrobacter spp, and others) escalated dramatically worldwide.
The escalation of AMR reflects the emergence and widespread dissemination of novel ESBLs and carbapenemase (CPase)-producing Enterobacteriaceae.
Several distinct CPase-encoding genes have been described among Enterobacteriaceae. Ominously, these CPase-encoding genes are usually associated with other AMR genes such as other β-lactamases, as well as genes conferring resistance to aminoglycosides, fluoroquinolones, and other antibiotic classes.
Clonal spread of resistant organisms via plasmids and mobile genetic elements among hospitals, geographic regions, and continents has fueled the explosive rise in CP resistance globally.
Some clones of MDR Enterobacteriaceae are essentially untreatable with existing antimicrobial agents.
Judicious use of antibiotics and aggressive infection-control measures are essential to minimize the spread of AMR.
This box summarizes key points contained in the article.
Declaration of interest
NM Clark has received research funding from Astellas, Shire, Ansun, Incyte, Janssen and BeiGene while GG Zhanel has received research funding from Achaogen, Astellas, Iterum, Merck & Co, Pfizer Inc, Shionogi, Sunovion, Tetraphase, The Medicines Company, Verity and Zambon. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17(10):1791–1798.
- El Salabi A, Walsh TR, Chouchani C. Extended spectrum β-lactamases, carbapenemases and mobile genetic elements responsible for antibiotics resistance in Gram-negative bacteria. Crit Rev Microbiol. 2013;39(2):113–122.
- Song J-H, Thamlikitkul V, Hsueh P-R. Clinical and economic burden of community-acquired pneumonia amongst adults in the Asia-Pacific region. Int J Antimicrob Agents. 2011;38(2):108–117.
- Livermore DM. Has the era of untreatable infections arrived? J Antimicrob Chemother. 2009;64(Suppl Supplement 1):i29–36.
- Taggar G, Attiq Rheman M, Boerlin P, et al. Molecular epidemiology of Carbapenemases in Enterobacteriales from humans, animals, food and the environment. Antibiotics (Basel). 2020;9(10):693.
- Luepke KH, Suda KJ, Boucher H, et al., Past, present, and future of antibacterial economics: increasing bacterial resistance, limited antibiotic pipeline, and societal implications. Pharmacotherapy. 2017;37(1):71–84.
- Bartsch SM, McKinnell JA, Mueller LE, et al. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect. 2017;23(1):e9- e16.
- Iredell J, Brown J, Tagg K. Antibiotic resistance in Enterobacteriaceae: mechanisms and clinical implications. BMJ. 2016;352:h6420.
- Lynch JP 3rd, Clark NM, Zhanel GG. Evolution of antimicrobial resistance among Enterobacteriaceae (focus on extended spectrum β-lactamases and carbapenemases). Expert Opin Pharmacother. 2013;14(2):199–210.
- Jamal AJ, Faheem A, Farooqi L, et al. Household transmission of Carbapenemase-Producing Enterobacterales (CPE) in Ontario, Canada. Clin Infect Dis. 2020:ciaa1295. DOI:10.1093
- Falagas ME, Tansarli GS, Karageorgopoulos DE, et al. Deaths attributable to Carbapenem-Resistant Enterobacteriaceae infections. Emerg Infect Dis. 2014;20(7):1170–1175.
- Logan LK, Weinstein RA. The epidemiology of Carbapenem-Resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis. 2017;215(suppl_1):S28–S36.
- Bush K, Fisher JF. Epidemiological expansion, structural studies, and clinical challenges of new β-Lactamases from Gram-Negative Bacteria. Annu Rev Microbiol. 2011;65(1):455–478.
- Livermore DM, Canton R, Gniadkowski M, et al. CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother. 2006;59(2):165–174.
- Laurent C, Rodriguez-Villalobos H, Rost F, et al. Intensive care unit outbreak of extended-spectrum β-Lactamase–Producing Klebsiella Pneumoniae controlled by cohorting patients and reinforcing infection control measures. Infect Control Hosp Epidemiol. 2008;29(6):517–524.
- Piednoir E, Thibon P, Borderan G-C, et al. Long-term clinical and economic benefits associated with the management of a nosocomial outbreak resulting from extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Crit Care Med. 2011;39(12):2672–2677.
- Tinelli M, Cataldo MA, Mantengoli E, et al. Epidemiology and genetic characteristics of extended-spectrum -lactamase-producing Gram-negative bacteria causing urinary tract infections in long-term care facilities. J Antimicrob Chemother. 2012;67(12):2982–2987.
- Urban C, Bradford PA, Tuckman M, et al. Carbapenem-resistant Escherichia coli Harboring Klebsiella pneumoniae Carbapenemase β-Lactamases associated with long-term care facilities. Clin Infect Dis. 2008;46(11):e127–30.
- Jimenez A, Abbo LM, Martinez O, et al. KPC-3–Producing Serratia marcescens Outbreak between acute and long-term care facilities, Florida, USA. Emerg Infect Dis. 2020;26(11):2746–2750.
- Azap ÖK, Arslan H, Serefhanoglu K, et al. Risk factors for extended-spectrum β-lactamase positivity in uropathogenic Escherichia coli isolated from community-acquired urinary tract infections. Clin Microbiol Infect. 2010;16(2):147–151.
- Ben‐Ami R, Rodríguez‐Baño J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum β-Lactamase–Producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49(5):682–690.
- Peirano G, Richardson D, Nigrin J, et al. High prevalence of ST131 isolates producing CTX-M-15 and CTX-M-14 among Extended-Spectrum-β-Lactamase-Producing Escherichia coli isolates from Canada. Antimicrob Agents Chemother. 2010;54(3):1327–1330.
- Rogers BA, Sidjabat HE, Paterson DL. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J Antimicrob Chemother. 2011;66(1):1–14.
- Chong Y, Ito Y, Kamimura T. Genetic evolution and clinical impact in extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect Genet Evol. 2011;11(7):1499–1504.
- Pitout JDD, Peirano G, Kock MM, et al. The Global Ascendency of OXA-48-Type Carbapenemases. Clin Microbiol Rev. 2019;33(1):e00102-19.
- Coque TM, Novais Â, Carattoli A, et al. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum β-Lactamase CTX-M-15. Emerg Infect Dis. 2008;14(2):195–200.
- Woodford N, Turton JF, Livermore DM. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev. 2011;35(5):736–755.
- Bush K, Jacoby GA. Updated functional classification of β-Lactamases. Antimicrob Agents Chemother. 2010;54(3):969–976.
- Paterson DL, Ko W-C, Von Gottberg A, et al. Antibiotic therapy for Klebsiella pneumoniae Bacteremia: implications of production of extended-spectrum -Lactamases. Clin Infect Dis. 2004;39(1):31–37.
- Canton R, Akova M, Carmeli Y, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect. 2012;18(5):413–431.
- Codjoe FS, Donkor ES. Carbapenem resistance: a review. Med Sci (Basel). 2017;6(1). DOI:https://doi.org/10.3390/medsci6010001
- Ito H, Arakawa Y, Ohsuka S, et al. Plasmid-mediated dissemination of the metallo-beta-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob Agents Chemother. 1995;39(4):824–829.
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new Metallo-β-Lactamase Gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53(12):5046–5054.
- Snyder BM, Montague BT, Anandan S, et al. Risk factors and epidemiologic predictors of blood stream infections with New Delhi Metallo-b-lactamase (NDM-1) producing Enterobacteriaceae. Epidemiol Infect. 2019;147:e137.
- Wu W, Feng Y, Tang G, et al. NDM Metallo-beta-Lactamases and their bacterial producers in health care settings. Clin Microbiol Rev. 2019;32(2);e00115-18.
- Castanheira M, Doyle TB, Kantro V, et al. Meropenem-Vaborbactam activity against Carbapenem-Resistant Enterobacterales isolates collected in U.S. hospitals during 2016 to 2018. Antimicrob Agents Chemother. 2020;64(4):e00305–20.
- Laolerd W, Akeda Y, Preeyanon L, et al. Carbapenemase-producing Carbapenem-Resistant Enterobacteriaceae from Bangkok, Thailand, and Their Detection by the Carba NP and Modified Carbapenem inactivation method tests. Microb Drug Resist. 2018;24(7):1006–1011.
- Aktas Z, Bal C, Midilli K, et al. First IMP-1-producing Klebsiella pneumoniae isolate in Turkey. Clin Microbiol Infect. 2006;12(7):695–696.
- Hammerum AM, Toleman MA, Hansen F, et al. Global spread of New Delhi metallo-β-lactamase 1. Lancet Infect Dis. 2010;10(12):829–830.
- Zarfel G, Hoenigl M, Leitner E, et al. Emergence of New Delhi Metallo-β-Lactamase, Austria. Emerg Infect Dis. 2011;17(1):129–130.
- Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10(9):597–602.
- Moellering RC Jr. NDM-1 — a Cause for Worldwide Concern. N Engl J Med. 2010;363(25):2377–2379.
- Stone NR, Woodford N, Livermore DM, et al. Breakthrough bacteraemia due to tigecycline-resistant Escherichia coli with New Delhi metallo- -lactamase (NDM)-1 successfully treated with colistin in a patient with calciphylaxis. J Antimicrob Chemother. 2011;66(11):2677–2678.
- Bonnin RA, Poirel L, Naas T, et al. Dissemination of New Delhi metallo-β-lactamase-1-producing Acinetobacter baumannii in Europe. Clin Microbiol Infect. 2012;18(9):E362–5.
- Pfeifer Y, Wilharm G, Zander E, et al. Molecular characterization of blaNDM-1 in an Acinetobacter baumannii strain isolated in Germany in 2007. J Antimicrob Chemother. 2011;66(9):1998–2001.
- Ghazawi A, Sonnevend Á, Bonnin RA, et al. NDM-2 carbapenemase-producing Acinetobacter baumannii in the United Arab Emirates. Clin Microbiol Infect. 2012;18(2):E34–6.
- Karthikeyan K, Thirunarayan MA, Krishnan P. Coexistence of blaOXA-23 with blaNDM-1 and armA in clinical isolates of Acinetobacter baumannii from India. J Antimicrob Chemother. 2010;65(10):2253–2254.
- Chen Y, Zhou Z, Jiang Y, et al. Emergence of NDM-1-producing Acinetobacter baumannii in China. J Antimicrob Chemother. 2011;66(6):1255–1259.
- Tavoschi L, Forni S, Porretta A, et al. Prolonged outbreak of New Delhi metallo-beta-lactamase-producing carbapenem-resistant Enterobacterales (NDM-CRE), Tuscany, Italy, 2018 to 2019. Euro Surveill. 2020;25(6). DOI:https://doi.org/10.2807/1560-7917.ES.2020.25.6.2000085.
- Baloch Z, Lv L, Yi L, et al. Emergence of almost identical F36:A-:B32 Plasmids carrying blaNDM-5 and qepA in Escherichia coli from both Pakistan and Canada. Infect Drug Resist. 2019;12:3981–3985.
- Hirsch EB, Tam VH. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother. 2010;65(6):1119–1125.
- Yigit H, Queenan AM, Anderson GJ, et al. Novel Carbapenem-Hydrolyzing β-Lactamase, KPC-1, from a Carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45(4):1151–1161.
- Kitchel B, Rasheed JK, Patel JB, et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother. 2009;53(8):3365–3370.
- Landman D, Babu E, Shah N, et al. Transmission of carbapenem-resistant pathogens in New York City hospitals: progress and frustration. J Antimicrob Chemother. 2012;67(6):1427–1431.
- Pavez M, Mamizuka EM, Lincopan N. Early dissemination of KPC-2-producing Klebsiella pneumoniae strains in Brazil. Antimicrob Agents Chemother. 2009;53(6):2702.
- Samuelsen O, Naseer U, Tofteland S, et al. Emergence of clonally related Klebsiella pneumoniae isolates of sequence type 258 producing plasmid-mediated KPC carbapenemase in Norway and Sweden. J Antimicrob Chemother. 2009;63(4):654–658.
- Leavitt A, Navon-Venezia S, Chmelnitsky I, et al. Emergence of KPC-2 and KPC-3 in carbapenem-resistant Klebsiella pneumoniae strains in an Israeli hospital. Antimicrob Agents Chemother. 2007;51(8):3026–3029.
- Brink AJ, Coetzee J, Clay CG, et al. Emergence of New Delhi metallo-beta-lactamase (NDM-1) and Klebsiella pneumoniae carbapenemase (KPC-2) in South Africa. J Clin Microbiol. 2012;50(2):525–527.
- Lauderdale T-L, Shi Z-Y, Lin C-F, et al. KPC-2-producing sequence type 11 Klebsiella pneumoniae detected in Taiwan. Antimicrob Agents Chemother. 2012;56(4):2207–2208.
- Venkatachalam I, Teo J, Balm MND, et al. Carbapenemase-Producing Klebsiella pneumoniae in Hospital, Singapore, 2011. Emerg Infect Dis. 2012;18(8):1381–1383.
- Jean -S-S, Hsueh P-R. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents. 2011;37(4):291–295.
- Hoard A, Montana S, Moriano A, et al. Genomic analysis of two NDM-1 providencia stuartii strains recovered from a single patient. Curr Microbiol. 2020;77(12):4029–4036.
- Prussing C, Snavely EA, Singh N, et al. Nanopore MinION sequencing reveals possible transfer of blaKPC–2 plasmid across bacterial species in two healthcare facilities. Front Microbiol. 2020;11:2007.
- Martins W, Nicolas MF, Yu Y, et al. Clinical and molecular description of a high-copy IncQ1 KPC-2 plasmid harbored by the international ST15 Klebsiella pneumoniae clone. mSphere. 2020;5(5):e00756-20.
- Oliveira ÉMD, Beltrao EMB, Scavuzzi AML, et al. High plasmid variability, and the presence of IncFIB, IncQ, IncA/C, IncHI1B, and IncL/M in clinical isolates of Klebsiella pneumoniae with bla KPC and bla NDM from patients at a public hospital in Brazil. Rev Soc Bras Med Trop. 2020;53:e20200397.
- Lombardi F, Gaia P, Valaperta R, et al. Emergence of Carbapenem-Resistant Klebsiella pneumoniae : progressive spread and four-year period of observation in a cardiac surgery division. Biomed Res Int. 2015;2015:871947.
- Martinez D, Cana L, Rodulfo H, et al. Characteristics of dual carbapenemase-producing Klebsiella pneumoniae strains from an outbreak in Venezuela: a retrospective study. Rev Panam Salud Publica. 2020;44:e50.
- Reyes JA, Melano R, Cardenas PA, et al. Mobile genetic elements associated with carbapenemase genes in South American Enterobacterales. Braz J Infect Dis. 2020;24(3):231–238.
- Falco A, Ramos Y, Franco E, et al. A cluster of KPC-2 and VIM-2-producing Klebsiella pneumoniae ST833 isolates from the pediatric service of a Venezuelan Hospital. BMC Infect Dis. 2016;16(1):595.
- Pournaras S, Protonotariou E, Voulgari E, et al. Clonal spread of KPC-2 carbapenemase-producing Klebsiella pneumoniae strains in Greece. J Antimicrob Chemother. 2009;64(2):348–352.
- Daikos GL, Tsaousi S, Tzouvelekis LS, et al., Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58(4):2322–2328.
- Poulou A, Voulgari E, Vrioni G, et al. Imported Klebsiella pneumoniae carbapenemase-producing K. pneumoniae clones in a Greek hospital: impact of infection control measures for restraining their dissemination. J Clin Microbiol. 2012;50:2618–2623.
- Del Franco M, Paone L, Novati R, et al. Molecular epidemiology of carbapenem resistant Enterobacteriaceae in Valle d’Aosta region, Italy, shows the emergence of KPC-2 producing Klebsiella pneumoniae clonal complex 101 (ST101 and ST1789). BMC Microbiol. 2015;15(1):260.
- Iacchini S, Sabbatucci M, Gagliotti C, et al. Bloodstream infections due to carbapenemase-producing Enterobacteriaceae in Italy: results from nationwide surveillance, 2014 to 2017. Euro Surveill. 2019;24(5):1800159.
- Brescini L, Morroni G, Valeriani C, et al. Clinical and epidemiological characteristics of KPC-producing Klebsiella pneumoniae from bloodstream infections in a tertiary referral center in Italy. BMC Infect Dis. 2019;19(1):611.
- Cristina ML, Alicino C, Sartini M, et al. Epidemiology, management, and outcome of carbapenem-resistant Klebsiella pneumoniae bloodstream infections in hospitals within the same endemic metropolitan area. J Infect Public Health. 2018;11(2):171–177.
- Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9(4):228–236.
- Poirel L, Héritier C, Tolün V, et al. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2004;48(1):15–22.
- Carrër A, Poirel L, Yilmaz M, et al. Spread of OXA-48-encoding plasmid in Turkey and beyond. Antimicrob Agents Chemother. 2010;54(3):1369–1373.
- Cuzon G, Ouanich J, Gondret R, et al. Outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in France. Antimicrob Agents Chemother. 2011;55(5):2420–2423.
- Poirel L, Ros A, Carrer A, et al. Cross-border transmission of OXA-48-producing Enterobacter cloacae from Morocco to France. J Antimicrob Chemother. 2011;66(5):1181–1182.
- Williamson DA, Heffernan H, Sidjabat H, et al. Intercontinental transfer of OXA-181-producing Klebsiella pneumoniae into New Zealand. J Antimicrob Chemother. 2011;66(12):2888–2890.
- Castanheira M, Deshpande LM, Mathai D, et al. Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: report from the SENTRY Antimicrobial Surveillance Program, 2006-2007. Antimicrob Agents Chemother. 2011;55:1274–1278.
- Koh TH, Cao DYH, Chan KS, et al. bla OXA-181 –positive Klebsiella pneumoniae, Singapore. Emerg Infect Dis. 2012;18(9):1524–1525.
- Mateos M, Hernandez-Garcia M, Del Campo R, et al. Emergence and persistence over time of Carbapenemase-Producing Enterobacter Isolates in a Spanish University Hospital in Madrid, Spain (2005–2018). Microb Drug Resist. 2020. DOI:https://doi.org/10.1089/mdr.2020.0265.
- Gong L, Tang N, Chen D, et al. A nosocomial respiratory infection outbreak of Carbapenem-Resistant Escherichia coli ST131 with multiple transmissible blaKPC–2 carrying plasmids. Front Microbiol. 2020;11:2068.
- Solgi H, Shahcheraghi F, Bolourchi N, et al. Molecular characterization of carbapenem-resistant serotype K1 hypervirulent Klebsiella pneumoniae ST11 harbouring blaNDM-1 and blaOXA-48 carbapenemases in Iran. Microb Pathog. 2020;104507.
- Mataseje LF, Boyd DA, Mulvey MR, et al. Two Hypervirulent Klebsiella pneumoniae Isolates Producing a bla KPC-2 Carbapenemase from a Canadian patient. Antimicrob Agents Chemother. 2019;63(7):e00517-19.
- Papadimitriou-Olivgeris M, Bartzavali C, Lambropoulou A, et al. Reversal of carbapenemase-producing Klebsiella pneumoniae epidemiology from blaKPC- to blaVIM-harbouring isolates in a Greek ICU after introduction of ceftazidime/avibactam. J Antimicrob Chemother. 2019;74(7):2051–2054.
- Frost I, Van Boeckel TP, Pires J, et al. Global geographic trends in antimicrobial resistance: the role of international travel. J Travel Med. 2019;26(8).
- Peirano G, Ahmed-Bentley J, Fuller J, et al. Travel-related carbapenemase-producing Gram-negative bacteria in Alberta, Canada: the first 3 years. J Clin Microbiol. 2014;52(5):1575–1581.
- Mataseje LF, Abdesselam K, Vachon J, et al. Results from the Canadian Nosocomial infection surveillance program on Carbapenemase-Producing Enterobacteriaceae, 2010 to 2014. Antimicrob Agents Chemother. 2016;60(11):6787–6794.
- Sellera FP, Lopes R, Monte DFM, et al. Genomic analysis of multidrug-resistant CTX-M-15-positive Klebsiella pneumoniae belonging to the highly successful ST15 clone isolated from a dog with chronic otitis. J Glob Antimicrob Resist. 2020;22:659–661.
- Heinemann C, Leubner CD, Savin M, et al. Research Note: tracing pathways of entry and persistence of facultative pathogenic and antibiotic-resistant bacteria in a commercial broiler farm with substantial health problems. Poult Sci. 2020;99(11):5481–5486.
- Aggarwal A, Bhalla M, Fatima KH. Detection of New Delhi metallo-beta-lactamase enzyme gene bla NDM-1 associated with the Int-1 gene in Gram-negative bacteria collected from the effluent treatment plant of a tuberculosis care hospital in Delhi, India. Access Microbiol. 2020;2(6):acmi000125.
- Reinke RA, Quach-Cu J, Allison N, et al. A method to quantify viable carbapenem resistant gram-negative bacteria in treated and untreated wastewater. J Microbiol Methods. 2020;179:106070.
- Aires-de-sousa M, Fournier C, Lopes E, et al. High colonization rate and heterogeneity of ESBL- and Carbapenemase-Producing Enterobacteriaceae Isolated from Gull Feces in Lisbon, Portugal. Microorganisms. 2020;8.
- Xiaomin S, Yiming L, Yuying Y, et al. Global impact of mcr-1-positive Enterobacteriaceae bacteria on “one health”. Crit Rev Microbiol. 2020;46(5):565-571.
- Irrgang A, Tausch SH, Pauly N, et al. First Detection of GES-5-Producing Escherichia coli from Livestock-An Increasing Diversity of Carbapenemases recognized from German Pig Production. Microorganisms. 2020;8(10):1593.
- Gay N, Lugagne N, Miltgen G, et al. a sentinel territory for antimicrobial-resistant bacteria surveillance in the South-Western Indian Ocean: a retrospective survey using hospitalized patient screening, 2015-2017. BMC Public Health. 2020;20(1):1488.
- Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115(15):E3463–E70.
- Adriaenssens N, Coenen S, Versporten A, et al. European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe (1997-2009). J Antimicrob Chemother. 2011;66(Suppl 6):vi3–12.
- Watkins RR, Bonomo RA. Overview: the ongoing threat of antimicrobial resistance. Infect Dis Clin North Am. 2020;34(4):649-658.
- Maltezou HC, Kontopidou F, Katerelos P, et al. Infections caused by carbapenem-resistant Gram-negative pathogens in hospitalized children. Pediatr Infect Dis J. 2013;32(4):e151–4.
- Guh AY, Bulens SN, Mu Y, et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae in 7 US Communities, 2012-2013. JAMA. 2015;314(14):1479–1487.
- Lautenbach E, Han J, Santana E, et al. Colonization with Extended-Spectrum β-Lactamase-Producing Escherichia coli and Klebsiella species in long-term care facility residents. Infect Control Hosp Epidemiol. 2012;33(3):302–304.
- Viau RA, Hujer AM, Marshall SH, et al. “Silent” dissemination of Klebsiella pneumoniae isolates bearing K. pneumoniae carbapenemase in a long-term care facility for children and young adults in Northeast Ohio. Clin Infect Dis. 2012;54:1314–1321.
- Zhang Y, Wang Q, Yin Y, et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. 2018;62(2):e01882-17.
- Micozzi A, Gentile G, Minotti C, et al. Carbapenem-resistant Klebsiella pneumoniae in high-risk haematological patients: factors favouring spread, risk factors and outcome of carbapenem-resistant Klebsiella pneumoniae bacteremias. BMC Infect Dis. 2017;17(1):203.
- Lalaoui R, Javelle E, Bakour S, et al. Infections due to Carbapenem-Resistant bacteria in patients with Hematologic Malignancies. Front Microbiol. 2020;11:1422.
- Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16:18.
- Ara-Montojo MF, Escosa-Garcia L, Alguacil-Guillen M, et al. Predictors of mortality and clinical characteristics among carbapenem-resistant or carbapenemase-producing Enterobacteriaceae bloodstream infections in Spanish children. J Antimicrob Chemother. 2020;76(1):220-225.
- Borer A, Saidel-Odes L, Eskira S, et al. Risk factors for developing clinical infection with carbapenem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant K pneumoniae. Am J Infect Control. 2012;40(5):421–425.
- Marimuthu K, Venkatachalam I, Khong WX, et al. Clinical and molecular Epidemiology of Carbapenem-Resistant Enterobacteriaceae among adult inpatients in Singapore. Clin Infect Dis. 2017;64(suppl_2):S68–S75.
- Trecarichi EM, Pagano L, Martino B, et al. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey. Am J Hematol. 2016;91(11):1076–1081.
- Del Puente F, Giacobbe DR, Salsano A, et al. Epidemiology and outcome of Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae (KPC-KP) infections in cardiac surgery patients: a brief narrative review. J Chemother. 2019;31(7–8):359–366.
- Giacobbe DR, Del Bono V, Mikulska M, et al. Impact of a mixed educational and semi-restrictive antimicrobial stewardship project in a large teaching hospital in Northern Italy. Infection. 2017;45(6):849–856.
- Schechner V, Kotlovsky T, Kazma M, et al. Asymptomatic rectal carriage of blaKPC producing carbapenem-resistant Enterobacteriaceae: who is prone to become clinically infected? Clin Microbiol Infect. 2013;19(5):451–456.
- Ozsurekci Y, Aykac K, Cengiz AB, et al. Bloodstream infections in children caused by carbapenem-resistant versus carbapenem-susceptible gram-negative microorganisms: risk factors and outcome. Diagn Microbiol Infect Dis. 2017;87(4):359–364.
- Litwin A, Fedorowicz O, Duszynska W. Characteristics of microbial factors of healthcare-associated infections including multidrug-resistant pathogens and antibiotic consumption at the university intensive care unit in Poland in the Years 2011-2018. Int J Environ Res Public Health. 2020;17(19):6943.
- Tumbarello M, Trecarichi EM, Tumietto F, et al. Predictive models for identification of hospitalized patients harboring KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2014;58(6):3514–3520.
- Giannella M, Trecarichi EM, De Rosa FG, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: a prospective observational multicentre study. Clin Microbiol Infect. 2014;20(12):1357–1362.
- Cano A, Gutierrez-Gutierrez B, Machuca I, et al. Risks of infection and mortality among patients colonized with Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae: validation of scores and proposal for management. Clin Infect Dis. 2018;66:1204–1210.
- Schechner V, Kotlovsky T, Tarabeia J, et al. Predictors of rectal carriage of carbapenem-resistant Enterobacteriaceae (CRE) among patients with known CRE carriage at their next hospital encounter. Infect Control Hosp Epidemiol. 2011;32(5):497–503.
- Fournier S, Desenfant L, Monteil C, et al. Efficiency of different control measures for preventing carbapenemase-producing enterobacteria and glycopeptide-resistant Enterococcus faecium outbreaks: a 6-year prospective study in a French multihospital institution, January 2010 to December 2015. Euro Surveill. 2018;23(8):17-00078.
- Vincent J-L, Sakr Y, Singer M, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–1487.
- Sekirov I, Croxen MA, Ng C, et al. Epidemiologic and genotypic review of carbapenemase-producing organisms in British Columbia, Canada, between 2008 and 2014. J Clin Microbiol. 2016;54(2):317–327.
- Surveillance CNI. Healthcare-associated infections and antimicrobial resistance in Canadian acute care hospitals, 2014-2018. Can Commun Dis Rep. 2020;46:99–112.
- Gauthier L, Dortet L, Cotellon G, et al. Diversity of Carbapenemase-producing Escherichia coli isolates in France in 2012-2013. Antimicrob Agents Chemother. 2018;62(8):e00266-18.
- Albiger B, Glasner C, Struelens MJ, et al. European survey of Carbapenemase-Producing Enterobacteriaceae working g. Carbapenemase-producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries, May 2015. Euro Surveill. 2015;20(45). DOI:https://doi.org/10.2807/1560-7917.ES.2015.20.45.30062
- Brolund A, Lagerqvist N, Byfors S, et al. Worsening epidemiological situation of carbapenemase-producing Enterobacteriaceae in Europe, assessment by national experts from 37 countries, July 2018. Euro Surveill. 2019;24(9):1900123.
- Giannella M, Trecarichi EM, Giacobbe DR, et al. Effect of combination therapy containing a high-dose carbapenem on mortality in patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Int J Antimicrob Agents. 2018;51(2):244–248.
- Falcone M, Giordano C, Barnini S, et al. Extremely drug-resistant NDM-9-producing ST147 Klebsiella pneumoniae causing infections in Italy, May 2020. Euro Surveill. 2020;25(48):2001779.
- Colomb-Cotinat M, Soing-Altrach S, Leon A, et al. Emerging extensively drug-resistant bacteria (eXDR) in France in 2018. Med Mal Infect. 2020;50(8):715–722.
- Wang Q, Wang X, Wang J, et al. Phenotypic and Genotypic Characterization of Carbapenem-resistant Enterobacteriaceae : data From a Longitudinal Large-scale CRE Study in China (2012–2016). Clin Infect Dis. 2018;67(suppl_2):S196–S205.
- Zhu J, Li Q, Li X, et al. Successful control of the first carbapenem-resistant Klebsiella pneumoniae outbreak in a Chinese hospital 2017–2019. Antimicrob Resist Infect Control. 2020;9(1):91.
- Mitgang EA, Hartley DM, Malchione MD, et al. Review and mapping of carbapenem-resistant Enterobacteriaceae in Africa: using diverse data to inform surveillance gaps. Int J Antimicrob Agents. 2018;52(3):372–384.
- Olowo-Okere A, Ibrahim YKE, Olayinka BO, et al. Phenotypic and genotypic characterization of clinical carbapenem-resistant Enterobacteriaceae isolates from Sokoto, northwest Nigeria. New Microbes New Infect. 2020;37:100727.
- Manenzhe RI, Zar HJ, Nicol MP, et al. The spread of carbapenemase-producing bacteria in Africa: a systematic review. J Antimicrob Chemother. 2015;70(1):23–40.
- Singh-Moodley A, Perovic O. Antimicrobial susceptibility testing in predicting the presence of carbapenemase genes in Enterobacteriaceae in South Africa. BMC Infect Dis. 2016;16(1):536.
- Ramos-Castaneda JA, Ruano-Ravina A, Barbosa-Lorenzo R, et al. Mortality due to KPC carbapenemase-producing Klebsiella pneumoniae infections: systematic review and meta-analysis: mortality due to KPC Klebsiella pneumoniae infections. J Infect. 2018;76(5):438–448.
- Chang H, Wei J, Zhou W, et al. Risk factors and mortality for patients with Bloodstream infections of Klebsiella pneumoniae during 2014–2018: clinical impact of carbapenem resistance in a large tertiary hospital of China. J Infect Public Health. 2020;13(5):784–790.
- Bassetti M, Giacobbe DR, Giamarellou H, et al. Management of KPC-producing Klebsiella pneumoniae infections. Clin Microbiol Infect. 2018;24(2):133–144.
- Tamma PD, Han JH, Rock C, et al. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis. 2015;60(9):1319–1325.
- Wu Y-E, Xu H-Y, Shi H-Y, et al. Carbapenem-Resistant Enterobacteriaceae bloodstream infection treated successfully with high-dose Meropenem in a preterm neonate. Front Pharmacol. 2020;11:566060.
- Bassetti M, Peghin M. How to manage KPC infections. Ther Adv Infect Dis. 2020;7:2049936120912049.
- Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55:943–950.
- Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56(4):2108–2113.
- Russo A, Giuliano S, Ceccarelli G, et al. Comparison of septic shock due to Multidrug-Resistant Acinetobacter baumannii or Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae in intensive care unit patients. Antimicrob Agents Chemother. 2018;62(6):e02562-17.
- Lodise TP, Berger A, Altincatal A, et al. Antimicrobial resistance or delayed appropriate therapy—does one influence outcomes more than the other among patients with serious infections due to Carbapenem-Resistant Versus Carbapenem-Susceptible Enterobacteriaceae? Open Forum Infect Dis. 2019;6(6):ofz194.
- Oliva A, Scorzolini L, Castaldi D, et al. Double-carbapenem regimen, alone or in combination with colistin, in the treatment of infections caused by carbapenem-resistant Klebsiella pneumoniae (CR-Kp). J Infect. 2017;74(1):103–106.
- Souli M, Karaiskos I, Masgala A, et al. Double-carbapenem combination as salvage therapy for untreatable infections by KPC-2-producing Klebsiella pneumoniae. Eur J Clin Microbiol Infect Dis. 2017;36(7):1305–1315.
- Giamarellou H, Galani L, Baziaka F, et al. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57(5):2388–2390.
- Cprek JB, Gallagher JC. Ertapenem-containing double-Carbapenem therapy for treatment of infections caused by Carbapenem-Resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2016;60(1):669–673.
- Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2011;55(6):3002–3004.
- Kollef MH, Ricard J-D, Roux D, et al. A randomized trial of the amikacin fosfomycin inhalation system for the adjunctive therapy of Gram-Negative Ventilator-associated pneumonia: IASIS trial. Chest. 2017;151(6):1239–1246.
- Rello J, Sole-Lleonart C, Rouby -J-J, et al. Use of nebulized antimicrobials for the treatment of respiratory infections in invasively mechanically ventilated adults: a position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin Microbiol Infect. 2017;23(9):629–639.
- Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect. 2011;17(12):1798–1803.
- Patel G, Huprikar S, Factor SH, et al. Outcomes of Carbapenem-Resistant Klebsiella pneumoniae Infection and the Impact of Antimicrobial and Adjunctive Therapies. Infect Control Hosp Epidemiol. 2008;29(12):1099–1106.
- Falcone M, Russo A, Iacovelli A, et al. Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase–producing K. pneumoniae. Clinical Microbiology and Infection. 2016;22(5):444–450.
- Doi Arakawa Y, Arakawa Y. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin Infect Dis. 2007;45(1):88–94.
- Zhanel GG, Lawson CD, Zelenitsky S, et al. Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert Rev Anti Infect Ther. 2012;10(4):459–473.
- Clark JA, Burgess DS. Plazomicin: a new aminoglycoside in the fight against antimicrobial resistance. Ther Adv Infect Dis. 2020;7:2049936120952604.
- Walkty A, Karlowsky JA, Baxter MR, et al. In Vitro Activity of Plazomicin against Gram-Negative and Gram-Positive Bacterial Pathogens Isolated from Patients in Canadian Hospitals from 2013 to 2017 as Part of the CANWARD Surveillance Study. Antimicrob Agents Chemother. 2019;63(1):e01832-18.
- Wagenlehner FME, Cloutier DJ, Komirenko AS, et al. Once-Daily Plazomicin for Complicated Urinary Tract Infections. N Engl J Med. 2019;380(8):729–740.
- Castanheira M, Deshpande LM, Woosley LN, et al. Activity of plazomicin compared with other aminoglycosides against isolates from European and adjacent countries, including Enterobacteriaceae molecularly characterized for aminoglycoside-modifying enzymes and other resistance mechanisms. J Antimicrob Chemother. 2018;73(12):3346–3354.
- McKinnell JA, Dwyer JP, Talbot GH, et al. Plazomicin for Infections Caused by Carbapenem-Resistant Enterobacteriaceae. N Engl J Med. 2019;380(8):791–793.
- Zhanel GG, Adam HJ, Baxter MR, et al. 42936 pathogens from Canadian hospitals: 10 years of results (2007–16) from the CANWARD surveillance study. J Antimicrob Chemother. 2019;74(Supplement_4):iv5–iv21.
- Bail L, Ito CAS, Arend L, et al. Distribution of genes encoding 16S rRNA methyltransferase in plazomicin-nonsusceptible carbapenemase-producing Enterobacterales in Brazil. Diagn Microbiol Infect Dis. 2021;99(2):115239.
- Tsuji BT, Pogue JM, Zavascki AP, et al., International Consensus Guidelines for the Optimal Use of the Polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39(1):10–39.
- Wistrand-Yuen P, Olsson A, Skarp K-P, et al. Evaluation of polymyxin B in combination with 13 other antibiotics against carbapenemase-producing Klebsiella pneumoniae in time-lapse microscopy and time-kill experiments. Clin Microbiol Infect. 2020;26(9):1214–1221.
- De Oliveira MS, De Assis DB, Freire MP, et al. Treatment of KPC-producing Enterobacteriaceae: suboptimal efficacy of polymyxins. Clin Microbiol Infect. 2015;21(2):e1–7.
- Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391–400.
- Zak-Doron Y, Dishon Benattar Y, Pfeffer I, et al. The association between empirical antibiotic treatment and mortality in severe infections caused by Carbapenem-resistant Gram-negative Bacteria: a Prospective Study. Clin Infect Dis. 2018;67(12):1815–1823.
- Andrade FF, Silva D, Rodrigues A, et al. Colistin update on its mechanism of action and resistance, present and future challenges. Microorganisms. 2020;8(11):1716. DOI:10.3390
- Perdigao Neto LV, Oliveira MS, Orsi TD, et al. Alternative drugs against multiresistant Gram-negative bacteria. J Glob Antimicrob Resist. 2020;23:33–37.
- Peleg AY, Potoski BA, Rea R, et al. Acinetobacter baumannii bloodstream infection while receiving tigecycline: a cautionary report. J Antimicrob Chemother. 2006;59(1):128–131.
- Freire AT, Melnyk V, Kim MJ, et al. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis. 2010;68(2):140–151.
- Cheng A, Chuang Y-C, Sun H-Y, et al. Excess mortality associated with Colistin-Tigecycline Compared with Colistin-Carbapenem Combination therapy for extensively Drug-Resistant Acinetobacter baumannii Bacteremia: a Multicenter prospective observational study. Crit Care Med. 2015;43(6):1194–1204.
- Zhanel GG, Cheung D, Adam H, et al. Review of Eravacycline, a novel Fluorocycline Antibacterial Agent. Drugs. 2016;76(5):567–588.
- Zhanel GG, Baxter MR, Adam HJ, et al. In vitro activity of eravacycline against 2213 Gram-negative and 2424 Gram-positive bacterial pathogens isolated in Canadian hospital laboratories: CANWARD surveillance study 2014–2015. Diagn Microbiol Infect Dis. 2018;91(1):55–62.
- Clark JA, Kulengowski B, Burgess DS. In-vitro activity of Eravacycline compared to Tigecycline and Carbapenem-resistant Enterobacteriaceae. Int J Antimicrob Agents. 2020;56(6):106178.
- Zhao C, Wang X, Zhang Y, et al. In vitro activities of Eravacycline against 336 isolates collected from 2012 to 2016 from 11 teaching hospitals in China. BMC Infect Dis. 2019;19(1):508.
- Morrissey I, Olesky M, Hawser S, et al. In vitro activity of Eravacycline against Gram-Negative Bacilli Isolated in Clinical Laboratories Worldwide from 2013 to 2017. Antimicrob Agents Chemother. 2020;22(5):556-561;64(3):e01699-19.
- Grant-Di Felice V, Efimova E, Izmailyan S, et al. Efficacy and Tolerability of Eravacycline in Bacteremic Patients with Complicated Intra-Abdominal Infection: a Pooled Analysis from the IGNITE1 and IGNITE4 Studies. Surg Infect (Larchmt). 2020;22(5):556-561. DOI:https://doi.org/10.1089/sur.2020.241
- Zhanel GG, Zhanel MA, Karlowsky JA. Intravenous Fosfomycin: an Assessment of Its Potential for Use in the Treatment of Systemic Infections in Canada. Can J Infect Dis Med Microbiol. 2018;2018:8912039.
- Aprile A, Scalia G, Stefani S, et al. In vitro fosfomycin study on concordance of susceptibility testing methods against ESBL and carbapenem-resistant Enterobacteriaceae. J Glob Antimicrob Resist. 2020;23:286–289.
- Leelawattanachai P, Wattanavijitkul T, Paiboonvong T, et al. Evaluation of Intravenous Fosfomycin Disodium Dosing Regimens in Critically Ill Patients for Treatment of Carbapenem-Resistant Enterobacterales Infections Using Monte Carlo Simulation. Antibiotics (Basel). 2020;9(9):615.
- Putensen C, Ellger B, Sakka SG, et al. Current clinical use of intravenous fosfomycin in ICU patients in two European countries. Infection. 2019;47(5):827–836.
- Falagas ME, Kastoris AC, Kapaskelis AM, et al. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum β-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis. 2010;10(1):43–50.
- Elliott ZS, Barry KE, Cox HL, et al. The Role of fosA in Challenges with Fosfomycin Susceptibility Testing of Multispecies Klebsiella pneumoniae Carbapenemase-Producing Clinical Isolates. J Clin Microbiol. 2019;57(10):57.
- Farfour E, Degand N, Riverain E, et al. Fosfomycin, from susceptibility to resistance: impact of the new guidelines on breakpoints. Med Mal Infect. 2020;50(7):611–616.
- Lee S-Y, Park Y-J, Yu JK, et al. Prevalence of acquired fosfomycin resistance among extended-spectrum -lactamase-producing Escherichia coli and Klebsiella pneumoniae clinical isolates in Korea and IS26-composite transposon surrounding fosA3. J Antimicrob Chemother. 2012;67(12):2843–2847.
- Karageorgopoulos DE, Miriagou V, Tzouvelekis LS, et al. Emergence of resistance to fosfomycin used as adjunct therapy in KPC Klebsiella pneumoniae bacteraemia: report of three cases. J Antimicrob Chemother. 2012;67:2777–2779.
- Onufrak NJ, Smith NM, Satlin MJ, et al. In pursuit of the triple crown: mechanism-based pharmacodynamic modelling for the optimization of three-drug combinations against KPC-producing Klebsiella pneumoniae. Clin Microbiol Infect. 2020;26(9):1256.e1–1256.e8.
- Montravers P, Bassetti M. The ideal patient profile for new beta-lactam/beta-lactamase inhibitors. Curr Opin Infect Dis. 2018;31(6):587–593.
- Zhanel GG, Lawson CD, Adam H, et al. Ceftazidime-Avibactam: a Novel Cephalosporin/β-lactamase Inhibitor Combination. Drugs. 2013;73(2):159–177.
- Shields RK, Potoski BA, Haidar G, et al. Clinical Outcomes, Drug Toxicity, and Emergence of Ceftazidime-Avibactam Resistance Among Patients Treated for Carbapenem-Resistant Enterobacteriaceae Infections: table 1. Clin Infect Dis. 2016;63(12):1615–1618.
- Ehmann DE, Jahic H, Ross PL, et al. Avibactam is a covalent, reversible, non- -lactam -lactamase inhibitor. Proc Natl Acad Sci U S A. 2012;109(29):11663–11668.
- Durand CR, Alsharhan M, Willett KC. New and emerging antibiotics for complicated intra-abdominal infections. Am J Ther. 2017;24(6):e763–e9.
- Sheu -C-C, Chang Y-T, Lin S-Y, et al. Infections caused by Carbapenem-Resistant Enterobacteriaceae: an update on therapeutic options. Front Microbiol. 2019;10:80.
- Pogue JM, Bonomo RA, Kaye KS. Ceftazidime/Avibactam, Meropenem/Vaborbactam, or Both? Clinical and Formulary Considerations. Clin Infect Dis. 2019;68(3):519–524.
- Tumbarello M, Trecarichi EM, Corona A, et al. Efficacy of Ceftazidime-Avibactam Salvage Therapy in Patients With Infections Caused by Klebsiella pneumoniae Carbapenemase–producing K. pneumoniae. Clinical Infectious Diseases. 2019;68(3):355–364.
- Marshall S, Hujer AM, Rojas LJ, et al. Can Ceftazidime-Avibactam and Aztreonam Overcome beta-Lactam Resistance Conferred by Metallo-beta-Lactamases in Enterobacteriaceae? Antimicrob Agents Chemother. 2017;61(4):e02243–16.
- Shaw E, Rombauts A, Tubau F, et al. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J Antimicrob Chemother. 2018;73(4):1104–1106.
- Yasmin M, Fouts DE, Jacobs MR, et al. Monitoring Ceftazidime-Avibactam and Aztreonam Concentrations in the Treatment of a Bloodstream Infection Caused by a Multidrug-Resistant Enterobacter sp. Carrying Both Klebsiella pneumoniae Carbapenemase–4 and New Delhi Metallo-β-Lactamase–1. Clin Infect Dis. 2020;71(4):1095–1098.
- Zhanel GG, Lawrence CK, Adam H, et al. Imipenem–Relebactam and Meropenem–Vaborbactam: two Novel Carbapenem-β-Lactamase Inhibitor Combinations. Drugs. 2018;78(1):65–98.
- Lob SH, Karlowsky JA, Young K, et al. In vitro activity of imipenem-relebactam against resistant phenotypes of Enterobacteriaceae and Pseudomonas aeruginosa isolated from intraabdominal and urinary tract infection samples – SMART Surveillance Europe 2015–2017. J Med Microbiol. 2020;69(2):207–217.
- Karlowsky JA, Lob SH, Kazmierczak KM, et al. In vitro activity of imipenem/relebactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated from intraabdominal and urinary tract infection samples: SMART Surveillance United States 2015–2017. J Glob Antimicrob Resist. 2020;21:223–228.
- Motsch J, Murta De Oliveira C, Stus V, et al. RESTORE-IMI 1: a multicenter, randomized, double-blind trial comparing efficacy and safety of Imipenem/Relebactam vs Colistin Plus Imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin Infect Dis. 2020;70(9):1799–1808.
- Wenzler E, Scoble PJ. An appraisal of the Pharmacokinetic and Pharmacodynamic Properties of Meropenem-Vaborbactam. Infect Dis Ther. 2020;9(4):769–784.
- Pfaller MA, Huband MD, Mendes RE, et al. In vitro activity of meropenem/vaborbactam and characterisation of carbapenem resistance mechanisms among carbapenem-resistant Enterobacteriaceae from the 2015 meropenem/vaborbactam surveillance programme. Int J Antimicrob Agents. 2018;52(2):144–150.
- Shoulders BR, Casapao AM, Venugopalan V. An Update on Existing and Emerging Data for Meropenem-Vaborbactam. Clin Ther. 2020;42(4):692–702.
- Bhowmick T, Weinstein MP. Microbiology of Meropenem-Vaborbactam: a novel Carbapenem Beta-Lactamase Inhibitor Combination for Carbapenem-Resistant Enterobacterales Infections. Infect Dis Ther. 2020;9(4):757–767.
- Petty LA, Henig O, Patel TS, et al. Overview of meropenem-vaborbactam and newer antimicrobial agents for the treatment of carbapenem-resistant Enterobacteriaceae. Infect Drug Resist. 2018;11:1461–1472.
- Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, et al. Effect and safety of Meropenem–Vaborbactam versus best-available therapy in patients with Carbapenem-resistant Enterobacteriaceae Infections: the TANGO II Randomized clinical trial. Infect Dis Ther. 2018;7(4):439–455.
- Ackley R, Roshdy D, Meredith J, et al. Meropenem-Vaborbactam versus Ceftazidime-Avibactam for Treatment of Carbapenem-Resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2020.(5):e02313–19.
- Athans V, Neuner EA, Hassouna H, et al. Meropenem-Vaborbactam as Salvage therapy for Ceftazidime-Avibactam-Resistant Klebsiella pneumoniae Bacteremia and Abscess in a Liver Transplant Recipient. Antimicrob Agents Chemother. 2019;63(1):e01551–18.
- Tsivkovski R, Lomovskaya O. Potency of Vaborbactam Is Less Affected than That of Avibactam in Strains Producing KPC-2 Mutations That Confer Resistance to Ceftazidime-Avibactam. Antimicrob Agents Chemother. 2020;64(4):e01936–19.
- Alosaimy S, Jorgensen SCJ, Lagnf AM, et al. Real-world multicenter analysis of clinical outcomes and safety of Meropenem-Vaborbactam in patients treated for serious Gram-Negative bacterial infections. Open Forum Infect Dis. 2020;7(3):ofaa051.
- Lasko MJ, Nicolau DP. Carbapenem-Resistant Enterobacterales: considerations for Treatment in the Era of New Antimicrobials and Evolving Enzymology. Curr Infect Dis Rep. 2020;22(3):6.
- Groft LM, Claeys KC, Heil EL. An evaluation of meropenem/vaborbactam for the treatment of nosocomial pneumonia. Expert Opin Pharmacother. 2020;22(3):265–271.
- Zhanel GG, Chung P, Adam H, et al. Ceftolozane/Tazobactam: a Novel Cephalosporin/β-Lactamase Inhibitor Combination with activity against Multidrug-Resistant Gram-Negative Bacilli. Drugs. 2014;74(1):31–51.
- Zhanel GG, Golden AR, Zelenitsky S, et al. Cefiderocol: a Siderophore Cephalosporin with activity against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli. Drugs. 2019;79(3):271–289.
- Abdul‐Mutakabbir JC, Alosaimy S, Morrisette T, et al. Cefiderocol: a novel Siderophore Cephalosporin against Multidrug-Resistant Gram-Negative Pathogens. Pharmacotherapy. 2020;40(12):1228–1247.
- Hackel MA, Tsuji M, Yamano Y, et al. In Vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against Carbapenem-Nonsusceptible and Multidrug-Resistant Isolates of Gram-Negative Bacilli Collected Worldwide in 2014 to 2016. Antimicrob Agents Chemother. 2018;62:e0196817.
- Lampejo T, Cherian BP, Tan MGM, et al. Cefiderocol in the treatment of systemic carbapenemase-producing multidrug-resistant Klebsiella pneumoniae infection. J Glob Antimicrob Resist. 2020;23:338–339.
- Falcone M, Tiseo G, Nicastro M, et al. Cefiderocol as Rescue Therapy for Acinetobacter baumannii and Other Carbapenem-resistant Gram-negative Infections in Intensive Care Unit Patients. Clin Infect Dis. 2020;72(11):2021–2024. DOI:https://doi.org/10.1093/cid/ciaa1410.
- Bassetti M, Echols R, Matsunaga Y, et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis. 2020;21(2);226-240.
- Wunderink RG, Matsunaga Y, Ariyasu M, et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2021:21(2);213–225.